Abstract
Approximately, 140 million people worldwide live permanently at high altitudes (HAs) and approximately another 40 million people travel to HA area (HAA) every year for reasons of occupation, sports or recreation. In India, whole of Ladakh region, part of Northwest Kashmir, Northern part of Sikkim and Tenga valley of Arunachal are considered inhabited areas of HAA. The low quantity of oxygen, high exposure of ultraviolet (UV) light, very low humidity, extreme subzero temperature in winter, high wind velocity, make this region difficult for lowlanders as well as for tourists. Acute mountain sickness, HA pulmonary edema, HA cerebral edema, and thromboembolic conditions are known to occur in HA. However, enough knowledge has not been shared on dermatoses peculiar to this region. Xerosis, UV-related skin disorders (tanning, photomelanosis, acute and chronic sunburn, polymorphic light eruption, chronic actinic dermatitis, actinic cheilitis, etc.), cold injuries (frostbite, chilblains, acrocyanosis, erythrocyanosis, etc.) nail changes (koilonychias), airborne contact dermatitis, insect bite reaction, and skin carcinoma (basal cell carcinomas, squamous cell carcinomas, and also rarely malignant melanoma) are the dermatoses seen in HAAs. Early diagnosis and knowledge of HA dermatoses may prevent serious consequences of disease and improve the quality of life for the visitors as well as for native of the place.
Keywords: Cold injuries, high altitude, Ladakh, nail changes, skin carcinoma, ultraviolet-related skin disorders, xerosis
What was known?
A unique environment of high altitude predisposes non acclimatized person to develop life threatening conditions like acute mountain sickness, high altitude pulmonary oedema, high altitude cerebral oedema and thrombotic conditions. Except few reports of common skin disorders of high altitude area like xerosis, ultraviolet related skin conditions and cold injuries there are not enough researches and knowledge shared on the high altitude dermatological conditions for native as well as lowlanders who happen to go into high altitude areas.
Introduction
Skin is the largest interface between humankind and the environment. Consequently, it is the sentinel site of onslaught of atmospheric determinants. It is estimated that 140 million people over the globe live permanently at altitudes of over 2500 m and approximately another 40 million enter high altitude (HA) area every year for reasons of occupation, sport, or recreation. The persons at high risk of being affected by HA dermatoses include native highlanders, mountaineers, soldiers, trekkers, miners, pilgrims, and porters.[1]
In India, whole of Ladakh region, part of Northwest Kashmir, Northern part of Sikkim and Tenga valley of Arunachal are considered inhabited areas of HA. There is no general consensus on the height from which HA starts, however, the height of 2700 m and above is considered a working definition of HA.[2] Because of extreme height, there are many peculiarities of the environment. The low quantity of oxygen, more than three times the exposure of ultraviolet (UV) light over the plains, very low humidity (14%–20%), extreme subzero temperature in winter (17°C–−30°C), high wind velocity, makes this region difficult for lowlanders as well as for tourists.[3] Acute mountain sickness,[4,5,6] HA pulmonary edema,[4,5,6,7] HA cerebral edema,[4,5,6] and thromboembolic conditions[8] are known to occur in HA. However, enough knowledge has not been shared on dermatoses peculiar to this region. This article aims to review the dermatoses encountered in the HA and their management.
Xerosis
Low relative humidity and temperature coupled with high wind velocity cause a significant reduction in water content of the skin.[9,10,11] Xerosis could lead to pruritus and subsequent cracks, fissuring and oozing [Figure 1].[9] which hampers daily activities. The ignorant may attribute this to bad hygiene and skin infection, motivating them to frequently use soap or antiseptic liquid in bath water, aggravating the problem. A study by Singh et al. revealed xerosis being common in both lowlanders and native of HA area (HAA). How does one avoid dryness at HAs? Soap application should be minimized and if possible it should be restricted to face, hands, armpits, and groin. Whole body soap application could be done at fortnightly intervals. Personal experience has shown that soaps having high fatty acid content are better suited for dry weather.[1]
Figure 1.
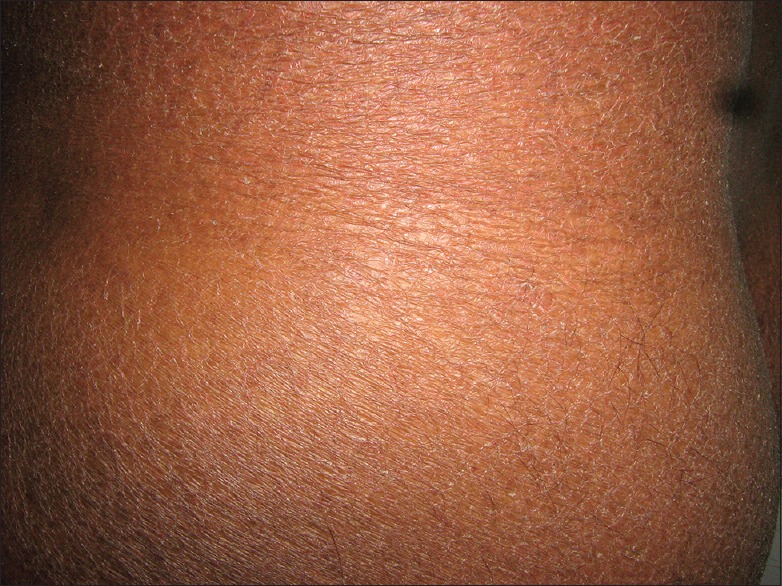
Dry skin of whole body
The daily bath should be followed by adequate and appropriate application of emollients such as a branded body lotion or oil, preferably coconut or olive oil.[12] Self-medication by way of applying topical steroid or antibiotic over affected areas is harmful, and should always be discouraged.[13]
Similar dryness could develop over the scalp that manifests as fine scaling with itching. This is usually confused with fungal infection/seborrhoeic dermatitis, prompting a trial of different brands of antifungal shampoo and treatments that cause further harm to the scalp and hair. Like soap, shampoo should also be used sparingly, preferably once a week. Milder shampoos available in the market may be used, followed by a conditioner.[14] Personal experience in this region has shown that additional weekly application of either coconut or olive oil over the scalp keeps it symptom-free.[1]
Ultraviolet-related skin disorders
Tanning, photomelanosis, acute and chronic sunburn, polymorphic light eruption, chronic actinic dermatitis, actinic cheilitis are common photodermatoses of this region caused by abnormal cutaneous reactions to solar radiation. While these diseases have different pathophysiologic mechanisms, not all of which have been clearly defined, photoprotection is an integral part of their management. The intensity of UV is maximum between 10 a.m. and 3 p.m. Although both UVA (320–400 nm) and B (280–320 nm) are harmful to the skin, it is the UVB component that is responsible for acute skin damage.[15]
Acute effects of ultraviolet radiation
The major acute clinical effects of UV radiation (UVR) on normal skin are sunburn inflammation (erythema) and tanning (enhanced melanogenesis). UVR has a wide range of other acute effects, such as DNA photodamage, immunosuppression, and Vitamin D synthesis.[16] UVR of short wavelength produces the severest degree of erythema on exposed areas [Figure 2]. The erythema usually takes 12–24 h to become maximal, fades after 1–3 days, and is then replaced by pigmentation. It may also proceed to vesiculation, crusting, and desquamation of skin affecting such areas as the unprotected scalp or chest.
Figure 2.
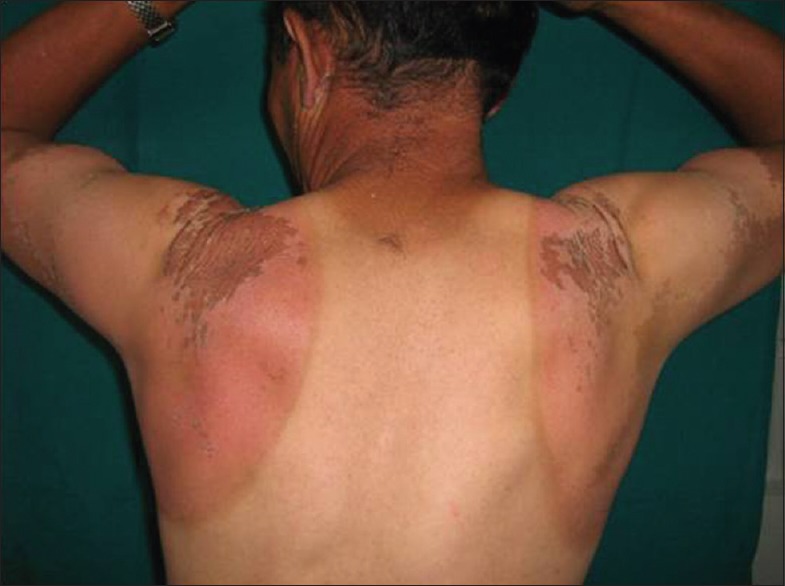
Acute sun burn of the back
The skin of most Caucasians initially shows an increase in size and functional activity of existing melanocytes and repeated exposure leads to greater dopa-positivity. Sunburn may be particularly severe in mountaineers, and this is greatly enhanced by reflected radiation from the snow, the so-called “albedo effect.” Snow reflects up to 90% of UVR compared to 9%–17% reflected from ground covered by grass. Hence, the reflected UVR may lead to severe sunburn in areas not usually exposed such as the inside of nostrils and ears, and under the chin.
Prolonged exposure to ultraviolet radiation
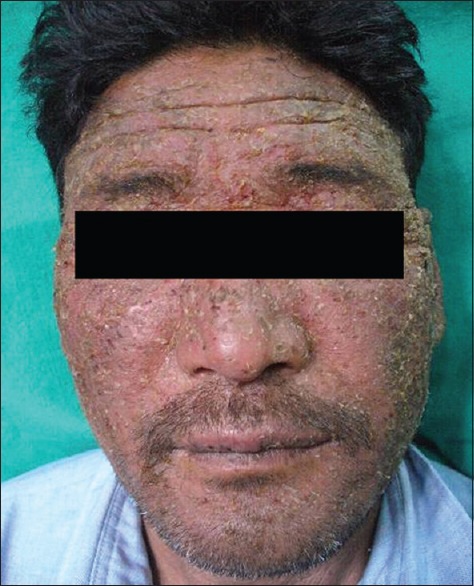
Chronic exposure to UVR leads to photoaging, immunosuppression, and ultimately photocarcinogenesis.[17] The clinical changes of chronic actinic dermopathy include hyperpigmentation, telangiectasiae, thickening and furrowing of the skin exposed to prolonged UVR. These changes start right in the childhood. Due to chronic sunburn reactions, a high incidence of hyperpigmentation and telangiectasia has been seen even in the first decade of life. These changes continue to increase during second, third, and fourth decades of life. The incidence of telangiectasiae reaches its peak in the third decade [Figure 3]. Then, due to thickening and furrowing of the skin most commonly seen in the fifth and sixth decades, incidence of telangiectasia and hyperpigmentation decreases. These changes thus represent the process of acclimatization of the skin to high doses of short wavelength UVR. Native highlanders, especially of the farming and shepherd community exposed to fierce sunlight; develop pigmentation, thickening, and furrowing on the skin [Figure 4]. This is prominent on the face and the back of the neck where the characteristic pattern is referred to as cutis rhomboidalis nuchae. At first, the elastic fibers become thicker, curled, and tangled. Later, in patients with clinically evident solar elastosis, in the upper dermis, basophilic degeneration of the collagen appears, separated from a somewhat atrophic epidermis by a narrow band of normal collagen. Hyperpigmentation and leakage of melanin takes place into the dermis, where it is taken up by melanophores, constituting a form of long-term dermal acclimatization to HA. The histological changes induced in the exposed skin of the highlander are so characteristic that it is termed “high-altitude dermopathy.”[18]
Figure 3.
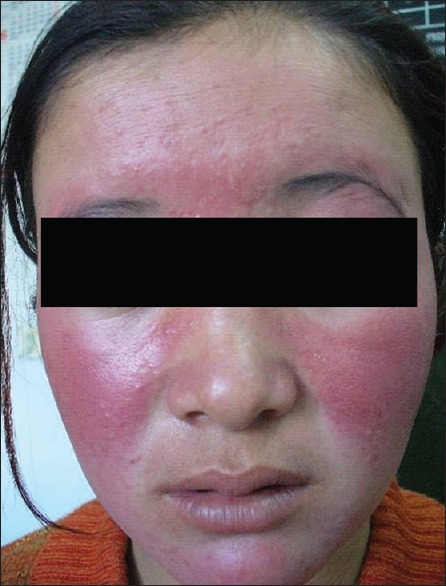
Telangiectasia of face in young girl
Figure 4.
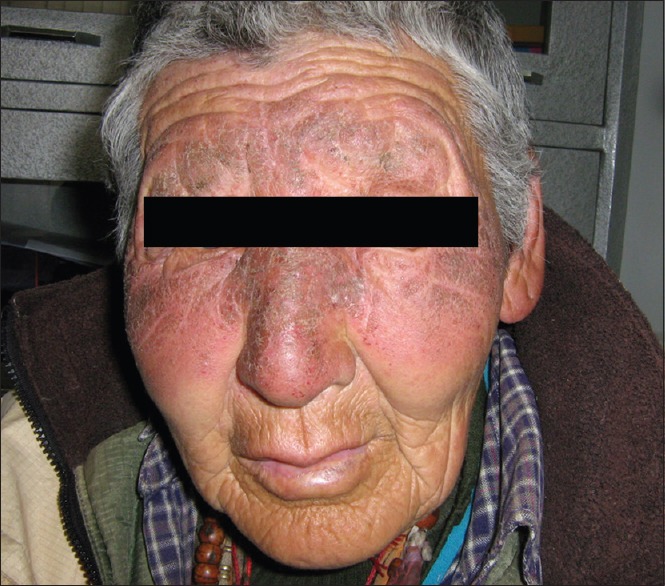
Pigmentation, thickening, and furrowing on native Ladakhi
Prolonged exposure of solar radiation at this altitude can manifest as tanning of exposed areas of body mainly face, “V” area of neck and dorsum of hands. Patients of polymorphic light eruption present with itchy, erythematous, and minute grouped papules or plaques over the exposed parts of the body mainly forearm, nape of the neck and face [Figure 5]. Chronic actinic dermatitis appears as thick erythematous to hyperpigmented lesions over the exposed areas of the body, the most common sites being the dorsum of both hands and nape of the neck [Figure 6]. Erosions or ulcers can develop over the lower lip, known as actinic cheilitis [Figure 7].
Figure 5.
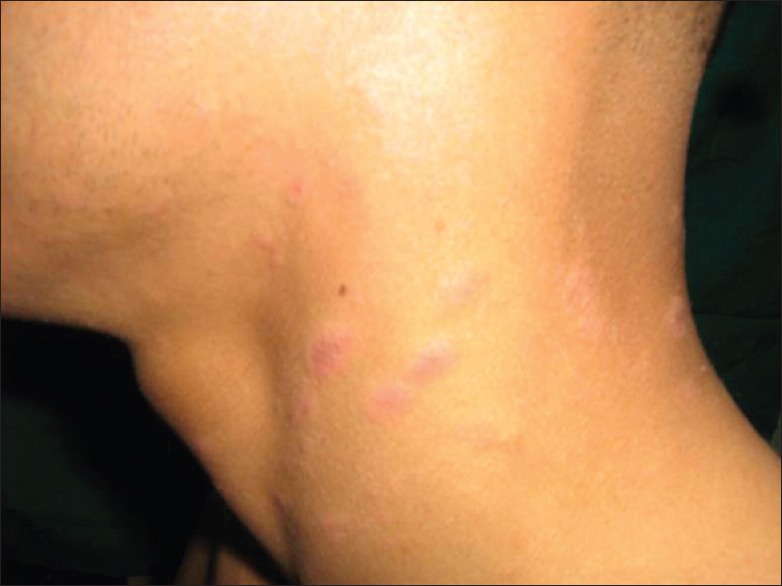
Polymorphic light eruption of the neck
Figure 6.
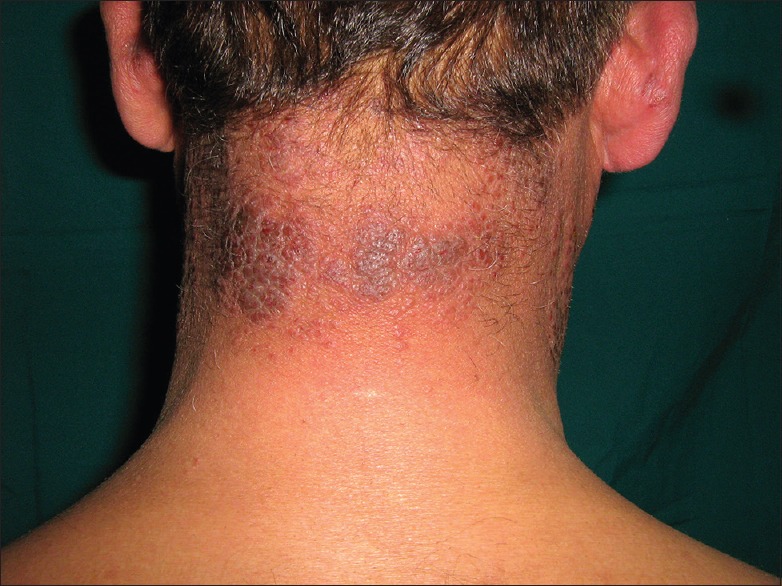
Chronic actinic dermatitis of nape of neck
Figure 7.
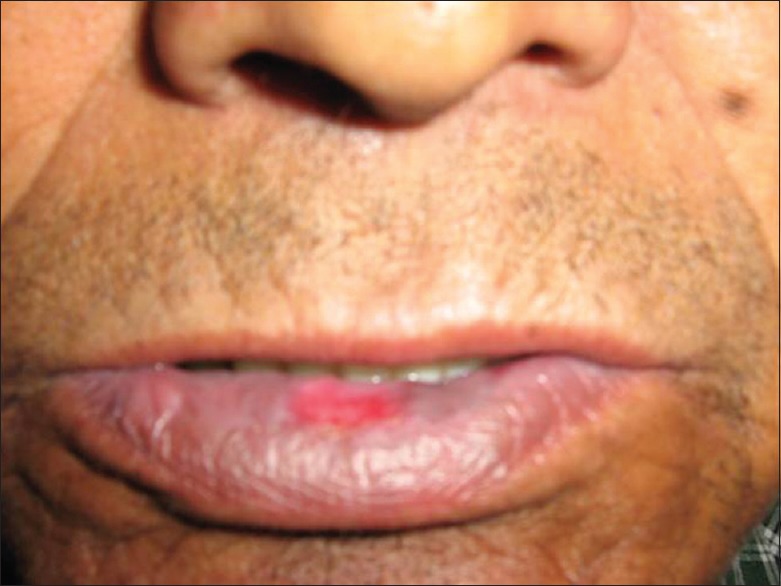
Actinic cheilitis of lower lip
Sun protective measures should be the first line of treatment. Persons are encouraged to use wide-brimmed headgear and full sleeved clothes with restricted outdoor movement between 10 a.m. and 3 p.m. Liberal use of broad spectrum sunscreen with sun protection factor (SPF) >30 at least 20 min before going out in the sun must be practiced.[19,20] The SPF value for sunscreens reflects the ability of the product to protect against UV-induced erythema, which is primarily the effect of UVB exposure and to a lesser extent from UVA-2 (320–340 nm). Broad spectrum sunscreen should have a mixture of organic UVB filters (cinnamates, PABA derivatives, salicylates, ensulizole), organic UVA filters (benzophenone, avobenzone, meradimate) and inorganic filters (titanium dioxide, zinc oxide).[19,20] Unlike other cosmetic products, sunscreen should not be massaged over the face or hands; instead, it should be applied by gently spreading the lotion on the surface, preferably over a cold cream in HA. It should be reapplied after 3–4 h if the person is intends to be outdoors for a longer duration.
Mild to moderate topical steroids depending on the area of involvement and severity of lesions are helpful. Oral Vitamin C taken in dosage of 100 mg daily has been found to be sun protective.[19] Chronic actinic dermatitis usually requires administration of oral steroid or immunosuppressant such as azathioprine.
Cold-related Injury (Chilblains, Frost Bite)
In winter, temperatures in HAA may dip down to −30°C; on glaciers and snowbound mountains, temperature may dip even to −60° C. If adequate precautions are not been taken against the cold, persons can suffer from chilblains or frostbite. Chilblains is an inflammatory disorder of skin seen where the skin is exposed to nonfreezing temperatures for a long time, characterized by redness, pain, and itching over the affected areas.[21] It usually affects the tips of toes [Figure 8], fingers, tip of the nose and ear pinna. It has been found that persons with low body mass index (BMI) and a genetic predisposition are more likely to develop chilblains.[21,22] In a study by Singh et al., all 79 cases of chilblains were seen either in lowlanders (n = 68) or tourists (n = 11) with not a single case among the native Ladakhi (P < 0.005). This could be explained by the genetic make-up of the native Ladakhi and protective lifestyle.[1] In another study by Singh et al., 108 patients of chilblains at Ladakh were studied. Only a single case of chilblains was found in local (P < 0.005) population. The family history of chilblains was present in 10 (9.2%) patients, there was recurrence in 12 (11.1%), and 21 patients (19.4%) were smokers. Most (63.8%) of the patients, had BMI between 20 and 22 kg/m2 (mean = 20.03 kg/m2; 95% confidence interval = 19.68–20.38 and standard deviation = 1.82). 42.1% of cases of chilblains also had hyperhidrosis (P < 0.05).[23]
Figure 8.
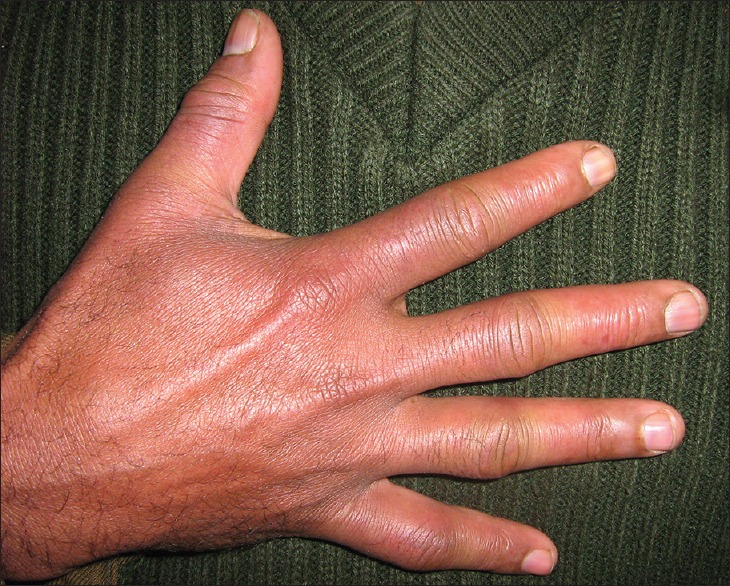
Chilblain of the right hand
Regular care of feet such as soaking in lukewarm water and drying them, application of emollients and wearing of nylon socks in layers are established precautionary measures against chilblains. Very tight or loose shoes should be avoided.[24] The practice of adding tea leaves and beet to warm water for soaking of feet in these areas requires scientific analysis. Although the Defence Research and Development Organization of India has introduced nitroglycerine and salbutamol sulfate creams for the prevention of cold injuries after pilot studies on soldiers and workers residing in HA,[25] larger clinical trials are needed to establish their efficacy. Oral nifedipine has been found to be effective but should be prescribed under supervision.[26]
Frostbite develops due to prolonged exposure to subzero temperature and consequent formation of ice crystals in the cells of tissues leading to blister formation.[27] The toes are usually affected [Figure 9], however, whole feet, fingers, pinna, tip of nose may be involved. Coldness, firmness, stinging, burning, numbness, clumsiness, pain, throbbing, burning or electric current-like sensations on rewarming on affected areas are the usual complaints of patients. Like in thermal burns, depending on the severity and duration of exposure, frostbite can be classified into four degrees. First-degree injury is superficial frostbite which involves the epidermis only; this is reversible. When it affects tissues deeper to the epidermis, it is labeled 2nd, 3rd, and 4th degree depending on depth. Fourth-degree frostbite is full-thickness damage affecting muscles, tendons, and bone, with resultant tissue loss.[27,28] Salvage of affected part is very difficult in 4th degree frostbite and the patient usually requires surgical intervention such as amputation. Surgical consultation should always be sought when there is the formation of multiple bullae, gangrene, loss of tissue, or evidence of infection.[27]
Figure 9.
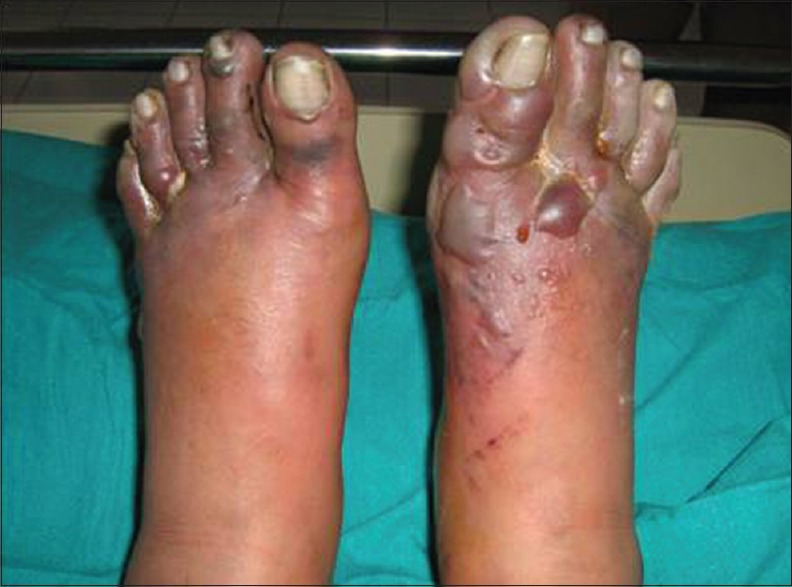
Frostbite of both the feet
The best preventive measure for frostbite is the wearing of protective clothing. For affected individuals, gradual warming of affected part of body (temperature from 37°C to 40°C–42°C) should be done for half an hour, three to four times daily. Affected areas should not be rubbed or massaged. Analgesic such as diclofenac sodium, ibuprofen or injection morphine sulfate can be given.[24,27] Such individuals must be de-inducted from extreme cold climate to prevent recurrence.
Acrocyanosis is a bilateral, dusky, mottled discoloring of the entire hands, feet, and sometimes the face that is persistent and accentuated by cold exposure. Erythrocyanosis is a cyanotic discoloration that occurs over areas with a thick layer of subcutaneous fat. Cold erythema, cold urticaria, and cold panniculitis are other effects of cold on the body tissues. Prevention and general supportive measures to keep the skin warm are helpful.[29]
Basic principles for the prevention of cold injury
Keeping warm in a cold environment requires several layers of clothing preferably wool or synthetics such as polypropylene because these materials insulate even when wet. Since the body loses a large amount of heat from the head, warm headgear is essential. Adequate food and fluid intake provide fuel to be burned, and warm fluids directly provide heat and prevent dehydration. Alcoholic beverages should be avoided, because alcohol causes cutaneous vasodilatation, which makes the body temporarily feel warm but actually causes greater heat loss. Similarly, nicotine in cigarette smoke has a vasoconstrictor action and aggravates cellular hypoxia. Thus, proper foot care, frequent changing of clothing, exercise of extremities, proper warm clothing, recognition of symptoms of cold injury and maintaining adequate hydration and nutritional status are key essentials for prevention against cold injuries.[30]
Nail Changes
Chronic hypoxia of HA causes increased erythropoiesis, depletion of iron stores and thinning of the nail plate. A high prevalence of koilonychias [Figure 10] has been reported in Ladakhis living in the region of Leh (3800 m) though its etiology is uncertain. There was no significant difference in hemoglobin levels in those with or without koilonychias.[31] It is due to retardation of nail plate growth and is more common in upper limbs.[32] Nail growth has been found to be slower in HA due to hypoxia and extreme cold climate. There are a few reports of Beau's lines also following HA exposure. The hypoxia associated with the hypobaric environment at HA could be sufficient to cause a disruption in nail matrix formation.[33]
Figure 10.
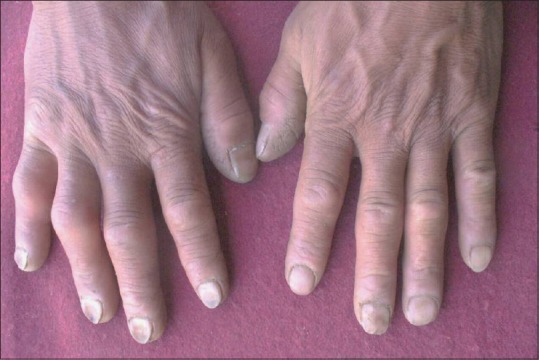
Koilonychia in native Ladakhi
Skin Carcinoma
Skin cancer is more common in Caucasians who have light skin and eyes and in those who burn rather than tan when exposed to sunlight (Fitzpatrick skin Types I and II). A combination of factors, including a predisposition of the immune system to be suppressed by UVR and a reduced capacity to repair UV-induced DNA damage, appears to increase the risk. The most serious long-term effect of UVR particularly for white-skinned populations is the induction of skin cancer, mainly nonmelanoma skin cancers, namely basal cell carcinomas (BCCs), squamous cell carcinomas (SCCs), and also malignant melanoma. UV exposure has been implicated in the development of all three of these malignancies, although the epidemiology of melanoma, BCC, and SCC differs.[17,34] In spite of the high level of UVR in HAA, a study by Singh et al. did not reveal any skin carcinoma or tumor in natives or lowlanders.[1]
Air Borne Contact Dermatitis
Plant allergies to Populus (Poplar) and Salix (Willow), reported to cause airborne contact dermatitis [Figure 11] in Indian HA studies, suggest their significant etiological association.[35] However, this entity is not peculiar to HAA as poplar and willow trees-related airborne contact dermatitis are commonly seen even in the other hilly terrain where they grow.
Figure 11.
Airborne contact dermatitis due to salicaceae allergy
Insect Bite Reaction (Papular Urticaria)
Afforestation of barren land at Ladakh has brought in ectoparasite insects into the habitat. These insects including ants, wasps, and moths (Hymenoptera species) attack aphids (Chaitophorus species) which are usually found on the willow trees (Salix species). There was no significant difference in the incidence of papular urticaria [Figure 12] in lowlanders, local Ladakhi or tourists.[1]
Figure 12.
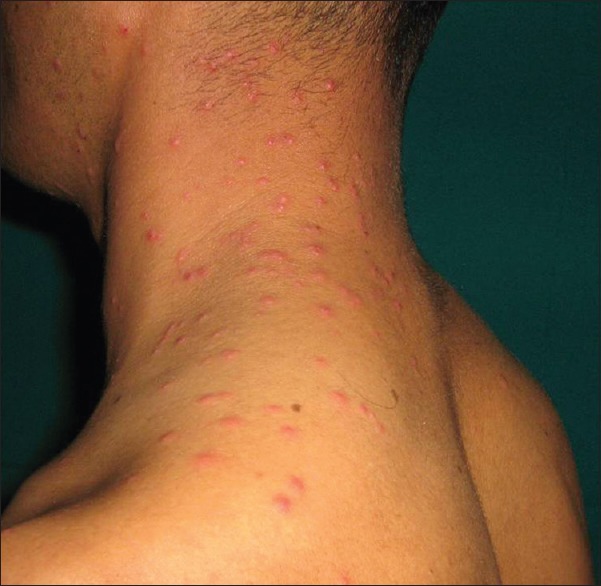
Papular urticaria of face and neck
Conclusion
Low humidity, high-velocity wind, excessive UV exposure, and extreme cold temperature are the determinants of various types of environmental dermatoses in HA. Xerosis, tanning, acute or chronic sunburn, polymorphic light reaction, chronic actinic dermatitis, chilblain, frostbite, airborne contact dermatitis, and insect bite reactions are common environmental dermatoses of this region. Natives are relatively protected from cold injuries due to their genetic makeup and protective lifestyle. Avoidance of frequent soap application, application of adequate and suitable oil or body lotion, effective sunscreen, wearing of protective clothing are important guidelines for skin care in HA. Understanding of HA dermatoses is thus essential for preventing environmental dermatoses in HAAs.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
What is new?
Xerosis, tanning, acute or chronic sunburn, polymorphic light reaction, chronic actinic dermatitis, insect bite reaction, koilonychias, chilblains and frostbite are common high altitude dermatoses. Incidence of cold injuries, skin carcinoma in natives of high altitude areas is almost negligible. Low body mass index predisposes person to develop chilblains in lowlanders. Knowledge of daily skin care and preventive measures are very essential to remain healthy in high altitude areas.
References
- 1.Singh G, Chatterjee M, Grewal R, Verma R. Incidence and care of environmental dermatoses in the high-altitude region of Ladakh, India. Indian J Dermatol. 2013;58:107–12. doi: 10.4103/0019-5154.108038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Director General Armed Forces Medical Service. Medical Memoranda in Problems of High Altitude. Vol. 140. New Delhi: Director General Armed Forces Medical Service; 1997. pp. 31–2. [Google Scholar]
- 3.West JB. The atmosphere. In: Hornbein TF, Schoene RB, editors. High Altitude an Exploration of Human Adaptation. Vol. 161. New York: Mercel Dekker Inc; 2001. pp. 25–41. [Google Scholar]
- 4.Basnyat B, Murdoch DR. High-altitude illness. Lancet. 2003;7(361):1967–74. doi: 10.1016/S0140-6736(03)13591-X. [DOI] [PubMed] [Google Scholar]
- 5.Hackett PH, Roach RC. High-altitude illness. N Engl J Med. 2001;345:107–14. doi: 10.1056/NEJM200107123450206. [DOI] [PubMed] [Google Scholar]
- 6.Hackett PH, Roach RC. High-altitude medicine. In: Auerbach PS, editor. Wilderness Medicine. St. Louis, MO: Mosby; 2001. pp. 2–43. [Google Scholar]
- 7.Hultgren HN. High-altitude pulmonary edema: Current concepts. Annu Rev Med. 1996;47:267–84. doi: 10.1146/annurev.med.47.1.267. [DOI] [PubMed] [Google Scholar]
- 8.Anand AC, Jha SK, Saha A, Sharma V, Adya CM. Thrombosis as a complication of extended stay at high altitude. Natl Med J India. 2001;14:197–201. [PubMed] [Google Scholar]
- 9.Pons-Guiraud A. Dry skin in dermatology: A complex physiopathology. J Eur Acad Dermatol Venereol. 2007;21(Suppl 2):1–4. doi: 10.1111/j.1468-3083.2007.02379.x. [DOI] [PubMed] [Google Scholar]
- 10.Blank IH. Further observations on factors which influence the water content of the stratum corneum. J Invest Dermatol. 1953;21:259–71. doi: 10.1038/jid.1953.100. [DOI] [PubMed] [Google Scholar]
- 11.Engelke M, Jensen JM, Ekanayake-Mudiyanselage S, Proksch E. Effects of xerosis and ageing on epidermal proliferation and differentiation. Br J Dermatol. 1997;137:219–25. doi: 10.1046/j.1365-2133.1997.18091892.x. [DOI] [PubMed] [Google Scholar]
- 12.Siddappa K. Dry skin conditions, eczema and emollients in their management. Indian J Dermatol Venereol Leprol. 2003;69:69–75. [PubMed] [Google Scholar]
- 13.Harding CR, Watkinson A, Rawlings AV, Scott IR. Dry skin, moisturization and corneodesmolysis. Int J Cosmet Sci. 2000;22:21–52. doi: 10.1046/j.1467-2494.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 14.Drovetskaya TV, Kreeger RL, Amos JL, Davis CB, Zhou S. Effects of low-level hydrophobic substitution on conditioning properties of cationic cellulosic polymers in shampoo systems. J Cosmet Sci. 2004;55:S195–205. [PubMed] [Google Scholar]
- 15.Hawk JL, Young AR, Ferguson J. Cutaneous photobiology. In: Burn T, Breathnach S, editors. Rook's Textbook of Dermatology. 7th ed. Vol. 2. Oxford: Blackwell Publishing; 2004. pp. 24.6–24.9. [Google Scholar]
- 16.Walker SL, Hawk JLM, Young AR. Acute and chronic effects of ultraviolet radiation on the skin. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Fitzpatrick's dermatology in general medicine. 6th ed. New York: McGraw-Hill Companies Inc; 2003. pp. 1275–82. [Google Scholar]
- 17.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Sawhney MP. Chronic actinic dermopathy – A clinical study in Ladakh. Indian J Dermatol Venereol Leprol. 2002;68:38–9. [PubMed] [Google Scholar]
- 19.Draelos ZD, Lim HW, Rougier A. Sunscreens and photodermatoses. In: Lim HW, Draelos ZD, editors. Clinical Guide to Sunscreens and Photoprotection. New York: Informa Healthcare; 2008. pp. 83–8. [Google Scholar]
- 20.Deleo V. Sunscreen use in photodermatoses. Dermatol Clin. 2006;24:27–33. doi: 10.1016/j.det.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Goette DK. Chilblains (perniosis) J Am Acad Dermatol. 1990;23(2 Pt 1):257–62. doi: 10.1016/0190-9622(90)70209-z. [DOI] [PubMed] [Google Scholar]
- 22.DeGroot DW, Castellani JW, Williams JO, Amoroso PJ. Epidemiology of U.S. army cold weather injuries, 1980-1999. Aviat Space Environ Med. 2003;74:564–70. [PubMed] [Google Scholar]
- 23.Singh GK, Datta A, Grewal RS, Suresh MS, Vaishampayan SS. Pattern of chilblains in a high altitude region of Ladakh, India. Med J Armed Forces India. 2015;71:265–9. doi: 10.1016/j.mjafi.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cappaert TA, Stone JA, Castellani JW, Krause BA, Smith D, Stephens BA. National Athletic Trainers’ Association. National Athletic Trainers’ Association position statement: Environmental cold injuries. J Athl Train. 2008;43:640–58. doi: 10.4085/1062-6050-43.6.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brochure-DRDO. [Last accessed in 2016 Nov 02]. Available from: http://www.drdo.gov.in/drdo/labs/INMAS/collaboration/brochure.htm .
- 26.Rustin MH, Newton JA, Smith NP, Dowd PM. The treatment of chilblains with nifedipine: The results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol. 1989;120:267–75. doi: 10.1111/j.1365-2133.1989.tb07792.x. [DOI] [PubMed] [Google Scholar]
- 27.Murphy JV, Banwell PE, Roberts AH, McGrouther DA. Frostbite: Pathogenesis and treatment. J Trauma. 2000;48:171–8. doi: 10.1097/00005373-200001000-00036. [DOI] [PubMed] [Google Scholar]
- 28.McCauley RL, Smith DJ, Jr, Robson MC, Heggers JP. Frostbite and other cold-induced injuries. In: Auerbach PA, editor. Wilderness Medicine: Management of Wilderness and Environmental Emergencies. 3rd ed. St. Louis, MO: Mosby; 1995. pp. 129–45. [Google Scholar]
- 29.Pierard G, Fumal I, Pierard-Franchimont C. Cold injuries. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Fitzpatrick's Dermatology in General Medicine. 6th ed. New York: McGraw-Hill; 2003. pp. 1211–9. [Google Scholar]
- 30.Nagpal BM, Sharma R. Cold injuries: The chill within. Med J Armed Forces India. 2004;60:165–71. doi: 10.1016/S0377-1237(04)80111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawhney MP. Ladakhi koilonychia. Indian J Dermatol Venereol Leprol. 2003;69:79–80. [PubMed] [Google Scholar]
- 32.Patial RK. High altitude koilonychia. J Assoc Physicians India. 1999;47:406–8. [PubMed] [Google Scholar]
- 33.Bellis F, Nickol A. Everest nails: A prospective study on the incidence of Beau's lines after time spent at high altitude. High Alt Med Biol. 2005;6:178–80. doi: 10.1089/ham.2005.6.178. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez CC, Federman DG, Kirsner RS. Skin cancer as an occupational disease: The effect of ultraviolet and other forms of radiation. Int J Dermatol. 2005;44:95–100. doi: 10.1111/j.1365-4632.2005.02301.x. [DOI] [PubMed] [Google Scholar]
- 35.Sawhney MS. Airborne salicaceae allergy in Ladakh. Indian J Dermatol Venereol Leprol. 1999;65:264–6. [PubMed] [Google Scholar]


