Abstract
Rickettsial diseases are some of the most covert reemerging infections of the present times. They are generally incapacitating and notoriously difficult to diagnose; untreated cases can have fatality rates as high as 30%–35%, but when diagnosed properly, they are often easily treated but lack of definite diagnostic tools and the hazards of handling these microorganisms aggravate the difficulties of diagnosis and treatment.
Keywords: Indian tick typhus, purpura fulminans, rickettsiae
What was known?
Rickettsial infections are one of the important causes of fever of unknown origin (FUO), often misdiagnosed leading to life threatening condition
Weil-Felix test will provide clue to the diagnosis.
Introduction
Purpura fulminans (PF) is a life-threatening emergency, in which there is skin necrosis and disseminated intravascular coagulation (DIC). It was first described by Guillotine 1884. There are tissue necrosis, small vessel thrombosis, and DIC. PF may be categorized as PF due to inherited abnormalities of protein C or other coagulation systems, acute infectious PF, and idiopathic PF.[1] Rickettsial infections are one of the important causes of fever of unknown origin (FUO) and this needs to be differentiated from other febrile illnesses such as enteric fever, malaria, dengue, leptospirosis, and infectious mononucleosis.[2] Rickettsial infections are prevalent worldwide but are often undiagnosed/misdiagnosed leading to a life-threatening condition.[3] Hereby, we report a life-threatening case of Indian tick typhus (ITT) presenting with atypical skin manifestations in the form of PF (retiform purpura all over the body). The Weil–Felix (W-F) test was useful in confirming the diagnosis.
Case Report
A 48-year-old female patient from Davanagere, Karnataka, presented to the medical emergency room of S. S. Institute of Medical Sciences and Research Centre, with fever of 1 week, headache and vomiting of 3 day duration, and altered sensorium of 1 day duration. On examination, vitals were normal. The patient was disoriented, not responding to verbal commands, but moving all the limbs. No lymphadenopathy, no skin manifestations were present during admission. The patient is a known case of Type 2 diabetes mellitus and hypertension since 3 years. Routine investigations showed total count – 32,900 cells/cumm (neutrophils - 86%; lymphocytes - 5%; eosinophils - 2%; monocytes - 7%), platelets 1.20 lakhs/cumm, decreased serum sodium (123.0 mmol/L) and chloride (90.4 mmol/L), and raised urea (64.8 mg/dl) and creatinine (2.8 mg/dl), raised liver enzymes (alkaline phosphatase 291.4 U/L; gamma-glutamyl transferase 70.3 U/L) with increased serum bilirubin (total bilirubin 4.4 mg/dl; direct bilirubin 2.9 mg/dl), activated partial thromboplastin time 57.6 s (normal 22–35 s). The patient was started on broad spectrum antibiotics (intravenous [IV] ceftriaxone 2 g and IV clindamycin 600 mg). On the second day, patient developed a maculopapular rash all over the body excluding face, palms, and soles. Later, during the day, the patient developed acute breathlessness, and her oxygen saturation started to drop to 90% and the patient was shifted to the Intensive Care Unit and started on inotropic drugs and supportive treatment. Four days later, a generalized retiform purpuric rash was noted all over the body along with high-grade fever [Figures 1 and 2]. Mucosa, palms, and soles were not involved. Histopathology of retiform purpura showed epidermal atrophy with dermal edema, perivascular inflammatory infiltrate in the dermis with microthrombi in the dermal vessels and extravasated erythrocytes. Cerebrospinal fluid cytology revealed 18 cells (lymphocytes - 16, neutrophils - 2); and biochemical parameters being proteins 65 mg/dl; glucose 90 mg/dl, ultrasound abdomen showed mild hepatosplenomegaly with fatty liver and right-sided mild pleural effusion. Blood was negative for malaria parasite; serology for dengue and leptospira was negative; W-F test showed a titer of 1:160 for OX-2; 1:40 for OX-19 and OX-K. The patient was started on doxycycline 100 mg twice daily. After about 5 days of treatment with doxycycline, the patient improved neurologically and was hemodynamically stable. The blood urea, serum creatinine improved, serum bilirubin decreased from 4.4 to 1.4 mg/dl, direct bilirubin from 2.9 to 0.7 mg/dl. Cutaneous manifestations improved with regress in the retiform rash and the patient was discharged after 10 days of hospital stay.
Figure 1.
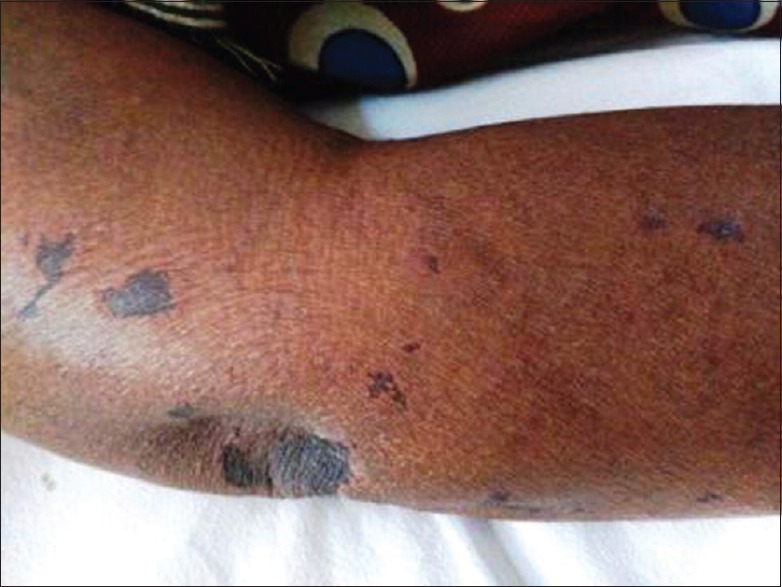
Retiform purpura over the elbow
Figure 2.
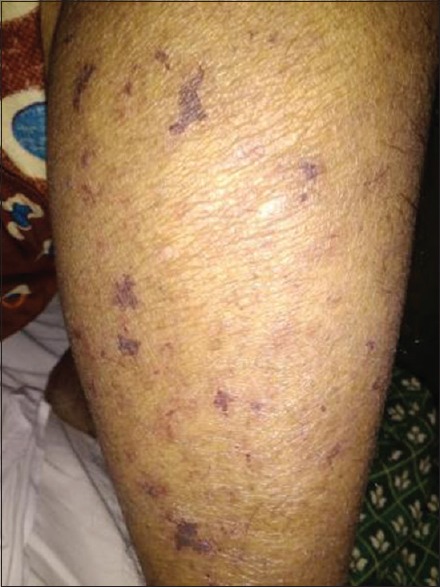
Retiform purpura over leg
Discussion
Rickettsial diseases are vector-borne diseases, vectors being ticks, flea, mite, and louse. They commonly affect the travelers to endemic areas. In India, they are reported from Maharashtra, Tamil Nadu, Karnataka, Kerala, Jammu and Kashmir, Uttarakhand, Himachal Pradesh, Rajasthan, Assam, and West Bengal.[4]
Rickettsial infections are caused by a variety of obligate intracellular, Gram-negative bacteria from the genera Rickettsia, Orientia, Ehrlichia, Neorickettsia, Neoehrlichia, and Anaplasma, belonging to the alphaproteobacteria. Rickettsia is classically divided into the typhus group and spotted fever group (SFG) although the genus has been subdivided further based on phylogenetic analysis. Orientia species makes up the scrub typhus group. Rickettsial diseases are zoonoses where human beings are accidentally involved in a chain of transmission between trombiculid mites (chiggers), ticks or fleas, and rodents.[5]
Case definition of rickettsial infection
Definition of suspected/clinical case
Acute undifferentiated febrile illness of 5 days or more with or without eschar should be suspected as a case of rickettsial infection (if eschar is present, fever of <5 days duration should be considered as scrub typhus). Other presenting features may be headache and rash (rash more often seen in fair persons), lymphadenopathy, multi-organ involvement such as liver, lung, and kidney, and acute respiratory distress. The differential diagnosis of dengue, malaria, pneumonia, leptospirosis, and typhoid should be kept in mind.
Definition of probable case
A suspected clinical case showing titers of 1:80 or above in OX-2, OX-19, and OX-K antigens by W-F test, and an optical density >0.5 for IgM by enzyme-linked immunosorbent assay (ELISA) is considered positive for members of typhus and SFGs of rickettsiae.
Definition of confirmed case
A confirmed case is the one in which (a) rickettsial DNA is detected in eschar samples or whole blood by polymerase chain reaction (PCR) or (b) rising antibody titers on acute and convalescent serum samples detected by indirect immunofluorescence assay (IFA).[5]
Epidemic Typhus
The causative agent of epidemic typhus is Rickettsia Prowazekii, named after von Prowazek, who died of typhus fever while investigating typhus fever. The infection is transmitted when the contaminated louse feces is rubbed through the minute abrasions caused by scratching. Occasionally, the infection may also be transmitted by aerosols of dried louse feces through inhalation or through conjunctiva. In some patients who recover from the disease, the rickettsiae may remain latent in the lymphoid tissue or organs for years. Such latent infection may, at times, be reactivated leading to recrudescent typhus (Brill–Zinsser disease).[6]
Murine Typhus
Murine or flea-borne typhus is a mild form of disease caused by Rickettsia typhi (Rickettsia mooseri). Humans acquire the disease usually through the bite of infected fleas when their saliva or feces is rubbed in or through aerosols of dried feces. Human infection is a dead end. Endemic typhus is worldwide in prevalence but not of much public health importance as the disease is mild and can be easily controlled now.[6]
Scrub Typhus
Scrub typhus, also called tsutsugamushi disease is caused by Orientia tsutsugamushi. It differs from other rickettsia species by its lack of polysaccharide cell wall and wide heterogeneity within the genus and thus deserves special emphasis during serological tests. In India, the disease was first noted among troops during World War II in Assam and West Bengal, during the Indo-Pak war in 1965. Virulent strains of O. tsutsugamushi are associated with hemorrhagic and intravascular coagulation, PF, atypical pneumonia, acute respiratory distress syndrome (ARDS), myocarditis, jaundice, and meningoencephalitis in addition to skin rash.[7]
Indian Tick Typhus
ITT is a tick-borne rickettsiosis prevalent in India. It is a rickettsial spotted fever (SF) similar to rocky mountain SF (RMSF) and is caused by Rickettsia conorii. The etiological agent was first isolated from India in 1950 from a brown dog tick, Rhipicephalus sanguineus. The disease is reported from Maharashtra, Tamil Nadu, Karnataka, Kerala, Jammu and Kashmir, Uttarakhand, Himachal Pradesh, Rajasthan, Assam, and West Bengal. The dog tick, R. sanguineus, is the principal vector of ITT although some species of Haemaphysalis and Hyalomma may also transmit the infection. An extensive study on tick-borne rickettsiosis in Pune district of Maharashtra revealed that ITT exists as zoonosis.[8]
Although the disease has been recognized clinically, cases have been documented only rarely, mainly by nonspecific serological tools, such as W-F test.[9] In ITT, maculopapular rash is present, inoculation eschar is rare, lymphadenopathy is generally absent. The disease needs to be differentiated from meningococcemia, brucellosis, malaria, and typhoid fever.[10] The disease is characterized by sudden onset of moderate- to high-grade fever, malaise, deep muscle pain, headache, and conjunctival suffusion.[11] Rash is usually maculopapular to start with, begins on the third day of fever in the extremities, moves centripetally, and involves rest of the body. ITT also differs from Mediterranean SF, the disease caused by the type strain of R. conorii, in that the rash is often purpuric and an inoculation eschar at the bite site is seldom identified.[12]
Rocky Mountain Spotted Fever
RMSF is a tick-borne zoonotic disease caused by infection with Rickettsia rickettsii. RMSF is reported throughout most of the contiguous United States, and the annual incidence rate has increased from 2.0 cases/1,000,000 persons in 1992 to 3.8 cases in 2002/1,000,000 persons annually. Symptoms of RMSF typically include acute-onset fever, headache, and myalgia within 14 days of a bite from an infected tick. Severe cases may include ARDS, disseminated intravascular coagulopathy, and multi-organ failure.[12]
Rickettsial Pox
Rickettsialpox is a worldwide mite-borne zoonosis caused by Rickettsia akari. Rickettsial pox has been isolated with homelessness and IV drug abuse. The incubation period lasts 6–15 days. Clinically, an eschar at the site of inoculation with eventual regional lymphadenopathy occurs first. Rickettsialpox is among the rare rickettsioses causing a vesicular eruption, which include Queensland tick typhus and African tick bite fever. Rickettsialpox is generally benign and self-limiting, but neurological symptoms such as photophobia, vertigo, pain on movement of the eyes, and nuchal rigidity may be severe enough to warrant lumbar puncture.[13]
Q Fever
Q fever is a bacterial infection affecting mainly the lungs, liver, and heart. It is found around the world and is caused by bacteria Coxiella burnetii. It usually takes about 20 days after exposure to the bacteria for symptoms to occur. Complications are cirrhosis, hepatitis, encephalitis, interstitial pulmonary fibrosis, meningitis, and pneumonia. People at risk should always carefully dispose of animal products that may be infected, disinfect any contaminated areas, and thoroughly wash their hands. Pasteurizing milk can also help prevent Q fever.[14]
Trench Fever
Trench fever, the first clinical manifestation attributed to Bartonella quintana, affected an estimated >1 million people during World War I. The name, trench fever, was mentioned for the first time in 1915. B. quintana is a facultative, intracellular, Gram-negative rod belonging to the α2 subgroup of proteobacteria. B. quintana is located in erythrocytes during asymptomatic bacteremia and has been observed in erythroblasts in bone marrow in bacteremic patients. The bacterium has a tropism for endothelial cells, leading to angioproliferative lesions, as observed in bacillary angiomatosis.[15]
Laboratory Findings
Blood parameters
Total leukocyte count during the early course of the disease is normal to low normal with marked shift to the left. Later, in the course of the disease, it shows leukocytosis in 30% of cases. Low platelet counts are present in about 60% of cases. Erythrocyte sedimentation rate is usually high. Hyponatremia and hypoalbuminemia, reflecting increased vascular permeability, are sometimes helpful in differentiating rickettsial infections from other acute infections. Hepatic transaminases values are frequently elevated. Blood urea is elevated due to prerenal mechanisms.[4]
Serology
Microimmunofluorescence, immunoperoxidase assay (IPA), latex agglutination, indirect hemagglutination, ELISA, dot blot immunoassay, and W-F are the various serological methods available for the diagnosis of rickettsial diseases.
Weil–Felix test
The diagnosis of rickettsial diseases was greatly aided when Weil and Felix discovered in 1916 that patient sera agglutinated certain Proteus antigens. The W-F reaction is still widely used and should be reserved only for situations in which other serological tests are not available. Extensive reports on the use of the W-F test have indicated that it detects many more positive cases than that are misdiagnosed. Broadly, the W-F test does provide at least some clues regarding the nature of the infection, which can be cross-confirmed using other techniques, if available.[16]
W-F test is the oldest assay based on detection of antibody to various Proteus antigens that cross-react with rickettsia. Although it lacks specificity and sensitivity, it may be used in developing countries as a first diagnostic step in the diagnosis of rickettsial diseases. W-F test detects IgM antibody detectable 5–10 days following the onset of symptoms. Whole cells of Proteus vulgaris OX-2 react strongly with serum from a person infected with SFG rickettsiae with the exception of those with RMSF, and whole cells of P. vulgaris OX-19 react with serum from persons infected from typhus group rickettsial as well as with RMSF. Furthermore, OX-K strains of Proteus mirabilis agglutinate with serum from scrub typhus patients.[17] Either a 4-fold rise in agglutinin titer in paired sera or a single titer of more than 1:320 is considered diagnostic for infection with these febrile agents.[4]
One of the major limitations of W-F test is the cross-reactivity among several rickettsial species. Specific investigations such as immunofluorescence, western blot, or PCR-based tests, which are helpful in making an accurate diagnosis of rickettsial diseases, are expensive and not readily available.[18]
Immunofluorescence assay
IFA is currently considered to be the reference serological method. However, it cannot determine the causative agent to the species level. PCR to detect rickettsiae in blood or tissue provides promise for early diagnosis. PCR testing and immunohistochemical staining of skin specimen obtained by performing a biopsy may help confirm the clinical diagnosis in patients with rash. However, serology remains the mainstay of diagnosis because these tests are expensive and less available to clinicians.[19]
Polymerase chain reaction assay
It is a rapid and specific test for diagnosis. It can be used to detect rickettsial DNA in whole blood, buffy coat fraction, or tissue specimen. It is the most rapid assay for the diagnosis.[4] The results are best within the first week for blood samples because of the presence of rickettsemia.[5]
Indirect immunoperoxidase assay
It gives comparable result as IFA but requires special instrument and experienced personnel for interpretation of the test.[5]
Treatment
There is paucity of evidence based on randomized controlled trials for the management of rickettsial diseases including scrub typhus. The following guidelines for treatment cover the most common infection, scrub typhus, murine typhus, and ITT and do not cover acute Q fever though the treatment of Q fever is on the similar lines. Without waiting for laboratory confirmation of the rickettsial infection, antibiotic therapy should be instituted when rickettsial disease is suspected.[5]
In vitro studies have shown that rickettsiae are susceptible to chloramphenicol, tetracycline, rifampicin, and some fluoroquinolones but are resistant to penicillins, cephalosporins, aminoglycosides, trimethoprim-sulfamethoxazole, and erythromycin.[20]
At primary level: The health-care provider needs to do the following:
Recognition of disease severity. If the patient comes with complications to primary health facility and treating physician considers it as rickettsial infection, treatment with doxycycline should be initiated before referring the patient
Referral to secondary or tertiary center in case of complications such as ARDS, acute renal failure, meningoencephalitis, and multi-organ dysfunction. In addition to recommended management of pneumonia, treatment of scrub typhus (doxycycline) is to be provided to the patient
-
In fever cases of duration of 5 days or more where malaria, dengue, and typhoid have been ruled out, the following drugs should be administered:
-
In adults: (a) Doxycycline 200 mg/day in two divided doses for individuals above 45 kg for a duration of 7 days or treatment may be terminated 2–3 days after the patient is afebrile. In louse-borne (epidemic) typhus, a single oral dose of 200 mg and repeat once later if necessary or in a short course of 100–200 mg daily for 3 days. (b) Chloramphenicol 500 mg 6 hourly orally for 7 days, (c) azithromycin 500 mg in a single dose for 5 days, may be used as an alternative[5,19]If the clinical sign and symptoms persist, an alternative diagnosis should be considered
- In children: In the pediatric population, shorter courses of doxycycline may be administered to minimize possible side effects therapy with doxycycline 4.5 mg/kg body weight in two divided doses administered orally for children below 45 kg or azithromycin in the dose of 10 mg/kg body weight for 5 days[5,20]
- In pregnant women: Azithromycin 500 mg in a single dose for 5 days. Azithromycin is the drug of choice in pregnant women as doxycycline is contraindicated.
-
At secondary and tertiary care level:
The treatment as specified above in uncomplicated cases
-
In complicated cases, the following treatment is to be initiated:
-
(a)IV Doxycycline (wherever available) 100 mg twice daily in 100 ml normal saline to be administered as an infusion over half an hour initially, followed by oral therapy to complete 7–15 days of therapy. Or (b) IV azithromycin in the dose of 500 mg IV in 250 ml normal saline over 1 h once daily for 1–2 days, followed by oral therapy to complete 5 days of therapy. Or (c) IV chloramphenicol 50–100 mg/kg/day 6-hourly doses to be administered as an infusion over 1 h initially, followed by oral therapy to complete 7–15 days of therapy
-
(a)
Management of the individual complications should be done as per the existing practices.[5]
Preventive antibiotic therapy for rickettsial infection is not indicated for patients who have had recent tick bites and are not ill. A limited number of ticks in areas, where tick-borne diseases are endemic, are infected with pathogenic rickettsiae. Therefore, the risk for such infections after a tick bite is low. Moreover, for RMSF, preventive therapy has been demonstrated to delay but not prevent the onset of symptoms.[21]
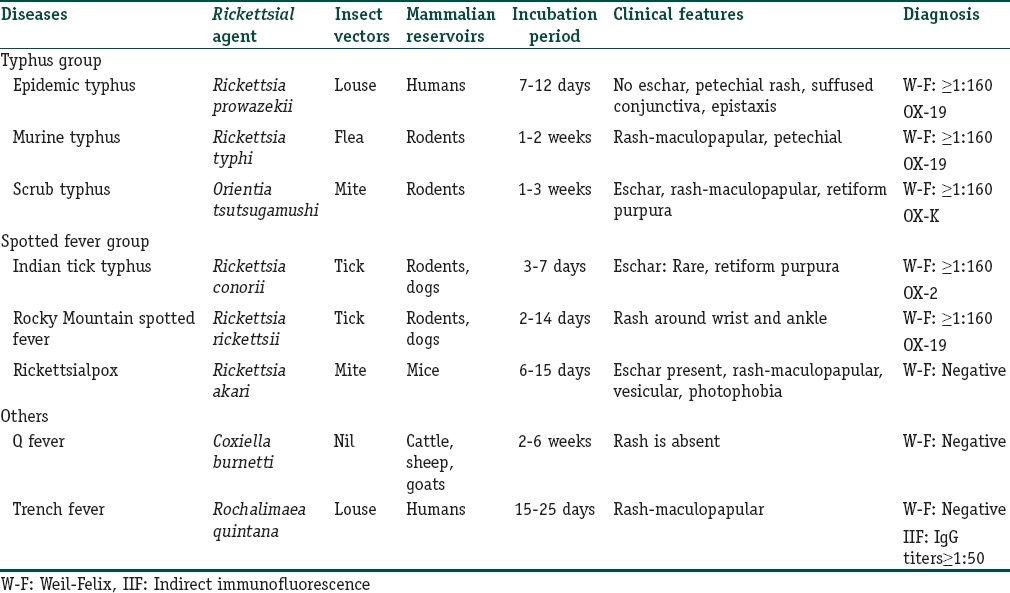
Table 1 summarizes the various differentiating factors for rickettsial infections.
Table 1.
Summary of rickettsial infections
Complications
Rickettsial infections sometimes produce severe life-threatening manifestations and take a fulminant course.[4]
Respiratory: Interstitial pneumonitis and noncardiogenic pulmonary edema secondary to pulmonary microvascular leakage are occasionally observed
Neurological: Meningoencephalitic syndrome is known to occur with rickettsial infections. In fact, rickettsial infections should be included in differential diagnosis of aseptic meningitis and encephalitis in patients exposed to endemic areas, especially when accompanied by renal insufficiency and/or jaundice
Renal: Acute renal failure is associated with bad prognosis and can be a presenting feature of the rickettsial disease. The possibility of scrub typhus should be borne in mind whenever a patient with varying degree of renal insufficiency particularly if eschar exists along with history of environmental exposure
DIC-like syndrome, hepatic failure, gangrene, and myocarditis are sometimes seen in rickettsioses.
Although rickettsial infections are rare, any patient with FUO with purpuric rash, rickettsial infection should be considered.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
What is new?
In cases of purpura fulminans, Rickettsial infections should be considered as an etiology
In India, Scrub typhus and Indian Tick Typhus are most common type of Rickettsial infections.
References
- 1.Adcock DM, Brozna J, Marlar RA. Proposed classification and pathologic mechanisms of purpura fulminans and skin necrosis. Semin Thromb Hemost. 1990;16:333–40. doi: 10.1055/s-2007-1002686. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan SK, Kashyap R, Kanga A, Sharma V, Prasher BS, Pal LS. Relevance of Weil-Felix test in diagnosis of scrub typhus in India. J Assoc Physicians India. 2006;54:619–21. [PubMed] [Google Scholar]
- 3.Walker DH, Dumler JS, Marrie T. Rickettsial diseases. In: Longo DL, Kasper DL, Jameson JL, Fauci AS, Hanser SL, Loscalgo J, editors. Harrison's Principle of Internal Medicine. 18th ed. New York: McGraw-Hill; 2011. pp. 1407–17. [Google Scholar]
- 4.Rathi N, Rathi A. Rickettsial infections: Indian perspective. Indian Pediatr. 2010;47:157–64. doi: 10.1007/s13312-010-0024-3. [DOI] [PubMed] [Google Scholar]
- 5.Rahi M, Gupte MD, Bhargava A, Varghese GM, Arora R. DHR-ICMR guidelines for diagnosis & management of Rickettsial diseases in India. Indian J Med Res. 2015;141:417–22. doi: 10.4103/0971-5916.159279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oberoi A, Singh N. Rickettsiae infection and classification. JK Sci. 2010;12:57–9. [Google Scholar]
- 7.Chaudhry D, Goyal S. Scrub typhus-resurgence of a forgotten killer. Indian J Anaesth. 2013;57:135–6. doi: 10.4103/0019-5049.111836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh S, Nagar G. Problem of ticks and tick-borne diseases in India with special emphasis on progress in tick control research: A review. J Vector Borne Dis. 2014;51:259–70. [PubMed] [Google Scholar]
- 9.Parola P, Fenollar F, Badiaga S, Brouqui P, Raoult D. First documentation of Rickettsia conorii infection (strain Indian tick typhus) in a traveler. Emerg Infect Dis. 2001;7:909–10. doi: 10.3201/eid0705.017527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah V, Vaidya V, Bang V, Shah I. Spotted fever in a child in Mumbai, India. J Vector Borne Dis. 2009;46:310–2. [PubMed] [Google Scholar]
- 11.Holman RC, McQuiston JH, Haberling DL, Cheek JE. Increasing incidence of Rocky Mountain spotted fever among the American Indian population in the United States. Am J Trop Med Hyg. 2009;80:601–5. [PubMed] [Google Scholar]
- 12.Jayaseelan E, Rajendran SC, Shariff S, Fishbein D, Keystone JS. Cutaneous eruptions in Indian tick typhus. Int J Dermatol. 1991;30:790–4. doi: 10.1111/j.1365-4362.1991.tb04788.x. [DOI] [PubMed] [Google Scholar]
- 13.Renvoisé A, van’t Wout JW, van der Schroeff JG, Beersma MF, Raoult D. A case of rickettsialpox in Northern Europe. Int J Infect Dis. 2012;16:e221–2. doi: 10.1016/j.ijid.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Honarmand H. Q Fever: An old but still a poorly understood disease. Interdiscip Perspect Infect Dis 2012. 2012 doi: 10.1155/2012/131932. 131932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217–23. doi: 10.3201/eid1202.050874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma A, Mahajan S, Gupta ML, Kanga A, Sharma V. Investigation of an outbreak of scrub typhus in the Himalayan region of India. Jpn J Infect Dis. 2005;58:208–10. [PubMed] [Google Scholar]
- 17.Mittal V, Gupta N, Bhattacharya D, Kumar K, Ichhpujani RL, Singh S, et al. Serological evidence of rickettsial infections in Delhi. Indian J Med Res. 2012;135:538–41. [PMC free article] [PubMed] [Google Scholar]
- 18.Tirumala S, Behera B, Jawalkar S, Mishra PK, Patalay PV, Ayyagari S, et al. Indian tick typhus presenting as purpura fulminans. Indian J Crit Care Med. 2014;18:476–8. doi: 10.4103/0972-5229.136081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahajan SK. Rickettsial diseases. J Assoc Physicians India. 2012;60:37–44. [PubMed] [Google Scholar]
- 20.Dana AN. Diagnosis and treatment of tick infestation and tick-borne diseases with cutaneous manifestations. Dermatol Ther. 2009;22:293–326. doi: 10.1111/j.1529-8019.2009.01244.x. [DOI] [PubMed] [Google Scholar]
- 21.Chapman AS, Bakken JS, Folk SM, Paddock CD, Bloch KC, Krusell A, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis – United States: A practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1–27. [PubMed] [Google Scholar]


