ABSTRACT
In a recent study, we found that protection following simian immunodeficiency virus (SIV) exposure correlated with rectal plasma cell frequency in vaccinated female rhesus macaques. We sought to determine if the same macaques maintained high mucosal plasma cell frequencies postinfection and if this translated to reduced viremia. Although delayed SIV acquisition did not predict subsequent viral control, alterations existed in the distribution of plasma cells and plasmablasts between macaques that exhibited high or low viremia. Flow cytometric analysis of cells from rectal biopsy specimens, bone marrow, and mesenteric lymph nodes of vaccinated infected, unvaccinated infected, and uninfected macaques identified two main IRF4hi subsets of interest: CD138+ plasma cells, and CD138− plasmablasts. In rectal tissue, plasma cell frequency positively correlated with plasma viremia and unvaccinated macaques had increased plasma cells and plasmablasts compared to vaccinated animals. Likewise, plasmablast frequency in the mesenteric lymph node correlated with viremia. However, in bone marrow, plasmablast frequency negatively correlated with viremia. Accordingly, low-viremic macaques had a higher frequency of both bone marrow IRF4hi subsets than did animals with high viremia. Significant reciprocal relationships between rectal and bone marrow plasmablasts suggested that efficient trafficking to the bone marrow as opposed to the rectal mucosa was linked to viral control. mRNA expression analysis of proteins involved in establishment of plasma cell niches in sorted bone marrow and rectal cell populations further supported this model and revealed differential mRNA expression patterns in these tissues.
IMPORTANCE As key antibody producers, plasma cells and plasmablasts are critical components of vaccine-induced immunity to human immunodeficiency virus type 1 (HIV-1) in humans and SIV in the macaque model; however, few have attempted to examine the role of these cells in viral suppression postinfection. Our results suggest that plasmablast trafficking to and retention in the bone marrow play a previously unappreciated role in viral control and contrast the potential contribution of mucosal plasma cells to mediate protection at sites of infection with that of bone marrow plasmablasts and plasma cells to control viremia during chronic infection. Manipulation of niche factors influencing the distribution and maintenance of these critical antibody-secreting cells may serve as potential therapeutic targets to enhance antiviral responses postvaccination and postinfection.
KEYWORDS: SIV rhesus macaque model, plasma cell, plasma cell niche factors, plasmablast
INTRODUCTION
As dedicated antibody producers, antigen-specific long-lived plasma cells (PC) are implicated in mediating protection against reinfection by vaccinia virus, poliovirus, and yellow fever virus as well as by some of our most effective vaccines (1). Although thought to reside primarily in the bone marrow niche (2–4), tissue-specific niches capable of supporting PC survival have recently been described (5–10). These niches are thought to support a limited number of PC within the physical confines of the niche itself through the secretion of cytokines and/or through direct cell-cell interactions (11). In the case of HIV vaccine design, generating a population of human immunodeficiency virus (HIV)-specific long-lived PC in the mucosa could provide a potent means of protecting against rectal or vaginal transmission, where the “window of vulnerability” prior to establishment of infection in local lymph nodes is a matter of hours to days (12–14). Similarly, memory B cells, which rapidly and sequentially differentiate into plasmablasts (PB) and PC upon stimulation, also provide a source of vaccine-induced protective antibodies. Memory B cells, however, may require interaction with memory T-follicular helper cells or follicular dendritic cells for differentiation and mucosal trafficking, depending on the nature of the antigen encountered (15, 16). In a recent vaccine study conducted by our group, mucosal B cell responses, including simian immunodeficiency virus (SIV) Env-specific rectal IgA, total rectal PC frequency, and rectal SIV-specific B memory cells measured 2 weeks postinfection correlated with delayed acquisition in females following a low-dose, repeated rectal challenge in the macaque model (17). After 9 challenges, all but one animal became infected. Postinfection, acute mean viral load in all vaccinated animals negatively correlated with total rectal PC frequency. Together, these data suggest that vaccination produced a population of mucosal PC that helped protect from infection and reduce peak viremia. Despite the potential of resident PC to mediate protection, few studies have attempted to characterize the mucosal PC niche in humans (6, 8–10) or macaques (18, 19). This follow-up study sought to phenotype PC and PB populations in the rectal mucosa, bone marrow, and mesenteric lymph node in uninfected and chronically SIV-infected macaques, assess their contribution to viral control, and examine the role of the PC niche in supporting PC/PB trafficking to and retention in tissues.
During chronic infection, total mucosal PC frequency was increased in unvaccinated compared to vaccinated macaques and directly correlated with plasma viral load. However, in the bone marrow, PB frequency inversely correlated with viral load and displayed a strong reciprocal relationship with rectal PB, possibly indicating preferred trafficking of PB to the bone marrow in animals with lower viremia. These results were supported by mRNA expression analysis of PC niche factors in sorted rectal and bone marrow populations. Data generated from this study provided a detailed analysis of PC and PB phenotypes and frequencies in multiple tissues of infected and uninfected macaques. The results further highlighted differences observed in vaccinated compared to unvaccinated and low-viremic compared to high-viremic SIV-infected animals, in addition to PC niche factors present in the bone marrow and rectal mucosa. Experimental manipulation of these niche factors may influence the recruitment and maintenance of PC at sites of infection and serve as potential targets for therapeutic enhancement or reduction of PC responses in infection, autoimmunity, and cancer (20–22).
RESULTS
Expression of IRF4 and CD138 identifies rhesus macaque PC and PB populations.
To assess the impact of total PB and PC on chronic SIV infection in previously vaccinated animals, 20 rhesus macaques were divided into four groups based on vaccination status, infection status, and viral load (Table 1). These included 4 uninfected macaques, 3 unvaccinated, infected controls (median viral load of 2.28 × 106 copies SIV RNA/ml plasma), 9 vaccinated macaques with high viral load (median viral load of 4.4 × 105 copies SIV RNA/ml plasma), and 4 vaccinated macaques with low viral load, defined as <104 copies SIV RNA/ml plasma (median viral load of 3.19 × 102 copies SIV RNA/ml plasma). Plasma viral loads for vaccinated animals with high viremia were not statistically different from those of the unvaccinated controls. Cells freshly isolated from bone marrow, mesenteric lymph node (MLN), and rectal pinch biopsy specimens were stained and analyzed using multiparameter flow cytometry. After first gating progressively on single cells, live cells, and a cellular gate that included cells with high forward scatter (FSC) and side scatter (SSC) to accommodate the morphology of PB/PC (23), we used CD2 and CD14 to remove T cells, NK cells, and monocytes. In order to focus on germinal-center-derived class-switched B cells, we then gated on IgM− cells. High expression of the transcription factor IRF4 is characteristic of both PB and PC (24–26), while expression of CD138 is most often associated with PC differentiation (27, 28). Consistent with other reports and through backgating analysis of IRF4hi populations of interest, these PC/PB subsets were identified as lacking expression of CD20 (29–32) but as CD19+/− (31, 33, 34). Thus, for all sites examined, PB were identified as CD20− IRF4hi CD138− and PC as CD20− IRF4hi CD138+ (Fig. 1A). In contrast to what we and others have observed (32, 35), the bone marrow lacked a clear CD20+ population (Fig. 1A). This result was surprising but consistent for the bone marrow and may have to do with differences in the way the cells were purified. Whereas other studies used a Ficoll gradient to isolate the cells, for this study, the polymorphonuclear cell (PMN) fraction was preserved for later sorting by performing a simple dextran sedimentation protocol. Additionally, high IRF4 expression has been noted in pro-B cells in the bone marrow (36). Therefore, it is possible that a fraction of these CD20− CD19+ CD10+ CD34+ IgM− cells (37, 38) were included in the bone marrow PB gate. For the rectal mucosa, bone marrow, and mesenteric lymph node, the frequency of PB was greater than that of PC (Fig. 1B). Nonetheless, PB and PC exhibited highly significant positive correlations in all tissues examined (Fig. 1C), emphasizing the common lineage of these two populations.
TABLE 1.
Rhesus macaque study subjectsa
| Macaque group and ID (sex) | No. of challenges for infection | Viral load at necropsy in: |
|
|---|---|---|---|
| Plasma (SIV RNA copies/ml plasma) | Rectal tissue (SIV RNA copies/μg RNA) | ||
| Vaccinated low viremic | |||
| R280* (F) | 6 | 482 | <50 |
| P180 (F) | 6 | 50 | |
| R647* (F) | 5 | 156 | Poor amplification |
| R249* (M) | 1 | 6,178 | <50 |
| Vaccinated high viremic | |||
| R666 (F) | 7 | 14,246 | |
| R651* (F) | 2 | 2,478,356 | |
| R248 (F) | 2 | 1,208,963 | 52,563 |
| R246 (F) | 1 | 50,967 | |
| R664 (F) | 9 | 36,560,431 | |
| R290* (F) | 1 | 2,067,152 | 43,734 |
| R657* (M) | 2 | 190,803 | |
| P602 (M) | 1 | 18,429 | <50 |
| R659 (M) | 1 | 443,914 | |
| Unvaccinated controls | |||
| R407** (M) | 3 | 7,298,622 | 864,017 |
| R648* (F) | 2 | 256,289 | 886,223 |
| P179 (F) | 1 | 2,280,082 | 85,022 |
| Uninfected seronegatives | |||
| R360 (M) | NA | NA | <50 |
| R361 (M) | NA | NA | |
| R362 (M) | NA | NA | <50 |
| R364 (F) | NA | NA | <50 |
F, female; M, male; NA, not applicable; *, frozen MLN and bone marrow cells, or **, frozen bone marrow used only for additional phenotyping.
FIG 1.
Flow-cytometric analysis of rhesus macaque PB and PC. Single-cell suspensions from fresh macaque rectal pinch biopsy specimens, bone marrow, and MLNs were analyzed by flow cytometry. (A) Representative gating strategy depicting a large lymphocyte gate within which PB were identified as CD2− CD14− IgM− CD20− IRF4hi CD138− and PC were identified as CD2− CD14− IgM− CD20− IRF4hi CD138+. BM, bone marrow. (B and C) Comparison of (B) and correlation analyses between (C) PB and PC frequencies in rectal (n = 18, gray circles), bone marrow (n = 20, white circles), and MLN cells (n = 20, black circles) from SIV+ and SIV− macaques. Rectal samples from animals R659 and R246 did not have sufficient cells postacquisition for reliable flow cytometry analysis. (D) Frozen bone marrow (n = 8; white squares, PB; white circles, PC) or MLN cells (n = 7; black squares, PB; black circles, PC) from SIV+ macaques identified with asterisks in Table 1 were analyzed for expression of markers associated with either a PB phenotype (top row) or a PC phenotype (bottom row). *, P < 0.05; **, P < 0.01, ****, P < 0.0001. PB and PC frequencies in panels B and C represent the averages for two separate staining assays performed side by side.
Analysis of additional markers on previously frozen bone marrow and MLN cells isolated at necropsy further supported the PB/PC designation, with the IRF4hi CD138− compartment containing a greater proportion of cells expressing Ki67 and HLA-DR, markers associated with a PB or immature PC phenotype, compared to the IRF4hi CD138+ compartment, while the IRF4hi CD138+ compartment contained a greater proportion of markers associated with a mature PC phenotype, namely, high expression of Bcl-2 and CD38 (Fig. 1D) (29, 31, 39). Expression of CD27 was low in both subsets, in agreement with previous findings (34, 35) (data not shown).
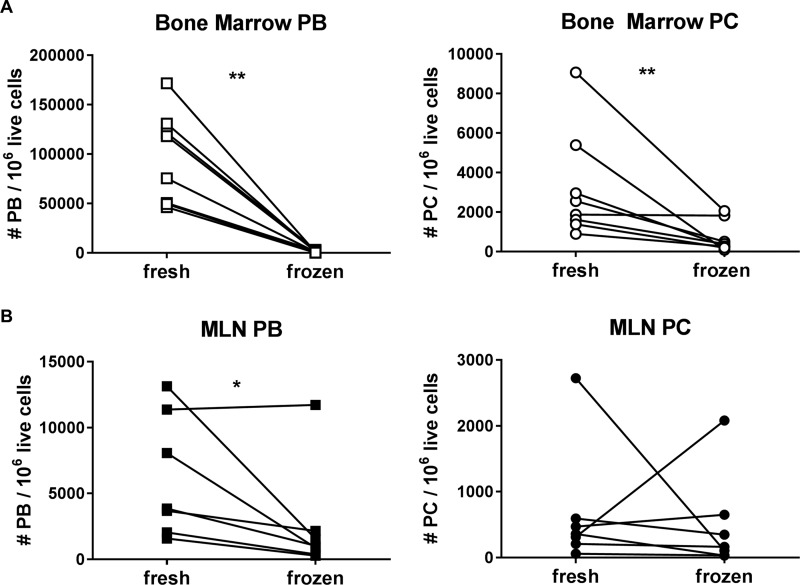
PC consistently had a higher frequency of Bcl-2+ cells than PB in all 3 tissues (see Fig. 4A). However, similar to what was reported by Klippert et al. (40), a significant loss of PB and PC was evident in previously frozen compared to fresh bone marrow cells (Fig. 2A), apparently due to the loss of cells with lower expression of the antiapoptotic molecule Bcl-2. The geometric mean fluorescence intensity (geoMFI) of Bcl-2 increased dramatically in frozen bone marrow PB and PC compared to fresh cells, as nearly all the Bcl-2−/low PB found in the fresh bone marrow were lost in frozen samples (Fig. 3A). The MLN also exhibited decreased PB in frozen samples (Fig. 2B, left panel), but this was less significant and not linked to a loss of Bcl-2−/low cells, which had a similarly high Bcl-2 geoMFI in both fresh and frozen PB populations (Fig. 3B).
FIG 4.
Tissue-specific distribution of retention and recruitment markers on macaque PB and PC. Freshly isolated cells from SIV+ and SIV− macaques were examined for expression of various phenotypic markers associated with retention and recruitment of PB (squares) and PC (circles) between and within tissues (gray symbols, rectal tissue; white symbols, bone marrow; black symbols, MLN). *, P < 0.05; **, P < 0.01, ***, P < 0.001; ****, P < 0.0001. For the PB versus PC comparisons, ## and # indicate those that remain significant at P values of <0.001 and 0.025, respectively, after Hochberg correction. For the PB versus PB or PC versus PC comparisons between tissues, “%” indicates those that remain significant at P values of <0.05 after Bonferroni correction. PB and PC frequencies represent the averages for two separate staining assays performed side by side.
FIG 2.
Comparison of fresh versus frozen PB and PC numbers in the bone marrow and MLN. The numbers of PB (left, squares) or PC (right, circles) per 106 live cells were determined by flow cytometry and compared in fresh and previously frozen samples (the latter are identified by an asterisk in Table 1) in bone marrow (white symbols) (A) and MLN (black symbols) (B). *, P < 0.05; **, P < 0.01. PB and PC numbers in fresh samples are presented as the averages for two separate staining assays performed side by side.
FIG 3.
Geometric mean fluorescence intensity of Bcl-2 and frequency of CD40 in fresh and frozen PB and PC subsets. The GeoMFI of Bcl-2 (A) and expression of CD40 (C) in bone marrow PB (white squares) and PC (white circles) and GeoMFI of Bcl-2 (B) and expression of CD40 (D) in MLN PB (black squares) and PC (black circles) were determined by flow cytometry and compared in fresh and previously frozen samples (the latter are identified by an asterisk in Table 1). *, P < 0.05; **, P < 0.01. PB and PC numbers in fresh samples are presented as the averages for two separate staining assays performed side by side.
Whereas bone marrow Bcl-2−/low PB were lost upon freezing, the frequency of bone marrow CD40+ PB increased sharply in frozen samples. In contrast, both frozen bone marrow and MLN exhibited a decrease in the frequency of CD40+ PC (Fig. 3C and D). The frequency of CD40+ cells was higher in PC than in PB in fresh bone marrow, but the reverse was seen in frozen bone marrow and MLN cells (Fig. 3C and D). These data emphasize the caveat of analyzing frozen cells alone and suggest caution in interpreting data obtained on cellular populations particularly sensitive to the freeze-thaw process.
Figure 4 depicts the expression of markers previously suggested to play a role in PB/PC trafficking and survival (41–44) on freshly isolated rectal, bone marrow, and MLN cells. Tissue-specific expression patterns are evident for a majority of the markers, most notably CXCR4 (highest in MLN PB and PC), CCR9 (lowest in MLN PB and PC), and CCR10 (highest in rectal PB and PC) (Fig. 4C, D, and E). Although less common, certain markers were also differentially expressed on PB compared to PC, with frequencies of cells expressing Bcl-2 and CCR10 consistently higher in PC than in PB in all tissues (Fig. 4A and E) and expression of α4β7 higher in PC than in PB (Fig. 4F) in both the bone marrow and the rectal compartment. Interestingly, the great majority of bone marrow PB expressed CCR9 and CD11a but lacked expression of Bcl-2, in contrast to what was observed on bone marrow PC (Fig. 4A, D, and G).
Vaccination status influences the frequency of PC and PB in rectal tissue of chronically SIV-infected macaques.
Given the protective effect of rectal PC observed in vaccinated female macaques prechallenge and 2 weeks postinfection (15) and the negative correlation between rectal PC in all vaccinated animals and peak viral load, we sought to determine if mucosal PC might play a role in controlling chronic viremia. However, delayed acquisition in SIV-vaccinated macaques did not translate to improved viral control during chronic infection (Fig. 5A), nor did we see any significant differences between males and females in terms of their PB/PC frequencies (data not shown). Indeed, mucosal PC directly correlated with viral load during chronic infection while PB showed a trend toward correlation with viral load (Fig. 5B). This effect was largely driven by the unvaccinated group, as any significance was lost when this group was removed from analysis (data not shown). Accordingly, no significant increase in either IRF4hi subset was noted in the rectal mucosa when comparing infected and uninfected animals (Fig. 5C); however, alterations were evident when infected animals were divided into vaccinated and unvaccinated groups. The unvaccinated group alone demonstrated significant increases in PBs and PCs compared to vaccinated macaques (Fig. 5D), suggesting that vaccination indirectly resulted in a reduction in PB recruited to and PC maintained in the mucosa during chronic infection. Notably, within the vaccinated group, no significant differences were found between animals with low or high plasma viremia; indeed, for both PC and PB, P values obtained by comparing low-viremic vaccinated or high-viremic vaccinated to unvaccinated animals were strengthened by grouping all vaccinated animals together.
FIG 5.
Influence of viral load and vaccination status on rectal, bone marrow, and MLN PB and PC frequencies. (A, B, E, G) Correlations between plasma viral load at necropsy and the number of challenges needed for infection of SIV-vaccinated macaques (A) or the frequency of PB (left, squares) and PC (right, circles) in rectal (B), bone marrow (E), or MLN (PB only) (G) of SIV+ macaques. (C) Frequency of rectal PB (left, gray squares) or PC (right, gray circles) in SIV+ versus SIV− macaques. (D) Frequency of rectal PB (left, gray squares) or PC (right, gray circles) in SIV+ vaccinated (VACC; low- and high-viremic groups), high-viremic unvaccinated (UNVACC), and SIV− macaques. (F and H) Frequency of PB (left, squares) or PC (right, circles) in bone marrow (F) and MLN (PB only) (H) of SIV+ low-viremic, high-viremic (vaccinated and unvaccinated groups) and SIV− macaques. (I) Correlation between frequency of bone marrow and rectal PB in macaque groups: white triangles, low viral load vaccinated; black squares, high viral load vaccinated; white diamonds, high viral load unvaccinated. *, P < 0.05; **, P < 0.01. PB and PC frequencies in panels B to I represent the averages for two separate staining assays performed side by side. INF, infected; UNINF, uninfected.
Plasma viral load differentially affects PB frequencies in the bone marrow compared to the mesenteric lymph node.
The role of the bone marrow as a hematopoietic organ and niche site of long-lived PC is well established; however, the bone marrow may also play a significant role in orchestrating immune responses (45). Acute and chronic HIV/SIV infection is known to severely impact mucosal immunity and lymphoid function, yet comparatively little is known about the impact of chronic infection on bone marrow immunity. Strikingly, in contrast to what we observed in the rectal mucosa, bone marrow PB inversely correlated with viral load, with PC demonstrating a strong trend toward an inverse correlation (Fig. 5E). PC and PB in the bone marrow were significantly increased in infected compared to uninfected animals (P = 0.037 and P = 0.0004, respectively; uninfected animals are represented by the 4 leftmost circles in Fig. 1C, middle panel), and as suggested by the correlation analyses, the low-viremic vaccinated macaques were largely responsible for this increase. Indeed, low-viremic macaques supported an increased frequency of PC and PB compared to uninfected macaques or high-viremic macaques (vaccinated and unvaccinated combined) (Fig. 5F).
Within the MLN, alterations were observed in PB, but not PC frequencies. This is consistent with the lymph node's primary role as an initiation site rather than an effector site of the immune response. Similar to the rectal mucosa, PB in the MLN directly correlated with viral load (Fig. 5G). Here, PB were increased in infected compared to uninfected macaques (P = 0.03, data not shown), but unlike what was observed for the rectal mucosa, this effect was most significant when dividing infected animals into high- versus low-viremic animals, as all high-viremic animals, vaccinated and unvaccinated examined together, had increased PB frequency compared to low-viral-load or uninfected macaques (P = 0.02 and P = 0.008, respectively) (Fig. 5H). Thus, albeit displaying a diametrical relationship with viral load, PB in both the MLN and bone marrow were more heavily influenced by plasma viral load than vaccination status per se compared to the rectal mucosa.
PB newly generated in the lymph node are generally believed to traffic to the bone marrow or to sites of inflammation, where they further differentiate into PC, depending on the local microenvironment (46). A strong reciprocal relationship exists between PB frequencies in the bone marrow and rectal mucosa (Fig. 5I), suggesting that PB of the low-viral-load animals preferentially traffic to the bone marrow and PB of high-viral-load unvaccinated animals preferentially traffic to the mucosa, while PB of vaccinated high-viral-load macaques are less biased either way.
Inflammation and viral replication in rectal tissue.
Inflammation plays a role in recruiting PB and memory B cells to sites of infection; therefore, we examined the level of inflammation present in rectal tissue sections from our infected and uninfected macaques. A hematoxylin and eosin (H&E) staining assay was performed on cryopreserved rectal tissue sections from 3 animals from each group (Fig. 6A), and a qualitative score of tissue inflammation was generated. The highest levels of inflammation were seen in unvaccinated animals, followed by vaccinated, high-viral-load macaques. Vaccinated low-viremic and uninfected macaques both had low levels of inflammation (Fig. 6B). The inflammation score directly correlated with rectal PB frequency (Fig. 6D) and had a strong trend toward correlation with the PC frequency (Fig. 6E).
FIG 6.
Inflammation and viral load in rectal tissues of SIV-infected and uninfected macaques. Frozen rectal tissue blocks from 12 macaques (n = 3 per group) were used for generating slides used for tissue staining and for the determination of tissue viral loads by NASBA. (A) One representative H&E stain of 3 frozen rectal tissue sections stained per group. Images are focused on the epithelial and lamina propria layers (magnification, ×10). (B) Twelve H&E-stained slides corresponding to 4 different macaque groupings (white bar, uninfected; horizontally striped bar, low viral load [v.l.] vaccinated; vertically striped bar, high viral load vaccinated; gray bar, high-viral-load unvaccinated macaques) were given a qualitative score of inflammation: 0.5 = none to minimal; 1.0 = minimal; 1.5 = mild; 2 = moderate. (C) Total RNA isolated from rectal tissue sections was used to quantify SIV RNA expressed in rectal tissues. Data are presented for SIV− (white circles), SIV+ vaccinated low- and high-viremic groups (gray circles), and SIV+ unvaccinated macaques (black circles). (D and E) Correlations between inflammation score and rectal PB (gray squares) (D) or rectal PC (gray circles) (E). (F and G) Correlations between rectal viral load at necropsy and percentage of rectal PB (gray squares) (F) or percentage of rectal PC (gray circles) (G). *, P < 0.05.
To explore the relationship between inflammation and tissue viral load, we extracted RNA from rectal tissue frozen in OCT for SIV viral load analysis. Without inclusion of the uninfected animals, rectal tissue viral load measurements did not correlate with the tissue inflammation score (P = 0.11); however, tissue viral loads did correlate with both rectal PB and PC frequencies (P = 0.025 and P = 0.041, respectively [Fig. 6F and G]). Due to low sample numbers, when examined alone, low-viremic vaccinated and high-viremic vaccinated animals did not differ significantly from the unvaccinated group; however, when combined, the vaccinated group maintained lower tissue viral loads than did the unvaccinated (Fig. 6C).
These data suggest that the vaccine regimen resulted in reduced inflammation and viral replication in the mucosa, possibly leading to better-preserved barrier function during chronic infection and reduced overall recruitment of PB and PC to the mucosa. In contrast, unvaccinated animals had higher levels of inflammation and tissue viral loads, which could have influenced PB/PC frequencies by promoting trafficking to the area and/or by inducing the formation of local isolated lymphoid follicles (47).
Trafficking, retention, and survival marker expression on PB/PC in the rectal mucosa, bone marrow, and MLN.
In order to gain a better understanding of the potential role of trafficking and retention in directing and supporting PB/PC accumulation in the rectal mucosa of unvaccinated macaques, the bone marrow of low-viremic macaques, and the MLN of high-viremic macaques, we examined the expression of α4β7, of the chemokine receptors CCR10, CCR9, CCR7, and CXCR4, and of CD11a, CD40, and Bcl-2 on the surface of PB/PC in different animal groups. Results that had a P value of ≤0.05 for both the Kruskal-Wallis and Mann-Whitney tests are presented in Table 2. Expression of the mucosal homing markers α4β7, CCR10, and CCR9 was higher in PB (α4β7 and CCR9 only) and PC of low-viremic and uninfected animals combined than in high-viremic animals in the MLN. This result is somewhat surprising given that we saw an increased frequency of PB in the MLN of high-viremic macaques. However, we did note an increased frequency of CCR7 and CD40 on PB and PC and of CXCR4 and Bcl-2 on PB in the MLN of infected compared to uninfected animals. Although CD11a expression was typical of PB/PC of the MLN, it was significantly lower in PB of vaccinated, infected than in those of uninfected or unvaccinated, infected animals. Thus, increased expression of markers associated with retention and engagement of PB/PC in the lymph node of infected macaques may facilitate their accumulation in the MLN. However, this effect may be mitigated in the low-viremic animals which, like the uninfected macaques, maintain higher expression of tissue homing markers, potentially promoting their egress from the MLN. Although α4β7, CCR10, and CCR9 are known to play a role in trafficking to the gut mucosa, we also noted expression of these markers, particularly CCR9, on PB/PC in the bone marrow (Fig. 4D to F).
TABLE 2.
Expression of markers associated with trafficking and maintenance of PB and PC in tissues of SIV-infected and uninfected macaquesa
| Tissue and marker | Cell type | Mean frequency (±SEM) within PB or PC subset |
P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HVL, Unvax | HVL, Vax + Unvax | HVL + LVL, Vax + Unvax | HVL + LVL, Vax | LVL, Vax | LVL, Vax + Uninf | Uninf | |||
| MLN | |||||||||
| α4β7 | PB | 36 (±5.2) | 57 (±5.9) | 0.01 | |||||
| PC | 36 (±5.4) | 64 (±5.3) | 0.004 | ||||||
| CCR10 | PC | 22 (±5.7) | 36 (±3.8) | 0.03 | |||||
| CCR9 | PB | 5.1 (±1.1) | 20 (±3.8) | 0.0008 | |||||
| PC | 7.5 (±1.9) | 17 (±2.5) | 0.005 | ||||||
| CCR7 | PB | 13 (±3.1) | 2.2 (±0.54) | 0.007 | |||||
| PC | 13 (±4.7) | 0.45 (±0.26) | 0.0004 | ||||||
| CXCR4 | PB | 47 (±6.6) | 4.9 (±1.0) | 0.005 | |||||
| CD40 | PB | 29 (±3.9) | 10 (±2.0) | 0.02 | |||||
| PC | 33 (±6.5) | 0 (±0) | 0.0004 | ||||||
| Bcl-2 | PB | 71 (±3.8) | 28 (±1.3) | 0.0004 | |||||
| CD11a | PB | 93 (±1.5) | 85 (±1.3) | 0.01 | |||||
| PB | 85 (±1.3) | 91 (±1.8) | 0.04 | ||||||
| Rectal | |||||||||
| CCR9 | PC | 46 (±8.2) | 6.0 (±4.0) | 0.02 | |||||
| PB | 12 (±4.3) | 44 (±7.7) | 0.05 | ||||||
| PB | 44 (±7.7) | 10 (±4.8) | 0.05 | ||||||
| CXCR4 | PB | 21 (±4.9) | 2.0 (±0.7) | 0.04 | |||||
| PB | 14 (±4.1) | 33 (±5.6) | 0.01 | ||||||
| PC | 10 (±5.7) | 34 (±11) | 0.01 | ||||||
| CCR7 | PC | 1.4 (±0.24) | 10 (±3.0) | 0.02 | |||||
| BM | |||||||||
| CCR7 | PB | 21 (±5.0) | 0.43 (±0.30) | 0.0008 | |||||
| CD40 | PC | 23 (±3.5) | 5.1 (±0.78) | 0.007 | |||||
| CD11a | PC | 44 (±5.9) | 86 (±4.2) | 0.002 | |||||
HVL, high viral load; LVL, low viral load; Vax, vaccinated; Unvax, unvaccinated; Inf, infected; Uninf, uninfected; BM, bone marrow; MLN, mesenteric lymph node; PB, plasmablast; PC, plasma cell.
In the rectal mucosa, PB of vaccinated macaques were more often CCR9+ or CXCR4+ than PB of uninfected macaques and more often CCR9+ than in unvaccinated, infected animals. Similarly, rectal PC of vaccinated macaques expressed more CCR7 than did those of unvaccinated, infected animals. Notably, when comparing low-viremic to all high-viremic animals, it was the vaccinated, low-viremic macaques that expressed the highest frequency of CXCR4+ PB and PC. Taken together, the data show that expressions of CCR9, CXCR4, and CCR7 were more specific to PB or PC of vaccinated macaques than to those of unvaccinated, infected animals, possibly resulting in their trafficking out of the rectal mucosa to the small intestine, bone marrow, or lymph nodes.
Bone marrow supported an increased frequency of CD40+ PC as well as CCR7+ PB in infected compared to uninfected macaques. The opposite was true for CD11a+ PC, for which the infected animals exhibited decreased expression compared to the uninfected. These results are difficult to interpret with respect to the observed increase in PB and PC in the low-viremic macaques compared to the uninfected or high-viremic macaques (Fig. 5F) and suggest a role for alternative markers not examined here in the trafficking and retention of PB/PC in the bone marrow, in addition to chemokines and other factors present in the bone marrow niche itself.
PC niche factors reflect differences found in PB recruitment and PC maintenance in SIV-infected rhesus macaques.
Extrinsic factors, including chemokines, adhesion molecules, and cytokines, are necessary for PC survival (48). In vivo these factors and the cells that produce them form what is known as the PC niche. The bone marrow niche is home to PC of various degrees of longevity, including the canonical long-lived PC (33). In addition to the bone marrow, other sites, including human tonsil (10), salivary glands (9), the intestinal mucosa (5, 6, 8), and lymph nodes (49), are also capable of supporting a viable PC niche, although these sites are less well characterized and their contribution to the long-lived PC pool is not well established. Studies suggest that a variety of cell types, including antigen-presenting cells (APC), like dendritic cells and macrophages, and polymorphonuclear cells (PMN), like eosinophils, neutrophils, and basophils, in addition to megakaryocytes and other stromal cells, play a role in creating the PC niche environment (11). In order to assess the contribution of the PC niche in the recruitment and maintenance of PB and PC during chronic SIV infection, freshly isolated bone marrow and rectal cells were sorted into two broad niche-supporting populations, CD2− CD20− CD19− HLA-DR+ APC and CD2− CD20− CD19− HLA-DR− PMN (50, 51). Total RNA was then extracted and analyzed without additional amplification for the expression of 44 PC niche genes of interest potentially involved in recruitment, adhesion, or maintenance of PB and PC in the bone marrow or rectal mucosa (see Table S1 in the supplemental material). Specific, barcoded primers generated by NanoString Technologies coupled with their nCounter analysis system were used to bind and detect expressed mRNAs.
In the bone marrow, where we observed an increase in PB/PC frequencies in low-viremic compared to high-viremic animals, we also found increased expression of PC niche factors in animals with low compared to high viremia. When expression patterns were analyzed as the ratio of SIV-low-viremic compared to SIV-high-viremic animals, all genes expressed with a greater than 2-fold difference and P values of <0.05 were increased specifically in the SIV-low-viremic group (Fig. 7A). These gene products include heparin-binding epidermal growth factor (EGF)-like growth factor (HBEGF), which binds CD138 (52) and was expressed by both APC and PMN, CXCL12, which recruits CXCR4-expressing PB and PC (53), and interleukin-10 (IL-10), IL-4, CD80, and ALDH1A2, which have all been reported to play a role in PC generation and maintenance (3, 27, 54–58).
FIG 7.
PC niche factors in the bone marrow and tissue-specific expression profiles of PC niche factors in bone marrow versus rectal mucosa during chronic SIV infection. Using a customized panel of PC niche factors (Table S1), mRNA expression analysis was performed on RNA isolated from sorted bone marrow or rectal cell populations representing either HLA-DR+ APC or HLA-DR− PMN. (A) Fold changes in expression of niche factors present in bone marrow samples are presented as a ratio of SIVlow (APC fraction, n = 4; PMN fraction, n = 4) to SIVhigh (APC fraction, n = 12; PMN fraction, n = 12) macaques. Only RNAs with a fold change in expression of >2 and P value of <0.05 are listed. (B and C) Fold changes in expression of niche factors are presented as the ratio between SIV+ bone marrow samples (APC fraction, n = 16; PMN fraction, n = 16) to SIV+ rectal samples (APC fraction, n = 7; PMN fraction, n = 3) (B) or SIV+ rectal samples to SIV+ bone marrow samples (C). Only RNAs with a fold change in expression of >10 and P value of <0.05 are listed. Striped bars, gray bars, black bars, and white bars represent specific cell types or niche factors associated with recruitment, adhesion, and maintenance, respectively.
Unfortunately, poor cell yields and RNA recovery in multiple rectal samples prohibited reliable comparisons between animals with high versus low viremia or an infected versus uninfected status. Rectal RNA in the APC fraction of 1 unvaccinated infected macaque showed greater expression of several PC niche RNAs than that of 6 vaccinated infected macaques. The protein products of these genes included HAS2, which leads to the production of hyaluronic acid, a factor that binds CD44-expressing PC (48), FN1 and VCAM1, which bind the integrins α4β7 and α4β1, respectively (53, 59), and CXCL12 as well as components of the platelet-derived growth factor receptor (PDGFR), whose expression on certain APC subsets has been linked to increased CXCL12 production (53) (data not shown). While suggestive of a role for the ACP niche fraction in promoting increased adhesion and maintenance of PB/PC in the rectal mucosa of unvaccinated macaques, additional experiments will be necessary to confirm these preliminary findings.
PC niche factors are differentially expressed in the bone marrow compared to the rectal mucosa.
Sufficient RNA was recovered from rectal tissue of all the SIV-infected macaques to allow comparison of PC niche genes of interest in the bone marrow and rectal mucosa. Notable differences (fold change of >10 and P values of <0.05) were apparent in a number of genes potentially involved in PC niche recruitment, adhesion, and maintenance (Fig. 7B). In terms of recruitment, CCL3, which attracts CCR1-expressing cells, was enriched in the bone marrow whereas CCL20, CXCL9 and CXCLl0, which recruit CCR6 and CXCR3-expressing cells to sites of inflammation, dominated in the rectal mucosa. In terms of adhesion to CD138, HBEGF expression was associated with the bone marrow whereas NGR1 and AREGB were expressed in the rectal mucosa. Receptors for the integrins α4β1 and α4β7 were likewise differentially expressed; CD82 was characteristic of the bone marrow, while expressions of FN1 and VCAM1 were more common in cells of the rectal mucosa. With the exception of the retinoic acid-producing enzyme encoded by ALDH1A2, factors associated with PC maintenance (or lack thereof) were found to be enriched in the bone marrow exclusively. This may speak to its role as the canonical niche site, particularly for long-lived PC. One of these factors, TNFSF13B, encodes APRIL, a cytokine known to support PC longevity. However, expression of IL-1β and tumor necrosis factor (TNF), which together promote expression of IL-8 (60), are indicative of a dynamic environment, suggesting active mobilization and turnover of PB and PC within the bone marrow during chronic SIV infection (61).
DISCUSSION
In a recent SIV vaccine study conducted in our lab, mucosal PC frequency was found to correlate with delayed SIV acquisition in female macaques (17) and with reduced mean viral load in early infection in all vaccinated macaques (unpublished data), presumably due to their production of SIV-specific antibodies. Many studies have found evidence for the protective effect of SIV- and HIV-specific antibodies early postchallenge (62–68), yet few have examined how the generation, recruitment, and maintenance of antibody-secreting cells themselves impact viral control in the chronic phase of infection. Unlike memory B cells, which express their receptor (BCR) on the cell surface, the majority of PC lack surface expression of their secreted immunoglobulin (33, 34), making consistent identification of SIV-specific PC a challenge. Furthermore, chronic HIV and SIV infection induces a number of B cell abnormalities affecting PB/PC, including hypergammaglobulinemia, reductions in memory B cells and antibody responses to recall antigens, progressive loss of lymphoid tissue architecture, and an altered isotype ratio in the gut, favoring IgG over IgA (69). Nevertheless, by examining a group of vaccinated and unvaccinated macaques with either low (all vaccinated) or high viremia, we were able to identify alterations in the PC niche environments present in the bone marrow and rectal mucosa that may influence how well the animals control viremia.
Low-viremic animals had increased PB and PC frequencies in the bone marrow compared to high-viremic animals or SIV-negative controls. Bone marrow PB showed a strong negative correlation with viral load and with rectal PB. Appropriately, the bone marrow also had increased expression of niche factors associated with PB/PC recruitment, adhesion, and survival, including CXCL12, HBEGF, and CD80 and factors associated with PB/PC generation from memory B cells like IL-10 and IL-4, as well as a factor most often associated with expression of the IgA isotype, the retinoic acid-producing enzyme encoded by ALDH1A2. In addition to trafficking of PB from the lymph nodes, resident memory B cells in the bone marrow and tissue sites may also contribute to elevated PB/PC frequencies. Whether the increase in bone marrow PB and PC is ultimately a cause or a consequence of reduced viremia is unclear. Flow-cytometric identification of SIV-specific PB and PC frequencies present in these animals at the time of challenge and over the course of disease progression would help address this question and is an approach that is actively being developed by members of our group.
In contrast to what was noted in early infection, during chronic infection total rectal PC frequency correlated with viral load; however, the vaccinated animals maintained a lower frequency of mucosal PB and PC than did the unvaccinated, and without the unvaccinated group, the correlation with viral load was lost. Thus, the vaccine regimen appeared to mitigate the accumulation of PC during chronic infection. A qualitative assessment of tissue inflammation and tissue viral load data suggest that vaccination led to better control of tissue viremia and limited inflammation, resulting in reduced trafficking and generation of PB/PC to or within mucosal tissues.
Why some vaccinated animals were able to maintain low plasma viral loads while supporting enhanced frequencies of bone marrow PB and PC is unclear; however, the answer may lie within the lymph nodes. The frequency of PB in the MLN correlated with viral load, similar to what was seen for the rectal mucosa; however, here the low-viremic vaccinated group, like the uninfected animals, maintained a lower frequency of PB than did high-viral-load animals, vaccinated animals included. Lymph nodes, like the gut-associated lymphoid tissue, are early sites of viral replication (12, 14). However, unlike the effector sites of the gut, which experience a profound and early loss of critical CD4+ T cell subsets, including resting, activated memory cells (70) and Th17 cells (71), inductive sites and lymph nodes contain a higher proportion of CD4+ naive and T follicular-helper (TFH) cells and are less sensitive to HIV-mediated cell death despite significant levels of infection (72). Indeed, lymph nodes have been described as viral “reservoirs” (73) during antiretroviral treatment and “sanctuaries” in the case of SIV viremic controllers (74). In our low-viremic animals, it is conceivable that low-level viral replication within the lymph nodes, in the context of a well-preserved mucosal barrier, helps protect the host by continually generating a dynamic pool of germinal center-derived, SIV-specific PB and memory B cells that efficiently traffic to the bone marrow, where newly generated PB compete with older PC for the survival niche (11, 33, 75, 76). This type of controlled yet evolving response may help limit viral escape. In the context of higher levels of viremia, which coincides with an increased frequency of TFH cells as well as increased nonspecific immune activation (77) and microbial translocation, a more permissive (78) and dysfunctional (79) germinal center response may contribute to the dysregulation of PC differentiation from memory B cells, hypergammaglobulinemia, and lack of viral control (80, 81).
Our results further suggest that it may be possible to alter specific PC niche microenvironments through vaccination or influence PC trafficking to and survival in bone marrow or tissue sites through direct manipulation of differentially expressed PC niche factors. In a model of multiple myeloma, thymoquinone, an established chemotherapeutic compound, inhibited chemotaxis to CXCL12, resulting in Fas-mediated apoptosis of the cancerous cells (82), and an earlier study showed a similar effect in mice treated with a CXCR4 inhibitor (83). Thus, in addition to the possibility of improving SIV-specific PC longevity, vaccine efficacy, or viral control postinfection, the development of novel therapeutic PC niche targets may help to mitigate the effects of autoimmunity or the progression of multiple myeloma.
MATERIALS AND METHODS
Animal cohort, immunizations, and challenge protocol.
The 20 Indian rhesus macaques (Macaca mulatta) used for these experiments were part of a larger study designed to assess the contribution of different SIV Env immunogens to vaccine efficacy. The care of the animals, vaccine regimens, and challenge protocols were described in detail elsewhere (17). Briefly, the macaques were housed and cared for at Advanced Bioscience Laboratories, Inc. (ABL, Rockville, MD), or at Bioqual, Inc. (Rockville, MD). Following challenge at the ABL facility, the macaques were maintained at the NIH Animal Facility, where all subsequent procedures were conducted. All animal care was in accordance with the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care and the recommendations of the Guide for the Care and Use of Laboratory Animals. Prior to initiation, all protocols and procedures were approved by the Institutional Animal Care and Use Committee of the respective facility. The animals were divided into groups based on viral load and vaccination status (Table 1). Unvaccinated control macaques were primed with empty Ad5hr vectors and boosted with adjuvant MF59 alone in the same manner as the vaccinated group. The uninfected seronegative macaques were used in two previous unpublished vaccine pilot studies, neither of which produced measurable anti-HIV or anti-SIV serum binding titers. In the first, which took place in 2012, animals were primed orally, intranasally, and intratracheally with Ad5hr-HIVTV-1gp160 followed by intramuscular boosting with a V3 scaffold protein. In the second study, which took place in 2014, animals received 100 to 200 μg of SIV gp120-containing nanoparticles delivered orally with 50 to 100 μg of mutant lymphotoxin.
Sample collection and cell isolation.
Mesenteric lymph node (MLN), rectal biopsy tissue, and bone marrow from the iliac crest were collected at necropsy from the animals identified in Table 1 and processed shortly after collection. In order to preserve granulocytes, 10 ml bone marrow was filtered through a 70-μm cell strainer and gently mixed with 1 vol 3% dextran in phosphate-buffered saline (PBS). Red blood cells (RBCs) were allowed to sediment at room temperature for 30 min. The leukocyte-rich upper layer was then aspirated and washed in RPMI containing 5 mM EDTA. Contaminating RBCs were lysed in 3 ml H2O for 20 s, immediately washed in RPMI plus 5 mM EDTA (12× volume), and resuspended in R10 medium (RPMI 1640 containing 10% fetal bovine serum [FBS], 2 mM l-glutamine, and antibiotics). MLNs were sliced, and cells were scraped out using a scalpel and filtered through a 40-μm cell strainer. Cells were washed, and RBCs were lysed in H2O as described above and resuspended in R10. Rectal pinch biopsy specimens were processed in the manner outlined by Demberg et al. (29). Rectal tissue sections (17 by 14 mm2) were frozen in OCT for subsequent SIV RNA analysis and tissue staining.
Characterization of PC and PB by flow cytometry.
Fresh or frozen cells (1.5 × 106) were stained with Aqua Live/Dead viability dye (Invitrogen, Carlsbad, CA) and a combination of fluorochrome-conjugated antibodies, including the following: Qdot605 α-CD14 (Tuk4) and α-CD2 (S5.5) (Invitrogen); eFL650NC α-CD20 (2H7), PECy7 α-CD138 (DL-101), phycoerythrin (PE) α-HLA-DR (L243), and APC α-CXCR4 (12G5) (eBioscience, San Diego, CA); PECy5 α-CD19 (J3-119) (Beckman Coulter, Brea, CA); polyclonal α-human IgD in Texas Red (Southern Biotech, Birmingham, AL); α-IgM in PerCPCy5.5 or PE-cF594 (G20-127), Alexa Fluor 488 α-CCR9 (112509) and BV711 α-CD11a (HI111) (BD Biosciences, San Jose, CA); fluorescein isothiocyanate (FITC) α-CD38 (AT-1) (StemCell Technologies, Inc., Vancouver, Canada); APC-Cy7 α-CD40 (5C3), APC-Cy7 α-CCR7 (G043H7), PE α-CCR10 (6588-5) (BioLegend, San Diego, CA); APC α-α4β7 (NIH Nonhuman Primate Reagent Resource). Following surface staining, intranuclear staining was performed using the BD Biosciences Transcription Factor Buffer Set according to the manufacturer's recommendations for eFluor440 α-IRF4 (3E4) (eBioscience) and PE-cF594 α-Bcl2 (Bcl2/100), with the later addition of Alexa Fluor 700 α-Ki67 (B56) (BD Biosciences) for analysis of frozen cells. Frequencies of PB/PC reported for these samples reflect the average of these two stains. A minimum of 50,000 live rectal cells and 200,000 live MLN and bone marrow cells were acquired on an LSRII (BD Biosciences) and analyzed using FlowJo Software (FlowJo LLC, Ashland, OR). Stained frozen cells were used exclusively for Fig. 1D and were compared with fresh cells in Fig. 2 and 3. Freshly isolated cells were used for all other flow cytometry-based figures. The fresh cells were stained side by side with two distinct phenotyping panels that contained common markers for the identification of PB and PC. Frequencies of PB/PC reported for these samples reflect the averages of these two stains.
FACS sorting and mRNA isolation of antigen-presenting cells and polymorphonuclear cell subsets.
Rectal cells (6 × 106 to 34 × 106) and 1 × 107 to 5 × 107 bone marrow cells isolated as described above were stained with Aqua Live/Dead viability dye, FITC α-CD2 (S5.5) (Invitrogen), FITC α-CD20 (2H7) (eBioscience), PECy5 α-CD19 (J3-119) (Beckman Coulter), and PE α-HLA-DR (L243) (eBioscience) and subjected to fluorescence-activated cell sorting on a FACSAria II (BD Biosciences) in order to isolate live cells lacking expression of markers for T, NK, and B cells. This population was then sorted into two broad populations representing HLA-DR+ antigen-presenting cells (APC) and HLA-DR− polymorphonuclear cells (50). For certain bone marrow samples, cells were depleted of CD2+ and CD19+ cells using the AutoMACS system (Miltenyi Biotech, Bergisch Gladbach, Germany) with PECy5-labeled antibodies and α-PECy5 magnetic beads prior to fluorescence-activated cell sorting (FACS). Cells were counted postsorting and frozen in buffer RLT (Qiagen, Valencia, CA). RNA was isolated from RLT lysates using the Quick-RNA MicroPrep kit from Zymo Research (Irvine, CA) and their provided protocol. RNA concentrations were determined by analysis on a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Grand Island, NY).
mRNA expression analysis.
A custom CodeSet containing color-coded probe pairs corresponding to 65 genes of interest from Macaca mulatta or, in 2 cases, Macaca fascicularis was compiled by NanoString Technologies, Inc. (Seattle, WA), using sequence information published in the NCBI Gene and Nucleotide databases. The CodeSet included 12 cell type-specific genes, 44 niche factor genes, and 9 reference genes selected using the Genevestigator reference expression database for human data. Isolated RNA was allowed to hybridize to the CodeSet overnight before being transferred to the nCounter Analysis System for mRNA quantification (84). Expression data were analyzed using NanoString's nSolver software (v2.0). Briefly, all data were background subtracted using the geometric mean of 8 negative-control probe sets and normalized for experimental variability according to 6 positive-control mRNA-probe combinations. Content was normalized based on the expression of 3 low-variance genes for bone marrow cells and/or rectal cells depending on the analyses performed. Samples with normalization flags were removed prior to final analysis.
Inflammation and SIV RNA in rectal tissues.
Slides were cut from frozen rectal tissue blocks and H&E stains performed by a trained professional at the Pathology/Histology Laboratory (PHL), Frederick National Lab for Cancer Research, Leidos Biomedical Research, Inc. (Frederick, MD). A senior staff pathologist from PHL analyzed the slides and provided a qualitative score of tissue inflammation in a blinded fashion. For RNA isolation, 10-μm sections were cut from the rectal blocks. Three to 10 sections were placed into prechilled 2.0-ml microcentrifuge tubes containing ∼6- by 3-mm zirconium oxide beads. RNA lysates were generated by adding 600 μl of buffer RLT (Qiagen) containing 0.1 volume 2-mercaptoethanol to each tube and homogenizing for 30 s at 4,500 rpm in a MicroSmash MS-100 (Tomy, Tokyo, Japan) bead mill homogenizer. Total RNA was purified using the RNeasy minikit (Qiagen) according to the manufacturer's instructions with an on-column DNase I treatment and the modification of adding 1.5 volumes of 100% ethanol to the lysate to copurify small RNA species. RNA was eluted in 100 μl of RNase-free water. RNA yield, purity, and quality were determined by 2100 Bioanalyzer RNA 6000 Picochip analysis (Agilent, Santa Clara, CA). Isolated RNA was stored at −80°C. RNAs from several extractions were pooled and concentrated using the Quick-RNA MicroPrep RNA Clean-Up protocol (Zymo Research) and 300 ng to 1 μg of RNA per sample was shipped frozen to ABL for SIV RNA quantification by nucleic acid sequence-based amplification (NASBA) as described previously (85).
Statistical analysis.
Unless otherwise stated above, data were analyzed using Prism (v6.01; GraphPad Software). The Wilcoxon matched-pair signed-rank test was used to analyze differences between PB and PC within the same sample and across tissues. Data for Fig. 4 were analyzed after arc-sine transformation, and the effects of Hochberg and Bonferroni corrections are noted in the figure legend. The markers associated with trafficking, retention, and survival were initially tested with the Kruskal-Wallis test and included in Results only if P values were ≤0.05 by this method. The Mann-Whitney test was used to analyze differences between the different macaque groups. The Spearman rank correlation test was used for all correlation analyses. A P value of ≤0.05 was considered statistically significant.
Accession number(s).
The NanoString data in this publication are accessible through NCBI's Gene Expression Omnibus GEO Series accession number GSE81609.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the skilled veterinarians Josh Kramer and Matthew Breed and to William Magnanelli and other staff at the NCI animal facility who collected all specimens for this project. We also acknowledge the dedicated employees at the Pathology/Histotechnology Laboratory at Frederick National Laboratory for Cancer Research, specifically Roberta Smith, who cut, mounted, and H&E stained the frozen rectal tissue blocks; Diana Haines, who performed a qualitative assessment of inflammation on the H&E-stained sections; and Andrew Warner, who isolated RNA from frozen rectal sections. We thank Ranajit Pal and Corinne O'Neill at Advanced Bioscience Laboratories, Inc., for performing the NASBA assays for plasma and tissue viral loads. APC-anti-α4β7 was provided by the NIH Nonhuman Primate Reagent Resource.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
We declare that we have no financial conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.01727-16.
REFERENCES
- 1.Slifka MK, Ahmed R. 1998. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol 10:252–258. doi: 10.1016/S0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 2.Moser K, Tokoyoda K, Radbruch A, MacLennan I, Manz RA. 2006. Stromal niches, plasma cell differentiation and survival. Curr Opin Immunol 18:265–270. doi: 10.1016/j.coi.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Chu VT, Berek C. 2013. The establishment of the plasma cell survival niche in the bone marrow. Immunol Rev 251:177–188. doi: 10.1111/imr.12011. [DOI] [PubMed] [Google Scholar]
- 4.Manz RA, Thiel A, Radbruch A. 1997. Lifetime of plasma cells in the bone marrow. Nature 388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 5.Di Niro R, Mesin L, Raki M, Zheng NY, Lund-Johansen F, Lundin KE, Charpilienne A, Poncet D, Wilson PC, Sollid LM. 2010. Rapid generation of rotavirus-specific human monoclonal antibodies from small-intestinal mucosa. J Immunol 185:5377–5383. doi: 10.4049/jimmunol.1001587. [DOI] [PubMed] [Google Scholar]
- 6.Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S, Donze O, Frossard C, Chizzolini C, Favre C, Zubler R, Guyot JP, Schneider P, Roosnek E. 2008. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest 118:2887–2895. doi: 10.1172/JCI33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang B, Hyland L, Hou S. 2001. Nasal-associated lymphoid tissue is a site of long-term virus-specific antibody production following respiratory virus infection of mice. J Virol 75:5416–5420. doi: 10.1128/JVI.75.11.5416-5420.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesin L, Di Niro R, Thompson KM, Lundin KE, Sollid LM. 2011. Long-lived plasma cells from human small intestine biopsies secrete immunoglobulins for many weeks in vitro. J Immunol 187:2867–2874. doi: 10.4049/jimmunol.1003181. [DOI] [PubMed] [Google Scholar]
- 9.Szyszko EA, Brokstad KA, Oijordsbakken G, Jonsson MV, Jonsson R, Skarstein K. 2011. Salivary glands of primary Sjogren's syndrome patients express factors vital for plasma cell survival. Arthritis Res Ther 13:R2. doi: 10.1186/ar3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Laar JM, Melchers M, Teng YK, van der Zouwen B, Mohammadi R, Fischer R, Margolis L, Fitzgerald W, Grivel JC, Breedveld FC, Lipsky PE, Grammer AC. 2007. Sustained secretion of immunoglobulin by long-lived human tonsil plasma cells. Am J Pathol 171:917–927. doi: 10.2353/ajpath.2007.070005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tangye SG. 2011. Staying alive: regulation of plasma cell survival. Trends Immunol 32:595–602. doi: 10.1016/j.it.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Haase AT. 2010. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 13.Barouch Dan H, Ghneim K, Bosche William J, Li Y, Berkemeier B, Hull M, Bhattacharyya S, Cameron M, Liu J, Smith K, Borducchi E, Cabral C, Peter L, Brinkman A, Shetty M, Li H, Gittens C, Baker C, Wagner W, Lewis Mark G, Colantonio A, Kang H-J, Li W, Lifson Jeffrey D, Piatak M Jr, Sekaly R-P. 2016. Rapid inflammasome activation following mucosal SIV infection of rhesus monkeys. Cell 165:656–667. doi: 10.1016/j.cell.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, Smith JY, Brinkman AL, Peter LE, Mathew SI, Smith KM, Borducchi EN, Rosenbloom DI, Lewis MG, Hattersley J, Li B, Hesselgesser J, Geleziunas R, Robb ML, Kim JH, Michael NL, Barouch DH. 2014. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 512:74–77. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebeis BJ, Klenovsek K, Rohwer P, Ritter U, Schneider A, Mach M, Winkler TH. 2004. Activation of virus-specific memory B cells in the absence of T cell help. J Exp Med 199:593–602. doi: 10.1084/jem.20030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurosaki T, Kometani K, Ise W. 2015. Memory B cells. Nat Rev Immunol 15:149–159. doi: 10.1038/nri3802. [DOI] [PubMed] [Google Scholar]
- 17.Tuero I, Mohanram V, Musich T, Miller L, Vargas-Inchaustegui DA, Demberg T, Venzon D, Kalisz I, Kalyanaraman VS, Pal R, Ferrari MG, LaBranche C, Montefiori DC, Rao M, Vaccari M, Franchini G, Barnett SW, Robert-Guroff M. 2015. Mucosal B cells are associated with delayed SIV acquisition in vaccinated female but not male rhesus macaques following SIVmac251 rectal challenge. PLoS Pathog 11:e1005101. doi: 10.1371/journal.ppat.1005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas MA, Demberg T, Vargas-Inchaustegui DA, Xiao P, Tuero I, Venzon D, Weiss D, Treece J, Robert-Guroff M. 2014. Rhesus macaque rectal and duodenal tissues exhibit B-cell sub-populations distinct from peripheral blood that continuously secrete antigen-specific IgA in short-term explant cultures. Vaccine 32:872–880. doi: 10.1016/j.vaccine.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaoul N, Burelout C, Peruchon S, van Buu BN, Laurent P, Proust A, Raphael M, Garraud O, Le Grand R, Prevot S, Richard Y. 2012. Default in plasma and intestinal IgA responses during acute infection by simian immunodeficiency virus. Retrovirology 9:43. doi: 10.1186/1742-4690-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corcoran LM, Nutt SL. 2015. The flavors of plasma cells. Oncotarget 6:32305–32306. doi: 10.18632/oncotarget.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiepe F, Radbruch A. 2016. Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat Rev Nephrol 12:232–240. doi: 10.1038/nrneph.2016.20. [DOI] [PubMed] [Google Scholar]
- 22.Winter O, Dame C, Jundt F, Hiepe F. 2012. Pathogenic long-lived plasma cells and their survival niches in autoimmunity, malignancy, and allergy. J Immunol 189:5105–5111. doi: 10.4049/jimmunol.1202317. [DOI] [PubMed] [Google Scholar]
- 23.Ghamlouch H, Ouled-Haddou H, Guyart A, Regnier A, Trudel S, Claisse J-F, Fuentes V, Royer B, Marolleau J-P, Gubler B. 2014. TLR9 ligand (CpG oligodeoxynucleotide) induces CLL B-cells to differentiate into CD20+ antibody-secreting cells. Front Immunol 5:292. doi: 10.3389/fimmu.2014.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H. 2006. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity 25:225–236. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Chaudhri VK, Wu Z, Biliouris K, Dienger-Stambaugh K, Rochman Y, Singh H. 2015. Regulation of bifurcating B cell trajectories by mutual antagonism between transcription factors IRF4 and IRF8. Nat Immunol 16:1274–1281. doi: 10.1038/ni.3287. [DOI] [PubMed] [Google Scholar]
- 26.Ochiai K, Maienschein-Cline M, Simonetti G, Chen J, Rosenthal R, Brink R, Chong AS, Klein U, Dinner AR, Singh H, Sciammas R. 2013. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity 38:918–929. doi: 10.1016/j.immuni.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jourdan M, Caraux A, De Vos J, Fiol G, Larroque M, Cognot C, Bret C, Duperray C, Hose D, Klein B. 2009. An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization. Blood 114:5173–5181. doi: 10.1182/blood-2009-07-235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid S, Yang S, Brown R, Kabani K, Aklilu E, Ho PJ, Woodland N, Joshua D. 2010. Characterisation and relevance of CD138-negative plasma cells in plasma cell myeloma. Int J Lab Hematol 32:e190–196. doi: 10.1111/j.1751-553X.2010.01222.x. [DOI] [PubMed] [Google Scholar]
- 29.Demberg T, Mohanram V, Venzon D, Robert-Guroff M. 2014. Phenotypes and distribution of mucosal memory B-cell populations in the SIV/SHIV rhesus macaque model. Clin Immunol 153:264–276. doi: 10.1016/j.clim.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckner CM, Moir S, Ho J, Wang W, Posada JG, Kardava L, Funk EK, Nelson AK, Li Y, Chun TW, Fauci AS. 2013. Characterization of plasmablasts in the blood of HIV-infected viremic individuals: evidence for nonspecific immune activation. J Virol 87:5800–5811. doi: 10.1128/JVI.00094-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medina F, Segundo C, Campos-Caro A, Salcedo I, Garcia-Poley A, Brieva JA. 2003. Isolation, maturational level, and functional capacity of human colon lamina propria plasma cells. Gut 52:383–389. doi: 10.1136/gut.52.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neumann B, Klippert A, Raue K, Sopper S, Stahl-Hennig C. 2015. Characterization of B and plasma cells in blood, bone marrow, and secondary lymphoid organs of rhesus macaques by multicolor flow cytometry. J Leukoc Biol 97:19–30. doi: 10.1189/jlb.1HI0514-243R. [DOI] [PubMed] [Google Scholar]
- 33.Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, Popova L, Kaminiski D, Fucile CF, Albizua I, Kyu S, Chiang KY, Bradley KT, Burack R, Slifka M, Hammarlund E, Wu H, Zhao L, Walsh EE, Falsey AR, Randall TD, Cheung WC, Sanz I, Lee FE. 2015. Long-lived plasma cells are contained within the CD19(-)CD38(hi)CD138(+) subset in human bone marrow. Immunity 43:132–145. doi: 10.1016/j.immuni.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silveira EL, Kasturi SP, Kovalenkov Y, Rasheed AU, Yeiser P, Jinnah ZS, Legere TH, Pulendran B, Villinger F, Wrammert J. 2015. Vaccine-induced plasmablast responses in rhesus macaques: phenotypic characterization and a source for generating antigen-specific monoclonal antibodies. J Immunol Methods 416:69–83. doi: 10.1016/j.jim.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demberg T, Brocca-Cofano E, Xiao P, Venzon D, Vargas-Inchaustegui D, Lee EM, Kalisz I, Kalyanaraman VS, Dipasquale J, McKinnon K, Robert-Guroff M. 2012. Dynamics of memory B-cell populations in blood, lymph nodes, and bone marrow during antiretroviral therapy and envelope boosting in simian immunodeficiency virus SIVmac251-infected rhesus macaques. J Virol 86:12591–12604. doi: 10.1128/JVI.00298-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hystad ME, Myklebust JH, Bo TH, Sivertsen EA, Rian E, Forfang L, Munthe E, Rosenwald A, Chiorazzi M, Jonassen I, Staudt LM, Smeland EB. 2007. Characterization of early stages of human B cell development by gene expression profiling. J Immunol 179:3662–3671. doi: 10.4049/jimmunol.179.6.3662. [DOI] [PubMed] [Google Scholar]
- 37.De Salort J, Sintes J, Llinas L, Matesanz-Isabel J, Engel P. 2011. Expression of SLAM (CD150) cell-surface receptors on human B-cell subsets: from pro-B to plasma cells. Immunol Lett 134:129–136. doi: 10.1016/j.imlet.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Leandro MJ. 2013. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies. Arthritis Res Ther 15(Suppl 1):S3. doi: 10.1186/ar3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oracki SA, Walker JA, Hibbs ML, Corcoran LM, Tarlinton DM. 2010. Plasma cell development and survival. Immunol Rev 237:140–159. doi: 10.1111/j.1600-065X.2010.00940.x. [DOI] [PubMed] [Google Scholar]
- 40.Klippert A, Neumann B, Stahl-Hennig C. 2016. Comparative phenotypical analysis of B cells in fresh and cryopreserved mononuclear cells from blood and tissue of rhesus macaques. J Immunol Methods 433:59–68. doi: 10.1016/j.jim.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Kunkel EJ, Butcher EC. 2003. Plasma-cell homing. Nat Rev Immunol 3:822–829. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- 42.Medina F, Segundo C, Campos-Caro A, González-Garcı′a I, Brieva JA. 2002. The heterogeneity shown by human plasma cells from tonsil, blood, and bone marrow reveals graded stages of increasing maturity, but local profiles of adhesion molecule expression. Blood 99:2154–2161. doi: 10.1182/blood.V99.6.2154. [DOI] [PubMed] [Google Scholar]
- 43.Fairfax KA, Kallies A, Nutt SL, Tarlinton DM. 2008. Plasma cell development: from B-cell subsets to long-term survival niches. Semin Immunol 20:49–58. doi: 10.1016/j.smim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. 2006. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol 6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 45.Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu Y, Wang G, Zou W. 2012. Bone marrow and the control of immunity. Cell Mol Immunol 9:11–19. doi: 10.1038/cmi.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Connor BP, Gleeson MW, Noelle RJ, Erickson LD. 2003. The rise and fall of long-lived humoral immunity: terminal differentiation of plasma cells in health and disease. Immunol Rev 194:61–76. doi: 10.1034/j.1600-065X.2003.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finke D. 2009. Induction of intestinal lymphoid tissue formation by intrinsic and extrinsic signals. Semin Immunopathol 31:151–169. doi: 10.1007/s00281-009-0163-6. [DOI] [PubMed] [Google Scholar]
- 48.Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, Muehlinghaus G, Szyska M, Radbruch A, Manz RA. 2003. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol 171:1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 49.Abe J, Ueha S, Yoneyama H, Shono Y, Kurachi M, Goto A, Fukayama M, Tomura M, Kakimi K, Matsushima K. 2012. B cells regulate antibody responses through the medullary remodeling of inflamed lymph nodes. Int Immunol 24:17–27. doi: 10.1093/intimm/dxr089. [DOI] [PubMed] [Google Scholar]
- 50.Elbim C, Monceaux V, Mueller YM, Lewis MG, Francois S, Diop O, Akarid K, Hurtrel B, Gougerot-Pocidalo MA, Levy Y, Katsikis PD, Estaquier J. 2008. Early divergence in neutrophil apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J Immunol 181:8613–8623. doi: 10.4049/jimmunol.181.12.8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vella A, Sartoris S, Bambara L, Ortolani R, Carletto A, Biasi D, Stefani E, Tridente G. 2002. Cell contact-dependent PMN HLA-DR and CD69 membrane expression induced by autologous mono-lymphocytes and cell lines. Inflammation 26:143–152. doi: 10.1023/A:1016514927365. [DOI] [PubMed] [Google Scholar]
- 52.De Vos J, Hose D, Reme T, Tarte K, Moreaux J, Mahtouk K, Jourdan M, Goldschmidt H, Rossi JF, Cremer FW, Klein B. 2006. Microarray-based understanding of normal and malignant plasma cells. Immunol Rev 210:86–104. doi: 10.1111/j.0105-2896.2006.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tokoyoda K, Hauser AE, Nakayama T, Radbruch A. 2010. Organization of immunological memory by bone marrow stroma. Nat Rev Immunol 10:193–200. doi: 10.1038/nri2727. [DOI] [PubMed] [Google Scholar]
- 54.Morikawa K, Nonaka M. 2005. All-trans-retinoic acid accelerates the differentiation of human B lymphocytes maturing into plasma cells. Int Immunopharmacol 5:1830–1838. doi: 10.1016/j.intimp.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Pantazi E, Marks E, Stolarczyk E, Lycke N, Noelle RJ, Elgueta R. 2015. Cutting edge: retinoic acid signaling in B cells is essential for oral immunization and microflora composition. J Immunol 195:1368–1371. doi: 10.4049/jimmunol.1500989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez Gomez M, Talke Y, Goebel N, Hermann F, Reich B, Mack M. 2010. Basophils support the survival of plasma cells in mice. J Immunol 185:7180–7185. doi: 10.4049/jimmunol.1002319. [DOI] [PubMed] [Google Scholar]
- 57.Rozanski CH, Arens R, Carlson LM, Nair J, Boise LH, Chanan-Khan AA, Schoenberger SP, Lee KP. 2011. Sustained antibody responses depend on CD28 function in bone marrow-resident plasma cells. J Exp Med 208:1435–1446. doi: 10.1084/jem.20110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoon SO, Zhang X, Berner P, Choi YS. 2009. IL-21 and IL-10 have redundant roles but differential capacities at different stages of plasma cell generation from human germinal center B cells. J Leukoc Biol 86:1311–1318. doi: 10.1189/jlb.0409268. [DOI] [PubMed] [Google Scholar]
- 59.Termini CM, Cotter ML, Marjon KD, Buranda T, Lidke KA, Gillette JM. 2014. The membrane scaffold CD82 regulates cell adhesion by altering alpha4 integrin stability and molecular density. Mol Biol Cell 25:1560–1573. doi: 10.1091/mbc.E13-11-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaudhary LR, Avioli LV. 1994. Dexamethasone regulates IL-1 beta and TNF-alpha-induced interleukin-8 production in human bone marrow stromal and osteoblast-like cells. Calcif Tissue Int 55:16–20. doi: 10.1007/BF00310163. [DOI] [PubMed] [Google Scholar]
- 61.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. 2004. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med 199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, Golijanin J, Buckler-White A, Sadjadpour R, Wang K, Mankoff Z, Schmidt SD, Lifson JD, Mascola JR, Nussenzweig MC, Martin MA. 2016. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog 5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, Burton DR. 2010. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol 84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyake A, Akagi T, Enose Y, Ueno M, Kawamura M, Horiuchi R, Hiraishi K, Adachi M, Serizawa T, Narayan O, Akashi M, Baba M, Hayami M. 2004. Induction of HIV-specific antibody response and protection against vaginal SHIV transmission by intranasal immunization with inactivated SHIV-capturing nanospheres in macaques. J Med Virol 73:368–377. doi: 10.1002/jmv.20100. [DOI] [PubMed] [Google Scholar]
- 67.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol 75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quinnan GV Jr, Yu XF, Lewis MG, Zhang PF, Sutter G, Silvera P, Dong M, Choudhary A, Sarkis PT, Bouma P, Zhang Z, Montefiori DC, Vancott TC, Broder CC. 2005. Protection of rhesus monkeys against infection with minimally pathogenic simian-human immunodeficiency virus: correlations with neutralizing antibodies and cytotoxic T cells. J Virol 79:3358–3369. doi: 10.1128/JVI.79.6.3358-3369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moir S, Fauci AS. 2009. B cells in HIV infection and disease. Nat Rev Immunol 9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 71.Favre D, Lederer S, Kanwar B, Ma Z-M, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR, Palermo R, Pandrea I, Miller CJ, Katze MG, McCune JM. 2009. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog 5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brenchley JM, Douek DC. 2008. HIV infection and the gastrointestinal immune system. Mucosal Immunol 1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horiike M, Iwami S, Kodama M, Sato A, Watanabe Y, Yasui M, Ishida Y, Kobayashi T, Miura T, Igarashi T. 2012. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology 423:107–118. doi: 10.1016/j.virol.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 74.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, Morcock D, Swanson T, Legasse AW, Axthelm MK, Hesselgesser J, Geleziunas R, Hirsch VM, Edlefsen PT, Piatak M Jr, Estes JD, Lifson JD, Picker LJ. 2015. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 21:132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, Berek C, Hiepe F, Manz R, Radbruch A, Dorner T. 2005. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood 105:1614–1621. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- 76.Slocombe T, Brown S, Miles K, Gray M, Barr TA, Gray D. 2013. Plasma cell homeostasis: the effects of chronic antigen stimulation and inflammation. J Immunol 191:3128–3138. doi: 10.4049/jimmunol.1301163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, Pan L, Poholek A, Rao SS, Brenchley JM, Alam SM, Tomaras GD, Roederer M, Douek DC, Seder RA, Germain RN, Haddad EK, Koup RA. 2012. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest 122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, Davis I, Farber D, Hartjen P, Haag F, Alter G, Schulze zur Wiesch J, Streeck H. 2012. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest 122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G Jr, Jacobson JM, Brooks AD, Crotty S, Estes JD, Pantaleo G, Lederman MM, Haddad EK. 2013. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 19:494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nagase H, Agematsu K, Kitano K, Takamoto M, Okubo Y, Komiyama A, Sugane K. 2001. Mechanism of hypergammaglobulinemia by HIV infection: circulating memory B-cell reduction with plasmacytosis. Clin Immunol 100:250–259. doi: 10.1006/clim.2001.5054. [DOI] [PubMed] [Google Scholar]
- 81.Moir S, Malaspina A, Ogwaro KM, Donoghue ET, Hallahan CW, Ehler LA, Liu S, Adelsberger J, Lapointe R, Hwu P, Baseler M, Orenstein JM, Chun TW, Mican JA, Fauci AS. 2001. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci U S A 98:10362–10367. doi: 10.1073/pnas.181347898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Badr G, Lefevre EA, Mohany M. 2011. Thymoquinone inhibits the CXCL12-induced chemotaxis of multiple myeloma cells and increases their susceptibility to Fas-mediated apoptosis. PLoS One 6:e23741. doi: 10.1371/journal.pone.0023741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Azab AK, Runnels JM, Pitsillides C, Moreau A-S, Azab F, Leleu X, Jia X, Wright R, Ospina B, Carlson AL, Alt C, Burwick N, Roccaro AM, Ngo HT, Farag M, Melhem MR, Sacco A, Munshi NC, Hideshima T, Rollins BJ, Anderson KC, Kung AL, Lin CP, Ghobrial IM. 2009. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 113:4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K. 2008. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 85.Romano JW, Shurtliff RN, Dobratz E, Gibson A, Hickman K, Markham PD, Pal R. 2000. Quantitative evaluation of simian immunodeficiency virus infection using NASBA technology. J Virol Methods 86:61–70. doi: 10.1016/S0166-0934(99)00184-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.