ABSTRACT
In this study, we investigated the effect of Toll-like receptor 2 (TLR2) ligation on the permissiveness of activated CD4+ T cells to HIV-1 infection by focusing our experiments on the relative susceptibility of cell subsets based on their expression of CCR6. Purified primary human CD4+ T cells were first subjected to a CD3/CD28 costimulation before treatment with the TLR2 agonist Pam3CSK4. Finally, cells were inoculated with R5-tropic HIV-1 particles that permit us to study the effect of TLR2 triggering on virus production at both population and single-cell levels. We report here that HIV-1 replication is augmented in CD3/CD28-costimulated CCR6+ CD4+ T cells upon engagement of the cell surface TLR2. Additional studies indicate that a higher virus entry and polymerization of the cortical actin are seen in this cell subset following TLR2 stimulation. A TLR2-mediated increase in the level of phosphorylated NF-κB p65 subunit was also detected in CD3/CD28-costimulated CCR6+ CD4+ T cells. We propose that, upon antigenic presentation, an engagement of TLR2 acts specifically on CCR6+ CD4+ T cells by promoting virus entry in an intracellular milieu more favorable for productive HIV-1 infection.
IMPORTANCE Following primary infection, HIV-1 induces an immunological and structural disruption of the gut mucosa, leading to bacterial translocation and release of microbial components in the bloodstream. These pathogen-derived constituents include several agonists of Toll-like receptors that may affect gut-homing CD4+ T cells, such as those expressing the chemokine receptor CCR6, which are highly permissive to HIV-1 infection. We demonstrate that TLR2 ligation in CD3/CD28-costimulated CCR6+ CD4+ T cells leads to enhanced virus production. Our results highlight the potential impact of bacterial translocation on the overall permissiveness of CCR6+ CD4+ T cells to productive HIV-1 infection.
KEYWORDS: CD4+ T cells, NF-κB, human immunodeficiency virus
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) infection triggers innate and adaptive immune responses that are suspected to play a role in the progression of the disease toward AIDS. Following infection, HIV-1 stimulates innate immunity via recognition of pathogen-associated molecular patterns such as genomic single-stranded RNA by pathogen recognition receptors, including endosomal Toll-like receptor 7 (TLR7) and TLR8, on innate immune cells. Engagement of such endosomal TLRs promotes secretion of several antiviral and immunomodulatory cytokines, such as alpha interferon (IFN-α), IFN-γ, tumor necrosis factor (TNF), IFN-γ-inducible protein 10 (IP-10), interleukin-10 (IL-10), and IL-18, which are involved in shaping the immune response following acute HIV-1 infection (1–4).
Immunological and structural alterations in the gut-associated lymphoid tissues (GALT) are seen and likely caused by direct and indirect effects of HIV-1 replication and the ensuing immune response (5, 6). Increased intraepithelial calcium concentrations (6, 7) and reduced sodium glucose cotransport (6) were suggested as factors implicated in abnormal epithelial cell differentiation and organization. Ineffective glucose uptake mediated by the HIV-1 protein Tat (8) and microtubule disruption induced by the viral protein gp120 (7) are also thought to play a dominant role in the high level of enterocyte apoptosis observed in HIV-1-infected individuals (5, 9). When associated with villous atrophy (10), this intestinal barrier disorganization causes disruption of epithelial tight junctions, resulting in the entry of microbial products into the bloodstream (a phenomenon also known as microbial translocation) (5, 11–15).
Simultaneously, induction of innate immune responses in the wake of HIV-1 replication induces differentiation of naive CD4+ T cells into effector subsets (16), which are the preferential targets of HIV-1. Infection of these cells contributes to production of newly formed infectious viral particles until their eventual depletion by apoptosis. This distinctive aspect of untreated HIV-1 infection is especially robust inside the GALT, where a significant fraction of effector CCR6+ CD4+ T cells reside (17). This cell subset includes Th17-polarized cells (18–20), which have been shown to be highly permissive to productive HIV-1 infection (17, 21–26). Importantly, these cells migrate into Peyer's patches of the distal small intestine via the homing chemokine receptor CCR6 by the action of CCL20, the single chemokine ligand for CCR6 (27–29). High surface expression of the HIV-1 coreceptor CCR5 (21, 22, 30), increased NF-κB DNA-binding activity (31), downregulated expression of RNase A superfamily members (26), and a higher expression of Lck, ZAP-70, MAP3K4, and PTPN13 (31) are thought to render gut-protective CCR6+ CD4+ T cells more susceptible to HIV-1 infection and promote their apoptosis-mediated elimination.
A lower frequency of fully functional intestinal CD13+ myelomonocytic cells (23, 32–34) and a poor neutrophil recruitment, due to the massive depletion or the impaired recruitment of CCR6+ CD4+ T cells following virus infection, contribute to a drastic reduction of antimicrobial peptide production, leading to an ineffective control of translocated commensal bacteria and fungi in the lamina propria (6, 35, 36). Large quantities of microorganisms may thus survive at extraintestinal sites where microbial components such as peptidoglycan, lipoteichoic acid, lipopolysaccharide, flagellin, ribosomal RNA, and unmethylated CpG-containing DNA are usually absent (37). Ligation of nucleotide-binding oligomerization domains 1 and 2 (NOD1 and NOD2) (38, 39) as well as TLR2, TLR4, TLR5, TLR6, and TLR9 (40–50) by these microbial products induces potent proinflammatory responses that have been proposed to mediate chronic immune activation and disease progression in the context of HIV-1 infection (11–13, 51–53).
Based on 16S rRNA sequence data, several studies have revealed that the human gut microbiota is varied within and between individual hosts, and its composition could influence how it activates the immune system upon translocation (54, 55). Lozupone and colleagues proposed that, despite a marked variation across healthy adults in the same population, Firmicutes (composed of Gram-positive bacteria) and Bacteroidetes (composed of Gram-negative bacteria) phyla dominate the composition of the gut microbiota (56). This group and others have also suggested that HIV-1 infection alters the gut microbiota of North American individuals in which Prevotella, a bacterial genus of the Bacteroidetes phylum, is significantly enriched (52, 57–59). Dillon and coworkers, for their part, have proposed that translocation of Prevotella species to mucosal tissues is significantly increased in HIV-1-infected subjects compared to uninfected individuals (5, 52). Despite the heterogeneity in the composition of the gut microbiota, this high abundance of the Prevotella genus and its enhanced capacity to translocate to extraintestinal sites thus suggest the predominance of Gram-negative bacteria in GALT of HIV-1-infected persons.
The translocated Gram-negative bacteria activate the immune system via the recognition of their wall components by many host cellular receptors. Among them, TLR2, which recognizes a great diversity of Gram-negative and -positive bacterium-derived compounds (47, 60–64), is known to induce the acquisition of an effector-like phenotype in both naive and memory CD4+ T cells, leading to an increase of their susceptibility to HIV-1 infection. Our group previously reported that TLR2 ligation in quiescent CD4+ T cells renders such cells susceptible to HIV-1 infection (47).
Given that the TLR2-dependent signaling pathway enhances HIV-1 replication by various means (44, 47, 49), and since CCR6-expressing CD4+ T cells are now considered preferential targets for the virus (17, 21, 31), we hypothesized that TLR2 ligation can affect HIV-1 replication in CCR6+ CD4+ T cells. We found that TLR2 engagement increases HIV-1 production in CD3/CD28-costimulated CCR6+ CD4+ T cells by allowing greater virus entry through remodelling of the cortical actin and creating a more favorable environment for virus gene expression.
RESULTS
HIV-1 replication is increased in CD3/CD28-costimulated CD4+ T cells by a TLR2 agonist.
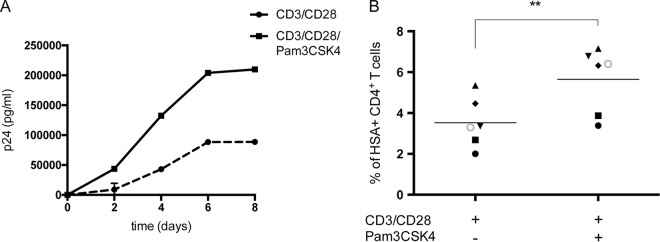
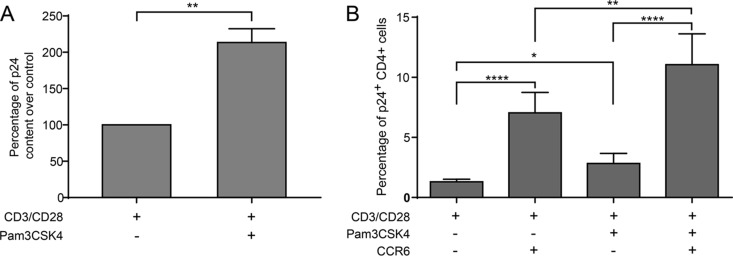
It has been shown previously that TLR2 engagement enhances susceptibility of quiescent CD4+ T cells to productive HIV-1 infection (47). To define further the contribution of TLR2-mediated signal transduction events in HIV-1 biology, kinetic infection studies were carried out in purified primary human CD4+ T cells that were first stimulated with anti-CD3 and anti-CD28 MAbs to partially mimic physiological antigen presentation before exposure to the TLR2 ligand Pam3CSK4. Our initial series of investigations was performed with fully infectious R5-tropic NL4.3 Balenv viruses, and HIV-1 replication was documented by measuring the p24 content in cell-free supernatants. Results shown in Fig. 1A indicate that TLR2 engagement augments virus production in CD3/CD28-costimulated CD4+ T cells. To monitor the effect of TLR2 triggering at the single-cell level, infection studies were also performed with a fully competent R5-using reporter virus called NL4.3 BAL-IRES-HSA, which contains all nine viral genes in addition to a gene coding for the murine reporter molecule HSA (65). Again, productive HIV-1 infection, as monitored by estimating the percentages of HSA-expressing cells, was increased in the presence of the TLR2 agonist Pam3CSK4 (Fig. 1B). Altogether, these results suggest that TLR2 ligation combined with CD3/CD28 costimulation increases HIV-1 replication in primary human CD4+ T cells.
FIG 1.
TLR2 triggering enhances HIV-1 replication in CD3/CD28-costimulated CD4+ T cells. Purified primary human CD4+ T cells were subjected to CD3/CD28 costimulation either in the absence or presence of the TLR2 agonist Pam3CSK4. (A) Cells were incubated with NL4.3 Balenv, and virus production was estimated at the indicated time points by measuring the p24 content in cell-free supernatants. (B) Cells were inoculated with NL4.3 Bal-IRES-HSA reporter virus, and the percentages of cells productively infected with HIV-1 (i.e., HSA+) were evaluated by flow cytometry. Data shown in panel A represent the means ± standard deviations (SD) of duplicates for a representative donor out of four. Each symbol shown in panel B represents a different donor, with the horizontal line depicting the means for all donors tested. Statistical analyses were made using ratio paired t test and one-way ANOVA, followed by a Dunnett's multiple-comparison test. Asterisks denote statistically significant data (**, P ≤ 0.01).
Cell proliferation and activation profiles are not affected by TLR2 ligation.
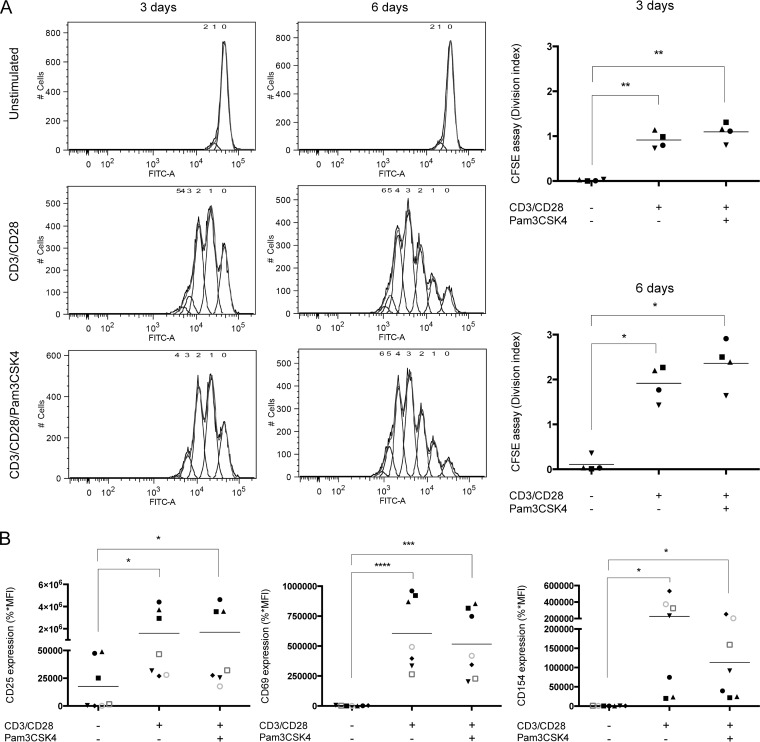
The process of CD4+ T cell activation is regulated by the relative expression and activity of some specific transcription factors, which are translocated into the nucleus to promote genes involved in the normal immune response (66) and sometimes also in HIV-1 gene expression (e.g., via NF-κB activation). Cell proliferation may also be implicated in viral dissemination by increasing the number of HIV-1-infected cells (67). Thus, cell proliferation was evaluated by use of a dilution assay that is based on the fluorescent cell staining dye carboxyfluorescein succinimidyl ester (CFSE). We also studied the cell activation status by measuring surface expression of some activation markers by flow cytometry (i.e., CD25, CD69, and CD154). As expected, cell proliferation was induced in a significant manner upon CD3/CD28 costimulation (Fig. 2A). However, proliferation of CD4+ T cells was not increased further by the presence of the TLR2 ligand. Similarly, surface expression of the activation-associated receptors CD25, CD69, and CD154 was significantly augmented by CD3/CD28 costimulation compared to untreated CD4+ T cells, but expression levels remained identical upon treatment with Pam3CSK4 (Fig. 2B). Hence, the effect of Pam3CSK4 combined with CD3/CD28 costimulation on the susceptibility of primary human CD4+ T cells to productive HIV-1 infection is not related to an effect on cell proliferation and/or activation.
FIG 2.
TLR2 ligation does not modulate proliferation and activation profiles in CD3/CD28-costimulated CD4+ T cells. Purified primary human CD4+ T cells were treated as indicated in the legend to Fig. 1. (A) A CFSE-based dilution assay was performed by flow cytometry to evaluate cell proliferation (as expressed by a division index) following 72 h and 6 days of stimulation. Representative proliferation profiles are depicted in the panels on the left, whereas division indices are shown in the panels on the right. (B) Surface expression of activation markers CD25, CD69, and CD154 was evaluated by flow cytometry following 72 h of stimulation (%*MFI, percentage of positive cells × mean fluorescence intensity). The data shown were obtained from CD4+ T cell preparations isolated from the peripheral blood of four (A) or seven (B) healthy participants. Each symbol represents a different donor, and the horizontal line depicts the means for all donors tested. Statistical analyses were made using ratio paired t test and one-way ANOVA, followed by a Dunnett's multiple-comparison test. Asterisks denote statistically significant data (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
TLR2 ligation increases susceptibility to HIV-1 infection of CD4+ T cells expressing the CCL20 receptor CCR6 without affecting cell distribution.
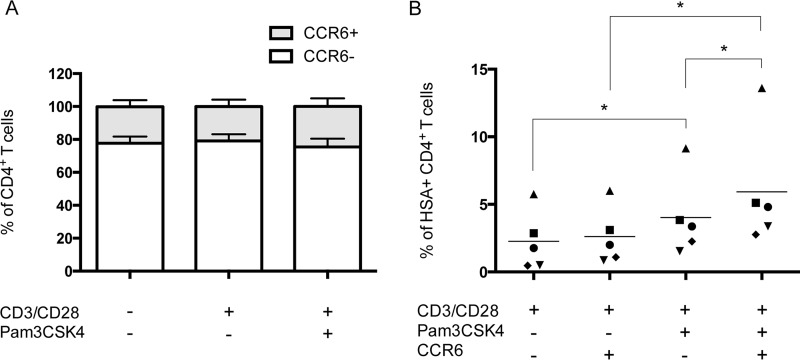
It has been shown that memory CD4+ T cells carrying the chemokine receptor CCR6 were more susceptible to virus infection due in part to their high expression of CCR5, which leads to a greater permissiveness to replication of R5-tropic HIV-1 variants (17, 21, 31). Consequently, the effect of the combination of TLR2 ligation and CD3/CD28 costimulation on the distribution and intrinsic susceptibility to productive virus infection of the HIV-1-targeted cell populations was analyzed, with a special emphasis being placed on the cell surface marker CCR6. These studies were rendered possible with the use of the HSA-encoding reporter virus. As shown in Fig. 3A, CD3/CD28 costimulation, combined or not with TLR2 ligation, did not affect cell distribution. Indeed, about 20% of total purified CD4+ T cells express the chemokine receptor CCR6, while the vast majority (i.e., 80%) of cells are CCR6 negative. Interestingly, infection experiments performed with fully competent reporter virus coding for the cell surface murine HSA molecule indicate that TLR2 ligation in CD3/CD28-costimulated cells enhances HIV-1 replication in a more significant manner in CCR6+ CD4+ T cells than in the CCR6− cell subset (Fig. 3B).
FIG 3.
TLR2 engagement increases susceptibility of the CD3/CD28-costimulated CCR6+ CD4+ T cell subset to productive HIV-1 infection. Purified primary human CD4+ T cells were treated as indicated in the legend to Fig. 1. (A) Surface expression of the CCR6 subset marker was analyzed by flow cytometry in the total CD4+ T cell population. (B) Cells were infected with NL4.3 Bal-IRES-HSA reporter virus, and percentages of HSA+ cells were estimated by flow cytometry in both CCR6− CD4+ and CCR6+ CD4+ T cell subsets. The data shown were obtained from CD4+ T cell preparations isolated from the peripheral blood of five healthy donors. Each symbol represents a different donor, and the horizontal line depicts the means for all donors tested. Statistical analyses were made using ratio paired t test and one-way ANOVA, followed by a Dunnett's multiple-comparison test. Asterisks denote statistically significant data (**, P ≤ 0.01).
TLR2 ligation in CD3/CD28-costimulated CD4+ T cells does not modulate CCR5 expression.
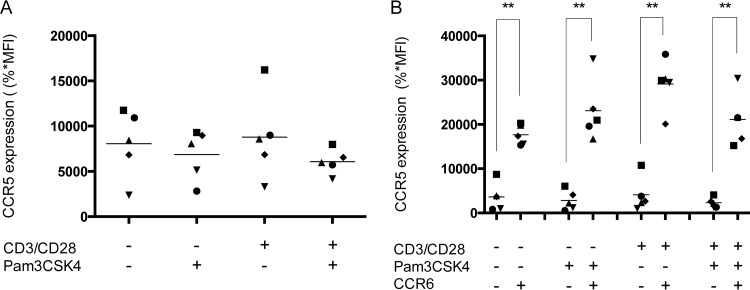
Based on previous studies demonstrating that the higher susceptibility of CCR6+ CD4+ T cells to HIV-1 infection is partly due to a greater expression of CCR5 (17, 21, 31), we analyzed the effect of TRL2 ligation associated with CD3/CD28 costimulation on the surface expression of this chemokine receptor. Data depicted in Fig. 4A demonstrate that TLR2 triggering, when combined with CD3/CD28 costimulation, does not affect CCR5 expression compared with CD3/CD28 costimulation alone in the total CD4+ T cell population. Moreover, our results also indicate that CCR6+ cells express basal levels of CCR5 that are significantly higher than levels seen in CCR6− cells, but these are not affected by the CD3/CD28 costimulation either in the absence or presence of the TLR2 ligand Pam3CSK4 (Fig. 4B). Surface expression of CD4 is also not modulated under all experimental conditions tested, including TLR2 ligation, as defined by flow cytometry analyses (data not shown).
FIG 4.
TLR2 engagement does not affect CCR5 expression in CD3/CD28-costimulated CCR6+ CD4+ T cells. Purified primary human CD4+ T cells were treated as indicated in the legend to Fig. 1. (A) Surface expression of CCR5 in the total CD4+ T cell population following activation was evaluated by flow cytometry. (B) Expression of CCR5 was also assessed in both CCR6− CD4+ and CCR6+ CD4+ T cell subpopulations. The data shown were obtained from CD4+ T cell preparations isolated from the peripheral blood of five healthy donors. Each symbol represents a different donor, and the horizontal line depicts the means for all donors tested. Statistical analyses were made using ratio paired t test and one-way ANOVA, followed by a Dunnett's multiple-comparison test. Asterisks denote statistically significant data (**, P ≤ 0.01).
Virus entry and polymerization of the cortical actin are both increased upon TLR2 engagement in CD3/CD28-costimulated CCR6+ CD4+ T cells.
Although TLR2 triggering does not affect surface expression levels of CCR5 and CD4, we quantified the extent of virus entry because it has been shown recently that Pam3CSK4 induces rearrangement of the F-actin cytoskeletal architecture (68). We performed an entry test using NL4.3 Balenv viruses and quantified the intracellular p24 content by enzyme-linked immunosorbent assay (ELISA) in CD3/CD28-costimulated CD4+ T cells that were either left untreated or treated with Pam3CSK4. We found that HIV-1 entry was increased in Pam3CSK4-treated CD3/CD28-costimulated CD4+ T cells compared to cells that were not treated with the TLR2 agonist (Fig. 5A). To determine the extent of virus entry in both CCR6+ and CCR6− CD4+ T cells, we performed a costaining of cell surface CCR6 together with the intracellular p24. HIV-1 entry was more significant in Pam3CSK4-treated CD3/CD28-costimulated CCR6+ CD4+ T cells compared to the similarly treated CCR6− CD4+ T cell subset (Fig. 5B).
FIG 5.
TLR2 triggering augments virus entry in CD3/CD28-costimulated CCR6+ CD4+ T cells. Purified primary human CD4+ T cells were treated as indicated in the legend to Fig. 1, and virus entry was estimated either by quantifying intracellular p24 contents by ELISA in the total cell population (A) or monitoring the percentage of p24-expressing cells by flow cytometry in both CCR6− CD4+ and CCR6+ CD4+ T cell subsets (B). Results depicted in panel A were obtained from cell preparations of seven healthy donors and show the percentage of increase of cell-associated p24 over the control ± standard errors of the means (SEM) (i.e., cells subjected to CD3/CD28 costimulation only) for each donor. Results depicted in panel B were obtained from cell preparations of 11 healthy donors and show the percentage of p24-positive cells ± SEM in CCR6− and CCR6+ CD4+ T cell populations stimulated with Pam3CSK4 or left unstimulated. Statistical analyses were made using ratio paired t test between selected pairs of data. Asterisks denote statistically significant data (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
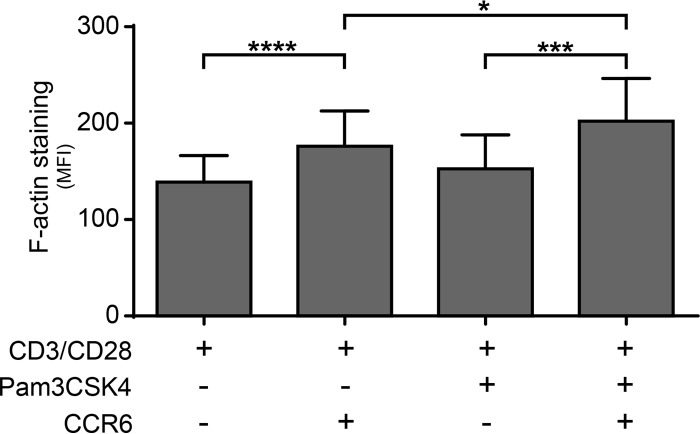
To shed light on the putative mechanism of action by which TLR2 triggering can enhance HIV-1 entry, we monitored the degree of actin polymerization by intracellular F-actin staining with phalloidin. Our data indicate that a more significant polymerization of the cortical actin is seen in Pam3CSK4-treated, CD3/CD28-costimulated CCR6+ CD4+ T cells compared to similarly treated CCR6− CD4+ T cells (Fig. 6).
FIG 6.
Pam3Csk4 enhances cortical actin polymerization in CD3/CD28-costimulated CCR6+ CD4+ T cells. Purified primary human CD4+ T cells were treated as indicated in the legend to Fig. 1, and F-actin staining was monitored by intracellular flow cytometry. The data shown were obtained from CD4+ T cell preparations isolated from the peripheral blood of five healthy donors. Results show mean fluorescence intensity of F-actin staining ± SEM in CCR6− and CCR6+ CD4+ T cell populations stimulated with Pam3CSK4 or left unstimulated. Statistical analyses were made using ratio paired t test between selected pairs of data. Asterisks denote statistically significant data (*, P < 0.05; ****, P < 0.0001).
Primary human CCR6+ CD4+ T cells express higher levels of cell surface TLR2 than the CCR6− subpopulation.
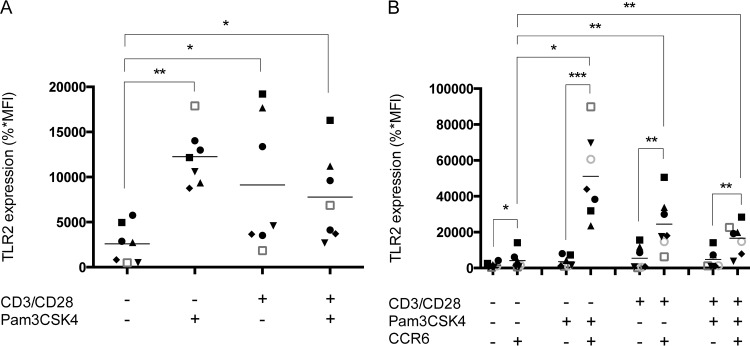
Given that a higher expression of cell surface TLR2 in CCR6+ CD4+ T cells could play a functional role in the observed phenomenon, we performed flow cytometry analysis to evaluate TLR2 levels on CD3/CD28-costimulated CD4+ T cells either in the absence or presence of Pam3CSK4. As illustrated in Fig. 7A, CD3/CD28 costimulation, whether or not it is combined with TLR2 ligation, induces a comparable induction of TLR2 expression. Although the enhanced TLR2 expression is limited to the CCR6+ CD4+ T cell subset, it is not modulated further by the TLR2 agonist in CD3/CD28-costimulated CD4+ T cells (Fig. 7B). These results suggest that TLR2 ligation shows no additional effect on CD3/CD28 costimulation on the induction of TLR2 expression in CD4+ T cells. However, these data also demonstrate that quiescent CCR6+ CD4+ T cells express higher levels of TLR2, which could lead to an enhanced sensitivity to Pam3CSK4, stronger NF-κB signaling, and higher virus gene expression.
FIG 7.
TLR2 agonist does not impact expression of its cognate receptor in CD3/CD28-costimulated CCR6+ CD4+ T cells. Purified primary human CD4+ T cells were treated as indicated in the legend to Fig. 1. (A) Surface expression of TLR2 was then analyzed by flow cytometry in the total CD4+ T cell population. (B) Expression of TLR2 was also monitored in both CCR6− CD4+ and CCR6+ CD4+ T cell subsets. The data shown were obtained from CD4+ T cell preparations isolated from the peripheral blood of seven healthy donors. Each symbol represents a different donor, and the horizontal line depicts the means for all donors tested. Statistical analyses were made using ratio paired t test and one-way ANOVA, followed by a Dunnett's multiple-comparison test. Asterisks denote statistically significant data (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).
Increased amounts of phosphorylated p65 are found in CD3/CD28-costimulated CCR6+ CD4+ T cells subjected to TLR2 ligation.
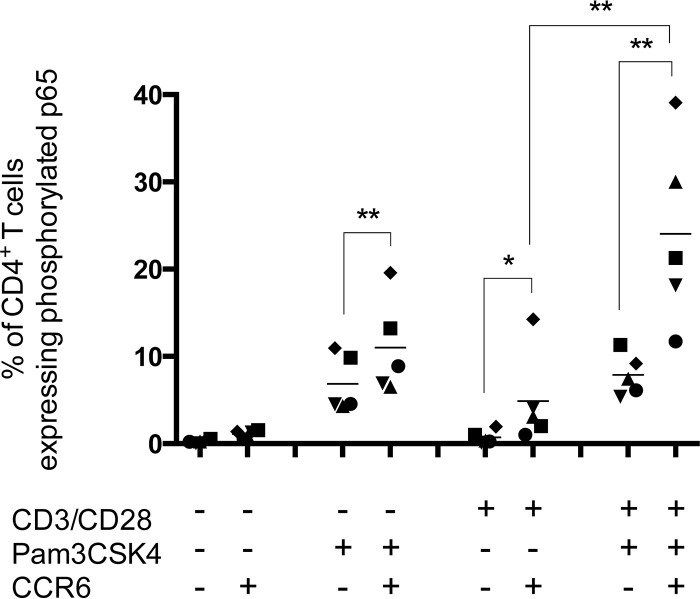
Pam3CSK4 is recognized as a potent activator of the proinflammatory transcription factor NF-κB (69). Interestingly, our group has previously suggested that TLR2 ligation increases the pool of cells permissive to HIV-1 infection by promoting differentiation of quiescent naive and memory CD4+ T cells into effector cells via NF-κB activation (47). Furthermore, Cleret-Buhot and colleagues recently showed that NF-κB DNA-binding activity is significantly higher in CCR6+ T cells following CD3/CD28 costimulation (31). Based on these published observations, we assessed by flow cytometry the overall effect of CD3/CD28 costimulation in combination with TLR2 ligation on the expression of phosphorylated p65, which is indicative of transcriptionally active NF-κB (70). Results presented in Fig. 8 show that TLR2 engagement is sufficient per se to induce phosphorylation of p65 in CCR6− CD4+ T cells, in contrast to CD3/CD28 costimulation alone, which has no effect. In contrast, CD3/CD28 costimulation and TLR2 ligation, either used alone or in combination, can significantly induce phosphorylation of p65 in the CCR6+ CD4+ T cell population. The highest levels of phosphorylated p65 were seen in CD3/CD28-costimulated CCR6+ CD4+ T cells that were also exposed to the TLR2 ligand Pam3CSK4.
FIG 8.
TLR2 agonist augments intracellular levels of phosphorylated p65 in CD3/CD28-costimulated CCR6+ CD4+ T cells. Purified primary human CD4+ T cells were subjected to a CD3/CD28 costimulation in the absence or presence of Pam3CSK4. Intracellular expression of phosphorylated p65 was then analyzed by flow cytometry in both CCR6− CD4+ and CCR6+ CD4+ T cell subpopulations. The data shown were obtained from CD4+ T cell preparations isolated from the peripheral blood of five healthy donors. Each symbol represents a different donor, and the horizontal line depicts the means for all donors tested. Statistical analyses were made using ratio paired t test and one-way ANOVA, followed by a Dunnett's multiple-comparison test. Asterisks denote statistically significant data (*, P ≤ 0.05; **, P ≤ 0.01).
DISCUSSION
In the past few years, several groups have scrutinized the modulatory effects of HIV-1 infection on translocation of gut-derived microbes and the possible impacts of such bacterial components found at extraintestinal sites on susceptibility to virus infection of circulating CD4+ T cells (6). It has been reported that permissiveness to HIV-1 infection of CD4+ T cells found at mucosal sites is modulated by their activation state, which is itself influenced by the intrinsic nature of gut-derived microbial elements. For example, data from in vitro studies indicate that TLR ligation of quiescent CD4+ T cells enhances their permissiveness to R5-tropic virions by increasing CCR5 expression and NF-κB activity (47) and by promoting HIV-1 nuclear import (44). In addition, it has been demonstrated that CD4+ T cells expressing CCR6, which have the potential to migrate into the GALT (17), are highly susceptible to HIV-1 infection due to their high expression of CCR5 (21) and increased ability to respond to a T-cell receptor agonist (21, 30, 31). No study has, however, established a correlation between the enhanced depletion of CD4+ T cells in the GALT during virus infection and the impact of bacterial components, such as TLR2 ligand, on the susceptibility of CCR6− CD4+ and CCR6+ CD4+ T cell subsets to HIV-1 infection. Considering the important role of microbial translocation on susceptibility of CD4+ T cells to HIV-1 infection, notably in the GALT, we hypothesized that a TLR2 agonist such as Pam3CSK4 can modulate receptiveness of CD4+ T cells to HIV-1 infection by acting specifically on the cell subset bearing CCR6.
Productive HIV-1 infection is occurring primarily in activated CD4+ T cells (71). Indeed, while cell activation is not mandatory to allow virus entry, it is nonetheless required for efficient reverse transcription and integration of proviral DNA into the host's genome (72). Moreover, activated CD4+ T cells express surface molecules that distinguish them from resting cells. Among these activation markers, some are receptor proteins, costimulatory molecules, adhesion molecules, and chemokine receptors (73). In our study, we investigated the effect of the TLR2 engagement on cell activation by measuring levels of CD25 (receptor protein), CD69 (receptor protein), and CD154 (costimulatory molecule) surface markers in CD3/CD28-costimulated cells. We demonstrate that the combination of CD3/CD28 costimulation and TLR2 ligation does not influence the overall activation state of CD4+ T cells compared to CD3/CD28 costimulation alone. Thus, as Pam3CSK4 did not seem to affect expression of the studied activation markers, we conclude that the TLR2 ligand-mediated increase in permissiveness of CD4+ T cells to productive HIV-1 infection is not due to a higher cell activation status. Following HIV-1 infection, inflammatory processes and the antiviral immune response promote T cell proliferation that is a driving force in viral dissemination by increasing the pool of HIV-1-infected cells (67). Therefore, we evaluated the effect of TLR2 engagement on cell division in CD3/CD28-costimulated CD4+ T cells. We found that the TLR2 ligand does not affect proliferation of such cells at either 3 or 6 days posttreatment.
Considering that CCR6+ CD4+ T cells are highly permissive to HIV-1 infection and that they have the potential to migrate to the GALT (17, 21, 30, 31), we thought it could be important to study the impact of TLR2 ligation on their distribution in the total CD4+ T cell population and cell susceptibility to virus infection. Cell distribution was analyzed by flow cytometry, and no significant differences were observed in the percentages of CD4+ T cells expressing CCR6 regardless of the stimuli tested (i.e., CD3/CD28 costimulation and/or TLR2 triggering). Of importance, we found that Pam3CSK4 specifically enhances susceptibility to HIV-1 infection of the CD3/CD28-costimulated CCR6+ CD4+ T cell subset. Indeed, while CD3/CD28 costimulation increases permissiveness of CCR6-expressing cells to HIV-1 infection, TLR2 ligation further augments this susceptibility, specifically in the CCR6+ cell population. Overall, these findings imply that the positive effect of TLR2 ligation with regard to HIV-1 production in the total population of CD3/CD28-costimulated CD4+ T cells is due to a specific effect of Pam3CSK4 in the CCR6+ CD4+ T cell population.
We performed additional experiments to shed light on the mechanisms underlying the increased susceptibility to HIV-1 infection of CD3/CD28-costimulated CCR6+ CD4+ T cells induced by the TLR2 agonist. As shown previously by Monteiro and coworkers, expression of the virus coreceptor CCR5, which is more important at the surface of CCR6+ CD4+ T cells, could play a major role in their permissiveness to virus infection (17). Thus, we assessed the effect of TLR2 ligation on CCR5 surface expression in CD3/CD28-costimulated CCR6+ CD4+ T cells. Levels of CCR5 were confirmed to be higher at the surface of the CCR6+ cell subpopulation than that of CCR6− cells regardless of stimulation. This observation is in agreement with a previous study showing a greater expression of CCR5 in the Th17 cell subset, which expresses CCR6 at its surface (31). However, none of the tested stimuli significantly increases CCR5 expression in CCR6+ CD4+ T cells. Thus, the higher susceptibility to productive HIV-1 infection of Pam3CSK4-treated, CD3/CD28-costimulated CCR6+ CD4+ T cells is not explained by a greater surface expression of CCR5. Similar observations were made when assessing surface expression of CD4.
It has been established that actin polymerization enhances the probability of HIV-1 Envelope-CD4/coreceptor interactions, thus potentiating fusion pore formation and, therefore, viral fusion and infection (74–76). Based on this information, we examined the possible modulatory effect of TLR2 triggering on HIV-1 entry. Our studies indicate that TLR2 ligation increases HIV-1 entry in CD3/CD28-costimulated CCR6+ CD4+ T cells. In addition, we report that TLR2 engagement induces a cytoskeletal rearrangement by augmenting the degree of actin polymerization. Our findings are in agreement with a previous work, which has revealed that Pam3CSK4 alters cytoskeletal dynamics (68). However, it should be noted that this work was carried out in human fibroblasts and not in CD4+ T cells, as shown here. Interestingly, some TLR ligands were reported to activate the actin-severing protein cofilin, a process leading to enhanced cytoskeletal dynamics in human immune cells (77).
We also investigated the effect of Pam3CSK4 exposure on expression of its main receptor, TLR2 (also designated CD282), at the surface of CD3/CD28-costimulated CCR6+ CD4+ T cells. Considering that TLR2 ligation triggers a signal leading to enhanced HIV-1 production in resting CD4+ T cells (47), we thought that a positive feedback loop between the ligand and its receptor could promote viral production specifically in cells expressing higher levels of TLR2. An increase in TLR2 expression was seen in CD4+ T cells following CD3/CD28 costimulation in the total population, which is in line with a previous study performed in a murine model (78). This higher TLR2 expression on CD3/CD28-costimulated CD4+ T cells is, however, not enhanced when the cells are also treated with Pam3CSK4. Multiparametric analyses discriminating for CCR6 expression demonstrate that CD3/CD28 costimulation significantly augments TLR2 expression at the surface of the CCR6+ cell subset compared to CCR6− cells, and TLR2 ligation does not increase this expression further. However, a trend toward higher expression of TLR2 could be observed in the unstimulated CCR6+ CD4+ T cell subpopulation compared to the CCR6− subset. It is possible that a difference in TLR2 expression levels between CCR6+ and CCR6− cells could explain the specific effect observed. While combination of the TLR2 agonist with CD3/CD28 costimulation did not increase TLR2 expression further by any subset, it is possible that CCR6-expressing CD4+ T cells are initially more sensitive to TLR2 ligation. Additional studies are needed to solve this issue.
One of the demonstrated functional outcomes of TLR2-mediated signaling pathways is the activation of the ubiquitous mammalian transcription factor NF-κB. Following its activation in the cytoplasm, this transcription factor translocates to the nucleus, where it induces several genes implicated in cellular responses to many stimuli (79, 80). As the promoter-proximal (enhancer) region of the HIV-1 long terminal repeat carries two adjacent NF-κB binding sites and since mutation of these regions greatly affects viral fitness, this transcription factor is considered a key element in the virus replicative cycle (81). It should be noted that it has been previously established that TLR2 ligation renders quiescent naive and memory CD4+ T cells more susceptible to productive infection by HIV-1 in part due to NF-κB activation (47). While this work was performed in cells in a resting state, we now show that, upon CD3/CD28 costimulation, TRL2 ligation in CD4+ T cells increases NF-κB activity specifically among the CCR6+ cell population. In accordance with a recent study, we also observe that CD3/CD28 costimulation alone significantly augments NF-κB activity in CCR6+ cells compared to the CCR6− cell subset. However, after addition of Pam3CSK4, the percentage of CCR6+ cells expressing phosphorylated p65 becomes significantly higher than when cells were stimulated through CD3/CD28 only. Altogether our data indicate that TLR2 ligation in CD3/CD28-costimulated CCR6+ CD4+ T cells creates an intracellular environment more favorable to achieving productive HIV-1 infection.
Our observations lead us to propose that peripheral blood CCR6+ CD4+ T cells, upon their arrival in the GALT due to their homing receptors (17), would be more sensitive to TRL2 ligation following an interaction with translocated bacteria and their derived metabolites. This phenomenon would contribute to their highly permissive state for HIV-1 infection (17, 21, 31, 82). Based on the current study and previous findings (45, 47, 83), we propose that microbial translocation seen in HIV-1-infected individuals enhances susceptibility of CCR6+ CD4+ T cells to virus infection, leading to a more important inflammatory status and a higher rate of disease progression. As each microorganism triggers highly adapted immune responses, a better understanding of the composition of the microbiota among HIV-1-infected persons is needed to accurately identify bacterial populations susceptible to translocate to extraintestinal sites and promote chronic inflammation.
MATERIALS AND METHODS
Ethics statement and cell culture.
The current study was approved by the Bioethics Committee from the Centre Hospitalier Universitaire de Québec-Université Laval. Peripheral blood samples were collected from healthy donors in accordance with the guidelines of the Institutional Bioethics Committee. To obtain purified primary human CD4+ T cells, peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque gradient centrifugation from healthy donors and kept frozen at −80°C at a final concentration of 1 × 108 cells/ml in freezing medium (90% fetal bovine serum [FBS] and 10% dimethyl sulfoxide [DMSO]) for a maximum of 3 months. Thereafter, purified CD4+ T cells were isolated from thawed PBMCs by immunomagnetic negative selection as described in the protocol provided by the manufacturer (STEMCELL Technologies, Vancouver, BC) and cultured in Roswell Park Memorial Institute (RPMI) 1640 culture medium (Fisher Scientific, Ottawa, ON) supplemented with 10% FBS at a concentration of 2 × 106 cells/ml.
Human embryonic kidney 293T cells were kindly provided by Warner C. Greene (The J. Gladstone Institutes, San Francisco, CA), and TZM-bl indicator cells were obtained from John C. Kappes, Xiaoyun Wu, and Tranzyme, Inc., via the AIDS Research Reagent Program (Germantown, MD) (84–88). These cell lines were cultured in Dulbecco's modified Eagle medium (DMEM) (Fisher Scientific, Ottawa, ON) supplemented with 10% FBS (Fisher Scientific, Ottawa, ON).
Antibodies, reagents, and plasmids.
The TLR2 agonist Pam3CSK4 was purchased from InvivoGen (San Diego, CA). Allophycocyanin (APC)-Cy7-conjugated anti-human CD196 (CCR6) was obtained from BioLegend (San Diego, CA), whereas APC-conjugated anti-mouse CD24 (HSA), APC-conjugated anti-human CD282 (TLR2), and eFluor660-conjugated anti-human phospho-NF-κB p65 were purchased from eBioscience (San Diego, CA). Fluorescein isothiocyanate (FITC)-conjugated anti-human CD4, APC-Cy7-conjugated anti-human CD195 (CCR5), phycoerythrin (PE)-Cy7-conjugated anti-human CD196 (CCR6), PE-conjugated anti-human CD25, FITC-conjugated anti-human CD69, and FITC-conjugated anti-human CD154 were all obtained from BD Biosciences (Mississauga, ON). The CellTrace carboxyfluorescein succinimidyl ester (CFSE) cell proliferation kit was purchased from Thermo Fisher Scientific (Waltham, MA). The FITC-conjugated KC57 monoclonal antibody (MAb), which is specific for the HIV-1 major viral core pin p24gag, was purchased from Beckman Coulter (Burlington, ON). Hybridomas producing 183-H12-5C and 31-90-25 antibodies, which recognize different epitopes of the HIV-1 major viral core protein p24gag, were supplied by the AIDS Research Reagent Program and the ATCC (Manassas, VA), respectively. The infectious molecular constructs pNL4.3Balenv and pNL4.3 BAL-IRES-HSA were previously described (65, 89).
Production of virus stocks.
The NL4.3Balenv vector codes for R5-tropic Bal Env glycoproteins inserted within the NL4.3 backbone and generates infectious HIV-1 particles (89). The NL4.3 BAL-IRES-HSA plasmid produces R5-tropic fully competent reporter virions coding for the murine HSA protein, which is expressed on the membrane of productively infected cells via a glycophosphatidylinositol anchor (65). Virus preparations were made by CaPO4 transfection of the infectious molecular clones pNL4.3 Balenv and pNL4.3 BAL-IRES-HSA. Briefly, 293T cells were transfected for 48 h with the expression vectors, and newly produced viral particles were ultracentrifuged at 100,000 × g for 45 min. The pellet was suspended in 2 ml of endotoxin-free phosphate-buffered saline (PBS; Fisher Scientific, Ottawa, ON) and frozen at −80°C until needed. Quantification of infectious titers harvested after ultracentrifugation was performed according to the Spearman-Karber method using TZM-bl indicator cells.
Stimulation and virus infection assays.
Purified primary human CD4+ T cells (1 × 106 cells/experimental condition) were cultured in RPMI 1640 culture medium supplemented with 10% FBS at a concentration of 2 × 106 cells/ml, seeded in 48-well flat-bottom tissue culture plates, and either left untreated or treated with anti-CD3 (clone OKT3; 5 μg/ml) and anti-CD28 (clone 9.3; 2 μg/ml) MAbs in the presence or absence of the TLR2 agonist Pam3CSK4 (10 μg/ml). After 72 h of incubation at 37°C under a 5% CO2 atmosphere, cells were either left uninfected to analyze surface expression of CCR5, CCR6, CD4, CD25, CD69, CD154, and TLR2 by flow cytometry or inoculated with HIV-1 to study the effect of anti-CD3/CD28 costimulation in the absence or presence of Pam3CSK4 stimuli on their susceptibility to virus infection. Virus infection assays were performed using NL4.3 Balenv or NL4.3 BAL-IRES-HSA virus stocks that were added to cells at a multiplicity of infection (MOI) of 0.1 for 2 h, after which cells were washed three times with PBS–2 mM EDTA–0.5% bovine serum albumin (BSA), resuspended in RPMI 1640 medium supplemented with 10% FBS, and incubated at 37°C under a 5% CO2 atmosphere. Virus replication was estimated by measuring the p24 content with an in-house enzymatic assay specific for the major viral p24gag protein (90) or quantifying the percentages of HSA+ cells by flow cytometry.
Flow cytometry studies.
Surface expression of CD4, CCR5, CCR6, CD25, CD69, CD154, TLR2, and virus-encoded HSA on the total population of purified CD4+ T cells and/or CCR6+ CD4+ and CCR6− CD4+ T cell subsets was analyzed by flow cytometry. In brief, cells were suspended in PBS–2 mM EDTA–0.5% BSA (2 × 106 cells/ml) and stained with appropriate MAbs at optimal concentrations for 15 min at room temperature under dark conditions. Cells were washed twice with PBS–2 mM EDTA–0.5% BSA and resuspended in 2% paraformaldehyde prior to flow cytometry analysis (BD FACSCanto; BD Biosciences, Mississauga, ON). The intracellular expression of phospho-NF-κB p65 in resting and activated CCR6+ CD4+ and CCR6− CD4+ T cell subsets has also been analyzed by flow cytometry. Briefly, cells (1 × 106 cells/experimental condition; 2 × 106 cells/ml) were stimulated with anti-CD3 (clone OKT3; 5 μg/ml) and anti-CD28 (clone 9.3; 2 μg/ml) MAbs in the absence or presence of prewarmed (40°C) TLR2 agonist Pam3CSK4 (10 μg/ml) for 30 min at 37°C under a 5% CO2 atmosphere. The TLR2 ligand was subjected to a heat treatment to reduce its natural ability to adopt a self-assembled nanostructure (91). Cells next were fixed in 1 ml of 2% paraformaldehyde for 20 min at 4°C before being stained with PE-Cy7-conjugated anti-human CCR6 in 200 μl of PBS–2 mM EDTA–0.5% BSA for 15 min at room temperature under dark conditions. Cells were washed twice with PBS–2 mM EDTA–0.5% BSA and stained in 50 μl of 1× permeabilization solution (BD Perm/Wash; BD Biosciences, Mississauga, ON) supplemented with eFluor660-conjugated anti-human phospho-NF-κB p65 for 30 min at 4°C under dark conditions. Cells were washed twice with permeabilization solution and resuspended in 250 μl of PBS–2 mM EDTA–0.5% BSA prior to flow cytometry analysis. Cellular proliferation assays were performed with the CellTrace CFSE cell proliferation kit (ThermoFisher Scientific, Waltham, MA). In brief, cells were treated with the fluorescent cell staining dye CFSE and stimulated with anti-CD3 (clone OKT3; 5 μg/ml) and anti-CD28 (clone 9.3; 2 μg/ml) MAbs in the absence or presence of Pam3CSK4 (10 μg/ml) for 72 h. Fluorescence emission was measured by cytometry analysis (BD FACSCanto; BD Biosciences, Mississauga, ON) to determine the effect of stimulations on cellular proliferation.
The degree of actin polymerization in both CCR6+ CD4+ and CCR6− CD4+ T cell subsets was assessed by intracellular F-actin staining. Briefly, cells were fixed with 150 μl of 2% paraformaldehyde for 30 min at 4°C in the dark, washed once with PBS–2 mM EDTA–0.5% BSA, and permeabilized with 100 μl of permeabilization buffer (eBioscience) for 15 min at room temperature. Cells were stained with Alexa Fluor 647-conjugated phalloidin (Sigma-Aldrich, Oakville, ON) for 30 min at 4°C in the dark. Cells were finally analyzed by flow cytometry.
Virus entry tests.
Cells were either left untreated or were treated as described above. Cells next were inoculated with NL4-3 Balenv (50 ng of p24 per 1 × 105 cells) for 2 h at 37°C. For assessing virus entry at the whole-CD4+-T-cell population level, cells were washed twice with an equal volume of PBS (pH 7.4), resuspended in 100 μl trypsin 0.05%–EDTA (Thermo Fisher Scientific), and incubated for 10 min at 37°C to remove unadsorbed/uninternalized virions. Finally, cells were washed three times with 1 ml of PBS (pH 7.4) and lysed using 500 μl of a disruption buffer (20 mmol/liter HEPES [pH 7.4], 150 mmol/liter sodium chloride, and 0.5% Triton X-100). Samples were kept at −80°C until assayed by an in-house p24 enzymatic test (90). For detection of intracellular p24 in both CCR6− and CCR6+ cell subpopulations, CCR6 was first stained as described above, and then cells were washed once with PBS–2 mM EDTA–0.5% BSA and permeabilized with 100 μl of permeabilization buffer (eBioscience) for 15 min at room temperature. Cells were then stained with KC57-FITC-conjugated antibody (Beckman Coulter) diluted 1:300 in permeabilization buffer for 30 min at 4°C under dark conditions. Cells were then washed twice with permeabilization buffer and resuspended in PBS–2 mM EDTA before analysis by flow cytometry.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism version 6. Ratio paired t test and one-way analysis of variance (ANOVA) with corrections for multiple comparisons (Dunnett's multiple-comparison test) were performed to define the statistical significance of our data. A threshold P value of ≤0.05 (*) was considered statistically significant, whereas P values of ≤0.01 (**), ≤0.001 (***), and ≤0.0001 (****) were considered highly significant.
ACKNOWLEDGMENTS
We acknowledge the Bioimaging Platform of the Infectious Disease Research Centre, funded by an equipment and infrastructure grant from the Canadian Foundation for Innovation.
This study was supported by funds allocated to M.J.T. from the Open Operating Grant Program of the Canadian Institutes of Health Research (CIHR) (HOP-143170). M.J.T. is the recipient of the Tier 1 CIHR-Canada Research Chair in Human Immunoretrovirology.
We have no competing financial interests to declare.
REFERENCES
- 1.Chang JJ, Altfeld M. 2010. Innate immune activation in primary HIV-1 infection. J Infect Dis 202(Suppl 2):S297–S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, Self SG, Borrow P. 2009. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diop OM, Ploquin MJ, Mortara L, Faye A, Jacquelin B, Kunkel D, Lebon P, Butor C, Hosmalin A, Barre-Sinoussi F, Muller-Trutwin MC. 2008. Plasmacytoid dendritic cell dynamics and alpha interferon production during simian immunodeficiency virus infection with a nonpathogenic outcome. J Virol 82:5145–5152. doi: 10.1128/JVI.02433-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malleret B, Maneglier B, Karlsson I, Lebon P, Nascimbeni M, Perie L, Brochard P, Delache B, Calvo J, Andrieu T, Spreux-Varoquaux O, Hosmalin A, Le Grand R, Vaslin B. 2008. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood 112:4598–4608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- 5.Dillon SM, Lee EJ, Donovan AM, Guo K, Harper MS, Frank DN, McCarter MD, Santiago ML, Wilson CC. 2016. Enhancement of HIV-1 infection and intestinal CD4+ T cell depletion ex vivo by gut microbes altered during chronic HIV-1 infection. Retrovirology 13:5. doi: 10.1186/s12977-016-0237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandler NG, Douek DC. 2012. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol 10:655–666. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 7.Maresca M, Mahfoud R, Garmy N, Kotler DP, Fantini J, Clayton F. 2003. The virotoxin model of HIV-1 enteropathy: involvement of GPR15/Bob and galactosylceramide in the cytopathic effects induced by HIV-1 gp120 in the HT-29-D4 intestinal cell line. J Biomed Sci 10:156–166. [DOI] [PubMed] [Google Scholar]
- 8.Di Sabatino A, Ciccocioppo R, D'Alo S, Parroni R, Millimaggi D, Cifone MG, Corazza GR. 2001. Intraepithelial and lamina propria lymphocytes show distinct patterns of apoptosis whereas both populations are active in Fas based cytotoxicity in coeliac disease. Gut 49:380–386. doi: 10.1136/gut.49.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steele AK, Lee EJ, Manuzak JA, Dillon SM, Beckham JD, McCarter MD, Santiago ML, Wilson CC. 2014. Microbial exposure alters HIV-1-induced mucosal CD4+ T cell death pathways ex vivo. Retrovirology 11:14. doi: 10.1186/1742-4690-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapembwa MS, Fleming SC, Sewankambo N, Serwadda D, Lucas S, Moody A, Griffin GE. 1991. Altered small-intestinal permeability associated with diarrhoea in human-immunodeficiency-virus-infected Caucasian and African subjects. Clin Sci (Lond) 81:327–334. doi: 10.1042/cs0810327. [DOI] [PubMed] [Google Scholar]
- 11.Zevin AS, McKinnon L, Burgener A, Klatt NR. 2016. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS 11:182–190. doi: 10.1097/COH.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, Wanke CA, Ward HD. 2015. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis 211:19–27. doi: 10.1093/infdis/jiu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klatt NR, Funderburg NT, Brenchley JM. 2013. Microbial translocation, immune activation, and HIV disease. Trends Microbiol 21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenchley JM, Douek DC. 2012. Microbial translocation across the GI tract. Annu Rev Immunol 30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchetti G, Cozzi-Lepri A, Merlini E, Bellistri GM, Castagna A, Galli M, Verucchi G, Antinori A, Costantini A, Giacometti A, di Caro A, D'Arminio Monforte A, ICONA Foundation Study Group . 2011. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS 25:1385–1394. doi: 10.1097/QAD.0b013e3283471d10. [DOI] [PubMed] [Google Scholar]
- 16.McKinstry KK, Strutt TM, Swain SL. 2010. The potential of CD4 T-cell memory. Immunology 130:1–9. doi: 10.1111/j.1365-2567.2010.03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monteiro P, Gosselin A, Wacleche VS, El-Far M, Said EA, Kared H, Grandvaux N, Boulassel MR, Routy JP, Ancuta P. 2011. Memory CCR6+CD4+ T cells are preferential targets for productive HIV type 1 infection regardless of their expression of integrin beta7. J Immunol 186:4618–4630. doi: 10.4049/jimmunol.1004151. [DOI] [PubMed] [Google Scholar]
- 18.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. 2012. Defining the human T helper 17 cell phenotype. Trends Immunol 33:505–512. doi: 10.1016/j.it.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. 2007. Phenotypic and functional features of human Th17 cells. J Exp Med 204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Annunziato F, Romagnani S. 2009. Do studies in humans better depict Th17 cells? Blood 114:2213–2219. doi: 10.1182/blood-2009-03-209189. [DOI] [PubMed] [Google Scholar]
- 21.Gosselin A, Monteiro P, Chomont N, Diaz-Griffero F, Said EA, Fonseca S, Wacleche V, El-Far M, Boulassel MR, Routy JP, Sekaly RP, Ancuta P. 2010. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J Immunol 184:1604–1616. doi: 10.4049/jimmunol.0903058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Hed A, Khaitan A, Kozhaya L, Manel N, Daskalakis D, Borkowsky W, Valentine F, Littman DR, Unutmaz D. 2010. Susceptibility of human Th17 cells to human immunodeficiency virus and their perturbation during infection. J Infect Dis 201:843–854. doi: 10.1086/651021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, Khoruts A, Frank I, Else J, Schacker T, Silvestri G, Douek DC. 2008. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim CJ, McKinnon LR, Kovacs C, Kandel G, Huibner S, Chege D, Shahabi K, Benko E, Loutfy M, Ostrowski M, Kaul R. 2013. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J Immunol 191:2164–2173. doi: 10.4049/jimmunol.1300829. [DOI] [PubMed] [Google Scholar]
- 25.Cecchinato V, Trindade CJ, Laurence A, Heraud JM, Brenchley JM, Ferrari MG, Zaffiri L, Tryniszewska E, Tsai WP, Vaccari M, Parks RW, Venzon D, Douek DC, O'Shea JJ, Franchini G. 2008. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol 1:279–288. doi: 10.1038/mi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen-Quick A, Lafferty M, Sun L, Marchionni L, DeVico A, Garzino-Demo A. 2016. Human Th17 cells lack HIV-inhibitory RNases and are highly permissive to productive HIV infection. J Virol 90:7833–7847. doi: 10.1128/JVI.02869-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Kang SG, Lee J, Sun Z, Kim CH. 2009. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol 2:173–183. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunkel EJ, Campbell DJ, Butcher EC. 2003. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation 10:313–323. doi: 10.1038/sj.mn.7800196. [DOI] [PubMed] [Google Scholar]
- 29.Westphal S, Lugering A, von Wedel J, von Eiff C, Maaser C, Spahn T, Heusipp G, Schmidt MA, Herbst H, Williams IR, Domschke W, Kucharzik T. 2008. Resistance of chemokine receptor 6-deficient mice to Yersinia enterocolitica infection: evidence of defective M-cell formation in vivo. Am J Pathol 172:671–680. doi: 10.2353/ajpath.2008.070393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez Y, Tuen M, Shen G, Nawaz F, Arthos J, Wolff MJ, Poles MA, Hioe CE. 2013. Preferential HIV infection of CCR6+ Th17 cells is associated with higher levels of virus receptor expression and lack of CCR5 ligands. J Virol 87:10843–10854. doi: 10.1128/JVI.01838-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cleret-Buhot A, Zhang Y, Planas D, Goulet JP, Monteiro P, Gosselin A, Wacleche VS, Tremblay CL, Jenabian MA, Routy JP, El-Far M, Chomont N, Haddad EK, Sekaly RP, Ancuta P. 2015. Identification of novel HIV-1 dependency factors in primary CCR4CCR6Th17 cells via a genome-wide transcriptional approach. Retrovirology 12:102. doi: 10.1186/s12977-015-0226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, Barclay GR, Smedley J, Pung R, Oliveira KM, Hirsch VM, Silvestri G, Douek DC, Miller CJ, Haase AT, Lifson J, Brenchley JM. 2010. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog 6:e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kedzierska K, Azzam R, Ellery P, Mak J, Jaworowski A, Crowe SM. 2003. Defective phagocytosis by human monocyte/macrophages following HIV-1 infection: underlying mechanisms and modulation by adjunctive cytokine therapy. J Clin Virol 26:247–263. doi: 10.1016/S1386-6532(02)00123-3. [DOI] [PubMed] [Google Scholar]
- 34.Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, Cervasi B, Yokomizo LK, Pan L, Vinton CL, Tabb B, Canary LA, Dang Q, Hirsch VM, Alter G, Belkaid Y, Lifson JD, Silvestri G, Milner JD, Paiardini M, Haddad EK, Brenchley JM. 2012. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol 5:646–657. doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bettelli E, Korn T, Oukka M, Kuchroo VK. 2008. Induction and effector functions of T(H)17 cells. Nature 453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loiseau C, Requena M, Mavigner M, Cazabat M, Carrere N, Suc B, Barange K, Alric L, Marchou B, Massip P, Izopet J, Delobel P. 2016. CCR6 regulatory T cells blunt the restoration of gut Th17 cells along the CCR6-CCL20 axis in treated HIV-1-infected individuals. Mucosal Immunol 9:1137–1150. doi: 10.1038/mi.2016.7. [DOI] [PubMed] [Google Scholar]
- 37.Marchetti G, Tincati C, Silvestri G. 2013. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev 26:2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanneganti TD, Lamkanfi M, Nunez G. 2007. Intracellular NOD-like receptors in host defense and disease. Immunity 27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. 2010. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svard J, Paquin-Proulx D, Buggert M, Noyan K, Barqasho B, Sonnerborg A, Nowak P. 2015. Role of translocated bacterial flagellin in monocyte activation among individuals with chronic HIV-1 infection. Clin Immunol 161:180–189. doi: 10.1016/j.clim.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Brandt KJ, Fickentscher C, Kruithof EK, de Moerloose P. 2013. TLR2 ligands induce NF-kappaB activation from endosomal compartments of human monocytes. PLoS One 8:e80743. doi: 10.1371/journal.pone.0080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nazli A, Kafka JK, Ferreira VH, Anipindi V, Mueller K, Osborne BJ, Dizzell S, Chauvin S, Mian MF, Ouellet M, Tremblay MJ, Mossman KL, Ashkar AA, Kovacs C, Bowdish DM, Snider DP, Kaul R, Kaushic C. 2013. HIV-1 gp120 induces TLR2- and TLR4-mediated innate immune activation in human female genital epithelium. J Immunol 191:4246–4258. doi: 10.4049/jimmunol.1301482. [DOI] [PubMed] [Google Scholar]
- 43.Thayil SM, Ho YC, Bollinger RC, Blankson JN, Siliciano RF, Karakousis PC, Page KR. 2012. Mycobacterium tuberculosis complex enhances susceptibility of CD4 T cells to HIV through a TLR2-mediated pathway. PLoS One 7:e41093. doi: 10.1371/journal.pone.0041093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding J, Chang TL. 2012. TLR2 activation enhances HIV nuclear import and infection through T cell activation-independent and -dependent pathways. J Immunol 188:992–1001. doi: 10.4049/jimmunol.1102098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thibault S, Fromentin R, Tardif MR, Tremblay MJ. 2009. TLR2 and TLR4 triggering exerts contrasting effects with regard to HIV-1 infection of human dendritic cells and subsequent virus transfer to CD4+ T cells. Retrovirology 6:42. doi: 10.1186/1742-4690-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogawa Y, Kawamura T, Kimura T, Ito M, Blauvelt A, Shimada S. 2009. Gram-positive bacteria enhance HIV-1 susceptibility in Langerhans cells, but not in dendritic cells, via Toll-like receptor activation. Blood 113:5157–5166. doi: 10.1182/blood-2008-10-185728. [DOI] [PubMed] [Google Scholar]
- 47.Thibault S, Tardif MR, Barat C, Tremblay MJ. 2007. TLR2 signaling renders quiescent naive and memory CD4+ T cells more susceptible to productive infection with X4 and R5 HIV-type 1. J Immunol 179:4357–4366. doi: 10.4049/jimmunol.179.7.4357. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Li G, Bafica A, Pantelic M, Zhang P, Broxmeyer H, Liu Y, Wetzler L, He JJ, Chen T. 2005. Neisseria gonorrhoeae enhances infection of dendritic cells by HIV type 1. J Immunol 174:7995–8002. doi: 10.4049/jimmunol.174.12.7995. [DOI] [PubMed] [Google Scholar]
- 49.Equils O, Schito ML, Karahashi H, Madak Z, Yarali A, Michelsen KS, Sher A, Arditi M. 2003. Toll-like receptor 2 (TLR2) and TLR9 signaling results in HIV-long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells: implications of simultaneous activation of TLRs on HIV replication. J Immunol 170:5159–5164. doi: 10.4049/jimmunol.170.10.5159. [DOI] [PubMed] [Google Scholar]
- 50.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem 274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 51.Krebs SJ, Ananworanich J. 2016. Immune activation during acute HIV infection and the impact of early antiretroviral therapy. Curr Opin HIV AIDS 11:163–172. doi: 10.1097/COH.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 52.Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, Gianella S, Siewe B, Smith DM, Landay AL, Robertson CE, Frank DN, Wilson CC. 2014. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol 7:983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korolevskaya LB, Shmagel KV, Shmagel NG, Saidakova EV. 2016. Systemic activation of the immune system in HIV infection: the role of the immune complexes (hypothesis). Med Hypotheses 88:53–56. doi: 10.1016/j.mehy.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Williams B, Landay A, Presti RM. 2016. Microbiome alterations in HIV infection a review. Cell Microbiol 18:645–651. doi: 10.1111/cmi.12588. [DOI] [PubMed] [Google Scholar]
- 55.Monaco CL, Gootenberg DB, Zhao G, Handley SA, Ghebremichael MS, Lim ES, Lankowski A, Baldridge MT, Wilen CB, Flagg M, Norman JM, Keller BC, Luevano JM, Wang D, Boum Y, Martin JN, Hunt PW, Bangsberg DR, Siedner MJ, Kwon DS, Virgin HW. 2016. Altered virome and bacterial microbiome in human immunodeficiency virus-associated acquired immunodeficiency syndrome. Cell Host Microbe 19:311–322. doi: 10.1016/j.chom.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. 2012. Diversity, stability and resilience of the human gut microbiota. Nature 489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, Knight R, Fontenot AP, Palmer BE. 2013. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe 14:329–339. doi: 10.1016/j.chom.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lozupone CA, Rhodes ME, Neff CP, Fontenot AP, Campbell TB, Palmer BE. 2014. HIV-induced alteration in gut microbiota: driving factors, consequences, and effects of antiretroviral therapy. Gut Microbes 5:562–570. doi: 10.4161/gmic.32132. [DOI] [PubMed] [Google Scholar]
- 59.Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, French A, Demarais P, Sun Y, Koenig L, Cox S, Engen P, Chakradeo P, Abbasi R, Gorenz A, Burns C, Landay A. 2014. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog 10:e1003829. doi: 10.1371/journal.ppat.1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zahringer U, Lindner B, Inamura S, Heine H, Alexander C. 2008. TLR2-promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology 213:205–224. [DOI] [PubMed] [Google Scholar]
- 61.Dziarski R, Wang Q, Miyake K, Kirschning CJ, Gupta D. 2001. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J Immunol 166:1938–1944. doi: 10.4049/jimmunol.166.3.1938. [DOI] [PubMed] [Google Scholar]
- 62.Kikkert R, Laine ML, Aarden LA, van Winkelhoff AJ. 2007. Activation of toll-like receptors 2 and 4 by Gram-negative periodontal bacteria. Oral Microbiol Immunol 22:145–151. doi: 10.1111/j.1399-302X.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 63.de Aquino SG, Abdollahi-Roodsaz S, Koenders MI, van de Loo FA, Pruijn GJ, Marijnissen RJ, Walgreen B, Helsen MM, van den Bersselaar LA, de Molon RS, Avila Campos MJ, Cunha FQ, Cirelli JA, van den Berg WB. 2014. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J Immunol 192:4103–4111. doi: 10.4049/jimmunol.1301970. [DOI] [PubMed] [Google Scholar]
- 64.Sun Y, Shu R, Li CL, Zhang MZ. 2010. Gram-negative periodontal bacteria induce the activation of Toll-like receptors 2 and 4, and cytokine production in human periodontal ligament cells. J Periodontol 81:1488–1496. doi: 10.1902/jop.2010.100004. [DOI] [PubMed] [Google Scholar]
- 65.Imbeault M, Lodge R, Ouellet M, Tremblay MJ. 2009. Efficient magnetic bead-based separation of HIV-1-infected cells using an improved reporter virus system reveals that p53 up-regulation occurs exclusively in the virus-expressing cell population. Virology 393:160–167. doi: 10.1016/j.virol.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 66.Zhu J, Paul WE. 2010. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev 238:247–262. doi: 10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ribeiro RM, Mohri H, Ho DD, Perelson AS. 2002. In vivo dynamics of T cell activation, proliferation, and death in HIV-1 infection: why are CD4+ but not CD8+ T cells depleted? Proc Natl Acad Sci U S A 99:15572–15577. doi: 10.1073/pnas.242358099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McGarry T, Veale DJ, Gao W, Orr C, Fearon U, Connolly M. 2015. Toll-like receptor 2 (TLR2) induces migration and invasive mechanisms in rheumatoid arthritis. Arthritis Res Ther 17:153. doi: 10.1186/s13075-015-0664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A 97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong H, May MJ, Jimi E, Ghosh S. 2002. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell 9:625–636. doi: 10.1016/S1097-2765(02)00477-X. [DOI] [PubMed] [Google Scholar]
- 71.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 72.Stevenson M, Stanwick TL, Dempsey MP, Lamonica CA. 1990. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J 9:1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shipkova M, Wieland E. 2012. Surface markers of lymphocyte activation and markers of cell proliferation. Clin Chim Acta 413:1338–1349. doi: 10.1016/j.cca.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y, Belkina NV, Shaw S. 2009. HIV infection of T cells: actin-in and actin-out. Sci Signal 2:pe23. [DOI] [PubMed] [Google Scholar]
- 75.Lehmann M, Nikolic DS, Piguet V. 2011. How HIV-1 takes advantage of the cytoskeleton during replication and cell-to-cell transmission. Viruses 3:1757–1776. doi: 10.3390/v3091757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blumenthal R, Durell S, Viard M. 2012. HIV entry and envelope glycoprotein-mediated fusion. J Biol Chem 287:40841–40849. doi: 10.1074/jbc.R112.406272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Freeman SA, Jaumouille V, Choi K, Hsu BE, Wong HS, Abraham L, Graves ML, Coombs D, Roskelley CD, Das R, Grinstein S, Gold MR. 2015. Toll-like receptor ligands sensitize B-cell receptor signalling by reducing actin-dependent spatial confinement of the receptor. Nat Commun 6:6168. doi: 10.1038/ncomms7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee SM, Joo YD, Seo SK. 2009. Expression and function of TLR2 on CD4 versus CD8 T cells. Immune Netw 9:127–132. doi: 10.4110/in.2009.9.4.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Napetschnig J, Wu H. 2013. Molecular basis of NF-kappaB signaling. Annu Rev Biophys 42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoesel B, Schmid JA. 2013. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nabel G, Baltimore D. 1987. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 82.Ancuta P, Monteiro P, Sekaly RP. 2010. Th17 lineage commitment and HIV-1 pathogenesis. Curr Opin HIV AIDS 5:158–165. doi: 10.1097/COH.0b013e3283364733. [DOI] [PubMed] [Google Scholar]
- 83.Cote SC, Plante A, Tardif MR, Tremblay MJ. 2013. Dectin-1/TLR2 and NOD2 agonists render dendritic cells susceptible to infection by X4-using HIV-1 and promote cis-infection of CD4(+) T cells. PLoS One 8:e67735. doi: 10.1371/journal.pone.0067735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Platt EJ, Bilska M, Kozak SL, Kabat D, Montefiori DC. 2009. Evidence that ecotropic murine leukemia virus contamination in TZM-bl cells does not affect the outcome of neutralizing antibody assays with human immunodeficiency virus type 1. J Virol 83:8289–8292. doi: 10.1128/JVI.00709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol 74:8358–8367. doi: 10.1128/JVI.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol 72:2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takeuchi Y, McClure MO, Pizzato M. 2008. Identification of gammaretroviruses constitutively released from cell lines used for human immunodeficiency virus research. J Virol 82:12585–12588. doi: 10.1128/JVI.01726-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dornadula G, Zhang H, Shetty S, Pomerantz RJ. 1999. HIV-1 virions produced from replicating peripheral blood lymphocytes are more infectious than those from nonproliferating macrophages due to higher levels of intravirion reverse transcripts: implications for pathogenesis and transmission. Virology 253:10–16. doi: 10.1006/viro.1998.9465. [DOI] [PubMed] [Google Scholar]
- 90.Bounou S, Leclerc JE, Tremblay MJ. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4(+)-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J Virol 76:1004–1014. doi: 10.1128/JVI.76.3.1004-1014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hamley IW, Kirkham S, Dehsorkhi A, Castelletto V, Reza M, Ruokolainen J. 2014. Toll-like receptor agonist lipopeptides self-assemble into distinct nanostructures. Chem Commun (Camb) 50:15948–15951. doi: 10.1039/C4CC07511K. [DOI] [PubMed] [Google Scholar]