ABSTRACT
Many enveloped viruses cause devastating disease in aquaculture, resulting in significant economic impact. LJ001 is a broad-spectrum antiviral compound that inhibits enveloped virus infections by specifically targeting phospholipids in the lipid bilayer via the production of singlet oxygen (1O2). This stabilizes positive curvature and decreases membrane fluidity, which inhibits virus-cell membrane fusion during viral entry. Based on data from previous mammalian studies and the requirement of light for the activation of LJ001, we hypothesized that LJ001 may be useful as a preventative and/or therapeutic agent for infections by enveloped viruses in aquaculture. Here, we report that LJ001 was more stable with a prolonged inhibitory half-life at relevant aquaculture temperatures (15°C), than in mammalian studies at 37°C. When LJ001 was preincubated with our model virus, infectious hematopoietic necrosis virus (IHNV), infectivity was significantly inhibited in vitro (using the epithelioma papulosum cyprini [EPC] fish cell line) and in vivo (using rainbow trout fry) in a dose-dependent and time-dependent manner. While horizontal transmission of IHNV in a static cohabitation challenge model was reduced by LJ001, transmission was not completely blocked at established antiviral doses. Therefore, LJ001 may be best suited as a therapeutic for aquaculture settings that include viral infections with lower virus-shedding rates than IHNV or where higher viral titers are required to initiate infection of naive fish. Importantly, our data also suggest that LJ001-inactivated IHNV elicited an innate immune response in the rainbow trout host, making LJ001 potentially useful for future vaccination approaches.
IMPORTANCE Viral diseases in aquaculture are challenging because there are few preventative measures and/or treatments. Broad-spectrum antivirals are highly sought after and studied because they target common components of viruses. In our studies, we used LJ001, a broad-spectrum antiviral compound that specifically inhibits enveloped viruses. We used the fish rhabdovirus infectious hematopoietic necrosis virus (IHNV) as a model to study aquatic enveloped virus diseases and their inhibition. We demonstrated inhibition of IHNV by LJ001 both in cell culture as well as in live fish. Additionally, we showed that LJ001 inhibited the transmission of IHNV from infected fish to healthy fish, which lays the groundwork for using LJ001 as a possible therapeutic for aquatic viruses. Our results also suggest that virus inactivated by LJ001 induces an immune response, showing potential for future preventative (e.g., vaccine) applications.
KEYWORDS: antiviral, aquaculture, aquatic virus, broad spectrum, enveloped, membrane fusion, rhabdovirus, virus
INTRODUCTION
Aquaculture is considered the fastest-growing food-producing sector, increasing at an average annual rate of 6.1%, from 36.8 million tons in 2002 to 66.6 million tons in 2012 (1). Aquaculture is estimated to account for more than 30% of the world's food fish, and this is projected to increase to 50% by 2030 (2). As fish and shellfish demands increase, it is critical to reduce losses from diseases that economically devastate the aquaculture industry. Viral pathogens, in particular, are challenging since there are few efficacious treatments or preventatives (3). A number of viruses are associated with significant economic losses for the aquaculture industry, including 11 enveloped viruses that cause diseases so severe that they are reportable to the World Organization for Animal Health (OIE) according to the 2016 aquatic animal health code (http://www.oie.int/en/). Examples of OIE-reportable viruses include infectious hematopoietic necrosis virus (IHNV) in the trout industry (4, 5), salmonid alphavirus in the salmon industry (3, 5), spring viremia of carp virus (SVCV) in the koi and carp industries (5, 6), and white spot syndrome virus (WSSV) in the shrimp industry (7, 8).
We used IHNV as a model to evaluate the broad-spectrum antiviral LJ001 for potential use in aquaculture. IHNV belongs to the family Rhabdoviridae and genus Novirhabdovirus (http://ictvonline.org/virusTaxonomy.asp) and infects a range of salmonid fish species (9) in addition to some nonsalmonid fish species (demonstrated experimentally) (10, 11). IHNV is an enveloped, bullet-shaped RNA virus encoding a negative-sense, single-stranded genome. The virus is enzootic in river systems throughout western North America and has spread to Asia and Europe, likely by movement of infected fish and eggs (12). Fry are typically most susceptible to disease, with cumulative mortality rates reported to be as high as 90 to 95% during epizootic outbreaks. Horizontal transmission of IHNV occurs when the virus is shed in feces, urine, sexual fluids, and external mucus (13), and the virus can survive in freshwater for up to 7 weeks (14). The only licensed vaccine is a DNA vaccine that encodes the IHNV glycoprotein (2, 15, 16) and is delivered by intramuscular injection. The vaccine elicits both early, nonspecific, and cross-protective antiviral immunity mediated by interferon as well as a long-term, specific adaptive response (17) and is considered effective (18). However, the vaccine can be cost-prohibitive and has delivery constraints (19), which makes it impractical for large numbers of susceptible fry. There is still a significant problem with many aquatic enveloped viruses, including IHNV, and new therapy options are needed.
A novel small-molecule antiviral rhodanine derivative, LJ001, has been shown to inhibit infections by numerous enveloped viruses at micromolar concentrations, with no effect on nonenveloped viruses (20). The antiviral activity of LJ001 is light dependent and requires the presence of molecular oxygen. The mechanism of action for LJ001 is based on the production of singlet oxygen (1O2) molecules that modify phospholipids in the lipid bilayer, which results in the stabilization of positive spontaneous curvature, decreased membrane fluidity, and, ultimately, inhibition of virus-cell membrane fusion and viral entry (21). Unlike viruses, host cells possess a “biogenic” membrane that actively repairs its defects by lipid synthesis and replacement (22). In contrast, the host-derived viral envelope cannot be repaired and is a susceptible target for LJ001. It has been suggested that there is limited opportunity for viruses to evolve resistance to LJ001 based on its mechanism of action and specific target of the membrane envelope (20). LJ001 has been shown to have no overt toxicity in Vero cells or mouse models at effective antiviral doses (up to 10 μM), but as predicted, toxicity can be induced in the presence of the fatty acid synthesis inhibitor 5-(tetradecyloxy)-2-furoic acid (TOFA) (20).
The use of LJ001 in mammalian models is impractical due to a reported biological half-life of ∼4 h at 37°C (20) and a lack of exposure to light in most tissues to activate the compound in vivo. Aquaculture environments are unique because pathogens, including IHNV, are transmitted through water, which is transparent to light. In this study, we determined that IHNV infection and horizontal transmission were inhibited by LJ001 and that LJ001 had a prolonged inhibitory half-life in aquaculture environments. We also preliminarily investigated the ability of LJ001-inactivated IHNV to induce an innate immune response in the rainbow trout host.
RESULTS
LJ001 is not cytotoxic at antiviral concentrations.
We first evaluated the potential cytotoxicity of LJ001 via in vitro experiments in fish-derived epithelioma papulosum cyprini (EPC) cells. Microscopically, there were no changes in cellular morphology or growth at LJ001 concentrations of up to 10 μM for up to 7 days. Additionally, cell viability was quantitatively assessed by using a cell counting kit (CCK-8), which is based on dehydrogenase enzyme activity. EPC cells were exposed to increasing concentrations of LJ001, and no cytotoxicity was evident with up to 7 days of exposure to 10 μM LJ001 (Fig. 1A). Cytotoxicity was reached at 7 days of exposure to 20 μM LJ001 (P ≤ 0.001) and at all time points when cells were exposed to 50 μM LJ001. There was no cytotoxicity of the vehicle control (dimethyl sulfoxide [DMSO]) at concentrations of up to 0.1%, but cytotoxicity was established at 3 days (P ≤ 0.05) and 7 days (P ≤ 0.001) of exposure to 0.5% DMSO (Fig. 1B). In Fig. 1A, cells exposed to 10 μM LJ001 were simultaneously exposed to 0.1% DMSO (50 μM LJ001 in 0.5% DMSO).
FIG 1.
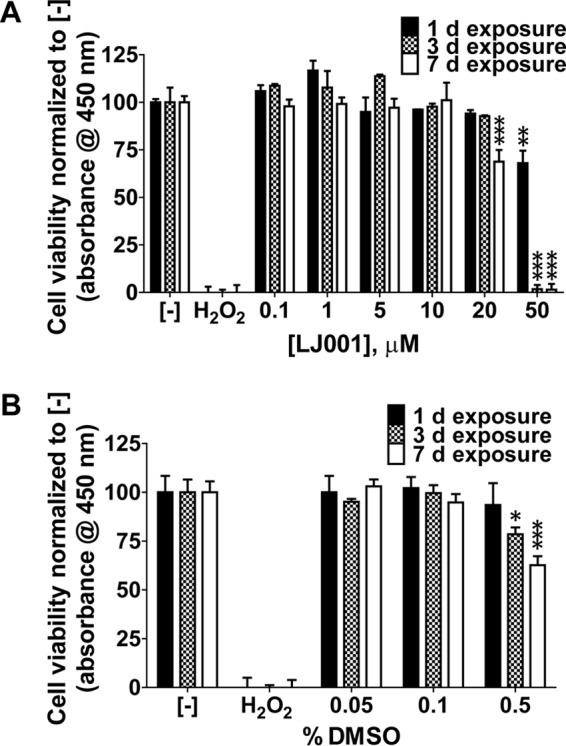
LJ001 is not cytotoxic at antiviral concentrations. EPC cells were exposed to increasing concentrations of LJ001 (A) or the vehicle control (DMSO) (B) for 1, 3, and 7 days in the presence of light at the time of addition. Formazan dye absorbance was measured at 450 nm. Positive-control wells were exposed to hydrogen peroxide (40 mM final concentration) for 1 day. The negative control (−) is no drug exposure. (A) Exposure to 0 to 50 μM LJ001 (50 μM LJ001 with 0.5% DMSO, 10 μM LJ001 with 0.1% DMSO, and 1.0 μM LJ001 with 0.01% DMSO). There was no cytotoxicity to EPC cells at up to 10 μM LJ001. Toxicity occurred when cells were exposed to 20 μM LJ001 for 7 days and 50 μM LJ001 at all time points. Data represent mean cell viabilities ± standard errors (n = 3) normalized to values for no treatment (negative control). **, P < 0.01; ***, P < 0.001. (B) Exposure to 0 to 0.5% DMSO. No cytotoxic effects were observed at concentrations of up to 0.1%. Cytotoxic effects were detected after exposure to 0.5% DMSO at 3 and 7 days. Data represent mean cell viabilities ± standard errors (n = 3) normalized to values for no treatment. *, P < 0.05; ***, P < 0.001.
Clinical parameters used to assess the potential toxicity of LJ001 during rainbow trout fry experiments included prolonged lethargy, circling, swimming slowly, preference for the bottom of challenge containers, and overt death, none of which were observed. Cytotoxic effects were also assessed via histopathology of whole, fixed fry (transverse sections). There was no histologic difference between three negative-control fish (histologic scores of 1, 2, and 1) and three fish immersed in 10 μM LJ001 (histologic scores of 1, 2, and 2). The scores were based on mild to moderate hyperplastic changes in the gills and skin (associated with various numbers of Ichthyobodo necator [“Costia”] parasites) with no internal organ abnormalities (data not shown) (P = 0.52 by an unpaired Student t test). Our results confirmed that LJ001 was not cytotoxic in vitro and in vivo at antiviral doses.
LJ001 blocks IHNV infection in vitro.
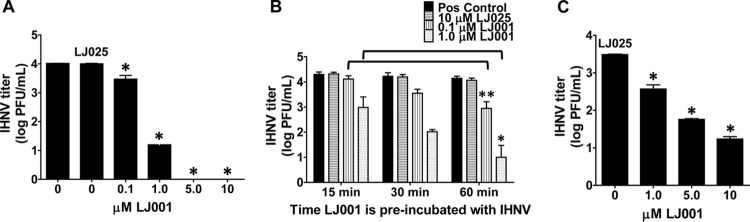
Next, we examined whether LJ001 could inhibit an aquatic rhabdovirus at previously established antiviral doses. LJ001 was preincubated with IHNV for 30 min prior to plaque assays using EPC cells. LJ001 was effective at inhibiting IHNV infection in vitro in a dose-dependent manner (Fig. 2A). There was significant inhibition of infection (IHNV at 1 × 104 PFU/ml) with 0.1 μM and 1.0 μM LJ001 and complete inhibition at both 5 μM and 10 μM LJ001 (P ≤ 0.001). The negative-control molecule, LJ025, had no inhibitory effect at a concentration of 10 μM (P > 0.05). Inhibition of infection was enhanced when LJ001 (0.1 and 1.0 μM) was preincubated with the virus for longer times (Fig. 2B). There were significant differences in inhibition at between 15 min of exposure and 60 min of exposure for both LJ001 concentrations used (P ≤ 0.01 with 0.1 μM LJ001 and P ≤ 0.05 with 1.0 μM LJ001). LJ001 was directly applied to EPC cells, followed by the addition of untreated virus. Inhibition of infection is similarly dose dependent (Fig. 2C). Inhibition is slightly decreased for direct application compared to virus/drug preincubation, without complete inhibition at 10 μM LJ001 when 3 × 103 PFU/ml virus were added. All mock controls had no detectable virus. Because of these encouraging results, particularly with preincubation, we then tested the inhibitory properties of LJ001 in vivo.
FIG 2.
LJ001 blocks IHNV infection in vitro. (A) Concentrations of up to 10 μM LJ001 (or 10 μM LJ025) were preincubated with 1 × 104 PFU/ml IHNV for 30 min during exposure to light. The IHNV titer was determined via a plaque assay. There was complete inhibition of infection with 5 and 10 μM LJ001, substantial and significant inhibition with 1.0 μM LJ001, and mild but significant inhibition with 0.1 μM LJ001 compared to the positive control (IHNV with no LJ001 treatment). The negative-control molecule LJ025 had no inhibitory effect. Mock controls had negative titers. Data represent mean IHNV titers ± standard errors (n = 3). *, P < 0.001. (B) LJ001 was preincubated with the virus for 15, 30, and 60 min. Inhibition was enhanced with an increased time of exposure of LJ001 to the virus. The difference in inhibition was significant at between 15 min and 60 min for both 1.0 μM and 0.1 μM LJ001. Mock controls had negative titers. Data represent mean IHNV titers ± standard errors (n = 4).*, P < 0.05; **, P < 0.01. (C) Up to 10 μM LJ001 (or 10 μM LJ025) was directly applied to EPC cells, followed by the addition of untreated virus at 3 × 103 PFU/ml. Inhibition of infection is dose dependent but decreased compared to that with virus/drug preincubation. Mock controls had negative titers. Data represent mean IHNV titers ± standard errors (n = 3). *, P < 0.001.
LJ001 blocks IHNV infection in vivo.
For in vivo studies, LJ001 was preincubated with IHNV for 15 min prior to immersion exposure to rainbow trout fry. LJ001 inhibited IHNV infection in a dose-dependent manner, where infection was measured by a plaque assay (Fig. 3A) to assess live-virus levels and confirmed by reverse transcriptase real-time PCR (RT-rPCR) (Fig. 3B) by probing for the IHNV N gene (viral RNA). There was substantial inhibition (>2 logs; P ≤ 0.001) of IHNV infection at 10 and 1.0 μM LJ001 via a plaque assay (only one of five fish had detectable virus, and the other four fish were negative at 10 μM LJ001; three of five fish had no detectable virus at 1.0 μM LJ001). There was also significant inhibition (P ≤ 0.05) of IHNV infection at 0.1 μM LJ001 (one of five fish was negative and one fish was positive at the lowest detection limit). All five fish in both the group receiving 0.01 μM LJ001 treatment and the group receiving virus with no treatment had positive viral titers. All mock-infected fish had negative titers. Our results demonstrated that IHNV infection can be inhibited in vivo by preincubation with low micromolar concentrations of LJ001.
FIG 3.
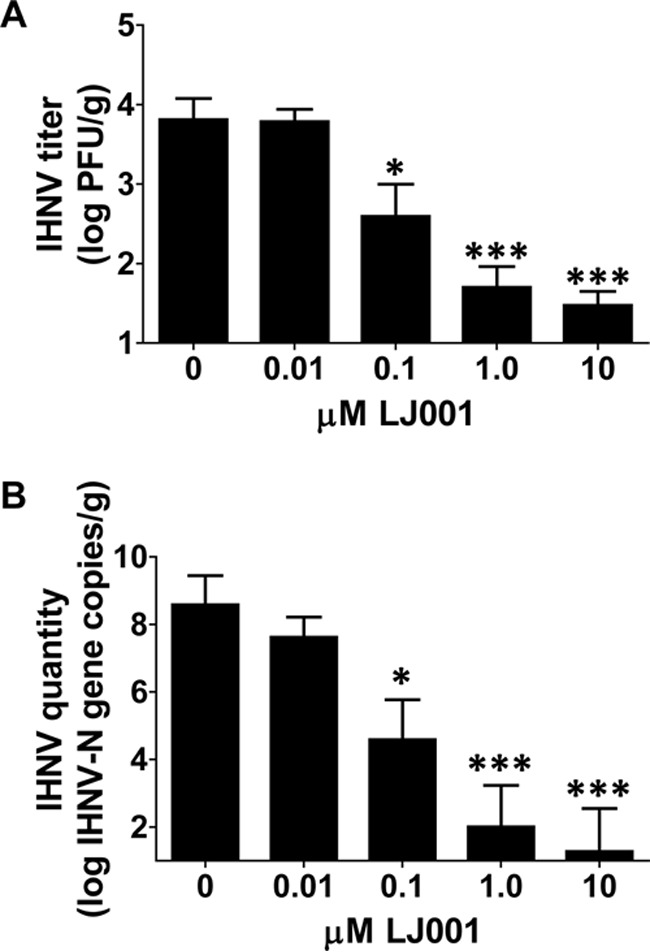
LJ001 blocks IHNV infection in vivo. Naive rainbow trout fry were immersed as a group in 1 × 104 PFU/ml IHNV that was preincubated for 15 min with 0 to 10 μM LJ001 while being exposed to light. Following 12 h, fish were separated into isolation beakers, and the virus was allowed to replicate for 72 h. The homogenate supernatant from each fish was used for a plaque assay (A), and RNA was isolated and quantified by RT-rPCR (B) to determine the IHNV titer or quantity, respectively. The positive control was IHNV and the vehicle control only (0 μM LJ001 and 0.01% DMSO, final concentration). The negative control was MEM and 0.01% DMSO. (A) There was a highly significant inhibition of infection with 10 μM and 1.0 μM LJ001 and significant inhibition with 0.1 μM LJ001. Data represent mean IHNV titers ± standard errors (n = 5 fish per group). *, P < 0.05; ***, P < 0.001. (B) RT-rPCR probing for the IHNV N gene confirmed the plaque assay results. Data represent mean numbers of IHNV N gene copies ± standard errors (n = 5). *, P < 0.05; ***, P < 0.001.
LJ001 is relatively stable in aquaculture environments.
We then tested the stability of LJ001 in aqueous environments. LJ001 was added to water samples (river, hatchery, or deionized water, with hatchery water at 15°C being the most aquaculture-relevant environment containing a medium level of organic material). The samples were placed for up to 7 days at 15°C or 4°C (with exposure to light) and finally preincubated with 1 × 104 PFU/ml IHNV, followed by a plaque assay. LJ001 was fairly stable in hatchery water at 15°C (Fig. 4A) and was less efficacious with increased amounts of organic material present (the river water had abundant particulate matter). LJ001 added to hatchery water at 15°C had a calculated inhibitory half-life of 1.9 days (R2 = 0.83) when a nonlinear regression, best-of-fit line was applied (data not shown). Additionally, the stability of LJ001 was further enhanced at even lower temperatures (4°C) (Fig. 4B). The calculated inhibitory half-lives of LJ001 at 4°C were 2.7 days (R2 = 0.76) in river water and 5.6 days (R2 = 0.72) in hatchery water. These results indicate that LJ001 is a relatively stable compound under aquaculture conditions.
FIG 4.
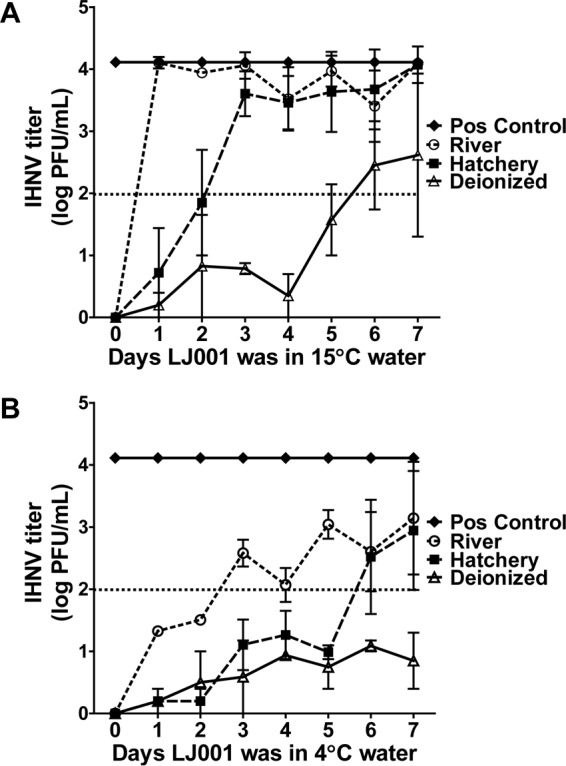
LJ001 is relatively stable in aquaculture environments. LJ001 was added to sterilized deionized, hatchery, or river water and placed at either 4°C or 15°C for 0 to 8 days (a new sample was created each day; not additive). On day 0 (final day), 1 × 104 PFU/ml IHNV (final concentration) was preincubated with each treated water sample (10 μM LJ001, final concentration), followed by a plaque assay to determine the IHNV titer. There was complete inhibition of infection by LJ001 for all water and temperature conditions on day 0. The dotted line represents the inhibitory half-life titer (>50% inhibition of the positive control). (A) In 15°C water, LJ001 had an inhibitory half-life of between 1 and 2 days in hatchery water. After day 0, LJ001 in river water had no inhibitory effect. The inhibitory half-life of LJ001 in DI water at 15°C was ∼5 to 6 days. (B) When placed in 4°C water, the inhibitory half-life of LJ001 was prolonged under all water conditions. Data represent mean IHNV titers ± standard errors (n = 2).
LJ001 inhibits horizontal transmission of IHNV.
Based on our in vivo pretreatment inhibition data, we explored the therapeutic applications of LJ001 and its ability to inhibit horizontal transmission of IHNV. Donor fish were immersion infected with IHNV, followed by the addition of LJ001 (5 μM final concentration), and finally, naive recipient fish were added after 15 min in a cohabitation experiment (see Fig. 5A for experimental groups). IHNV recipient fish from LJ001-treated groups had significantly lower (P = 0.002) viral titers than did DMSO-treated (control) IHNV recipient fish (Fig. 5B). None of the 36 LJ001-treated IHNV recipient fish had negative titers, but 19/36 fish had titers of <3.5 log PFU/g, whereas only 4/36 DMSO control IHNV recipients had titers of <3.5 log PFU/g. IHNV-infected donor fish from LJ001-treated groups had slightly lower, but not significantly lower (P = 0.38), viral titers. Our results revealed a significant reduction, but not complete blockage, of horizontal transmission of IHNV when fish were treated with LJ001.
FIG 5.
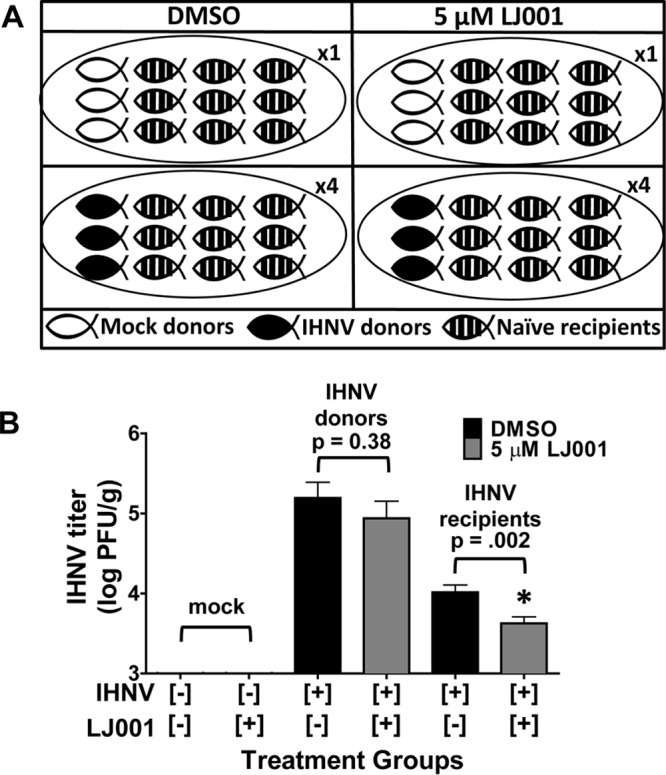
LJ001 inhibits horizontal transmission of IHNV. Rainbow trout were immersion infected with 2 × 105 PFU/ml IHNV (donor fish) or MEM (mock) and remained in the flowthrough for 24 h. (A) Experimental groups. Three IHNV- or mock-infected donor fish were placed into static challenge containers, followed by the addition of LJ001 (5 μM final concentration) or 0.005% DMSO (vehicle control); after 15 min, nine naive recipient fish were added to each challenge container for cohabitation (n = 1 challenge container per mock-infected group, and n = 4 challenge containers per IHNV-infected group). Water exchanges and fresh LJ001 or DMSO dosing occurred every 24 h. (B) For donor fish that were immersion infected with IHNV, there was a nonsignificant (P = 0.38, as determined by Student's t test) decrease in viral loads for the LJ001-treated donor fish (12 fish) compared to DMSO-treated (vehicle control) donor fish (12 fish). There was a significant decrease in viral loads for LJ001-treated IHNV recipient fish (36 fish) compared to DMSO-treated (control) IHNV recipient fish (36 fish) (P = 0.002). All mock-infected fish had negative titers. Data represents mean IHNV titers ± standard errors (n = 12 for mock groups, n = 12 for IHNV donors, and n = 36 for IHNV recipients).
Mx-1 gene expression levels are elevated with LJ001-inactivated IHNV.
To determine whether LJ001-inactivated virus may elicit an immune response and thus may be promising as a vaccine vehicle, we measured Mx-1 gene expression levels in rainbow trout fry exposed to LJ001-inactivated IHNV. Up to 10 μM LJ001 was preincubated with IHNV for 15 min prior to immersion exposure of rainbow trout fry. Pectoral fins were bead homogenized to isolate RNA for Mx-1 gene expression via quantitative PCR (qPCR) and for IHNV N gene copy numbers via RT-rPCR. As expected, Mx-1 gene expression was significantly upregulated in fin tissues of fish immersed in untreated virus (IHNV and vehicle control only [0.0001% DMSO, final concentration]) compared to the negative-control group (no virus and DMSO) (Fig. 6). Similarly, Mx-1 expression was significantly upregulated in the 0.01 μM LJ001 treatment group. Fish immersed in IHNV pretreated with higher doses of LJ001 (0.1, 1.0, and 10 μM) had higher Mx-1 gene expression levels than did those in the negative-control group, although upregulation was not statistically significant when the data were subjected to a multiple-group statistical comparison. At 10 μM LJ001, the Mx-1 expression level was elevated 7.5-fold over the that of negative-control group, despite a lack of detectable viral RNA in the fin tissues. Our results suggest that LJ001-inactivated virus may elicit an innate immune response that could provide protection to the host. Future studies are aimed at testing this potential prophylactic approach.
FIG 6.
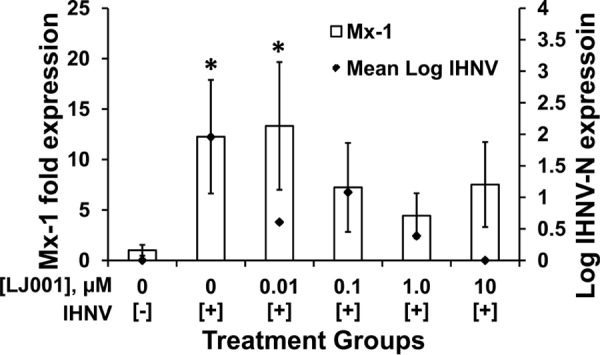
Mx-1 gene expression levels are elevated with LJ001-inactivated IHNV (10 μM). Naive rainbow trout fry (n = 5 fish per treatment group) were exposed in batch by immersion delivery of 1 × 104 PFU/ml IHNV preincubated with 0 to 10 μM LJ001. Following 12 h, fish were separated into isolation beakers, and the virus was allowed to replicate for 72 h. Pectoral fins were sampled for Mx-1 gene expression and corresponding IHNV N gene expression levels. The positive control was IHNV and the vehicle control only (0.01% DMSO, final concentration). The negative control was MEM (mock) and 0.01% DMSO. Mx-1 gene expression was significantly upregulated in the positive-control and 0.01 μM LJ001 treatment groups compared to the negative control. *, P < 0.05. Data represent means ± standard errors for Mx-1 expression and mean IHNV titers.
DISCUSSION
LJ001 is established as a broad-spectrum antiviral against enveloped viruses in mammalian systems (20). The present study revealed that LJ001 was efficacious against a fish virus in vitro (Fig. 2) and in vivo (Fig. 3). Additionally, LJ001 was not cytotoxic to fish cells (Fig. 1A) or juvenile rainbow trout (histopathology data not shown) at antiviral concentrations. High concentrations of LJ001 (≥50 μM), however, overwhelm the system and establish cytotoxic effects, which is compounded by the cytotoxic effects of DMSO at concentrations of >0.1% (Fig. 1B). The main limitations for the use of LJ001 in mammalian systems are the requirement of light for activation and a relatively short biological half-life at 37°C (reported to be ∼90 min) (20). Generally, compounds degrade more slowly at lower temperatures, and the normal physiological temperature range for salmonids and their viral pathogens includes 15°C (23). LJ001 also binds to any organic material that has a lipid membrane (20, 21, 24), which likely accounts for the decrease in efficacy shown in Fig. 2C, where some LJ001 was presumed to immediately bind to the cells before the addition of untreated virus. Pathogens in aquaculture are transmitted in water, and water is typically transparent to light. Thus, water is a more favorable medium for LJ001 applications during daylight hours or under artificial illumination. Our model virus, IHNV, preferentially infects trout at 15°C (25), and we demonstrated that LJ001 was more stable at these temperatures (Fig. 4A), with an inhibitory half-life of ∼1.9 days (R2 = 0.83). Additionally, the amount of organic material present in natural water environments will vary. We showed that increased amounts of organic material in the water decreased the efficacy of LJ001 but that the efficacy of LJ001 remained relatively high in the tested hatchery water.
Horizontal transmission of IHNV occurs during epizootic outbreaks, in which a small proportion of fish become infected initially and begin shedding virus in the water, which perpetuates the epizootic outbreak (26, 27). Often, two peaks of mortality are observed following acute rhabdovirus infection, which indicates that horizontal transmission plays an important role in outbreaks. Our data demonstrated that LJ001 significantly inhibited horizontal transmission from IHNV-infected donor fish to naive recipient fish in a cohabitation challenge model (Fig. 5), which was likely due to direct LJ001 inactivation of the virus being shed by donor fish. The amount of virus shed by multiple fish (three infected fish were used in each experimental group) is expected to be massive and should mimic horizontal transmission in a natural setting. Peak shedding rates in Atlantic salmon are upwards of 3.2 × 107 PFU/fish/h (27), and horizontal transmission to naive fish requires only 10 PFU/ml (multiplicity of infection [MOI]). Therefore, small amounts of virus in the water can infect fish, followed by rapid viral replication in the host. Although longer exposures and higher doses of LJ001 would likely enhance the inhibition of horizontal transmission, inhibition below the MOI (essentially complete inhibition) is unlikely to be achieved for this virus and antiviral combination. We chose this difficult model system (high shedding levels and low MOI needed for infection) to demonstrate the potential of LJ001 as a therapeutic, and we suspect that there is great promise for the compound in systems with lower levels of virus shedding and higher MOIs required for infection. Real-world conditions of variable amounts of organic material and high water flow rates/flowthrough systems are also challenges for therapeutic applications in aquaculture. For example, LJ001 might be suitable to inhibit viral transmission in pond aquaculture settings or when fish are housed short-term under static conditions, as occurs when fish are transported, tagged, or vaccinated, and in tanks for ornamental fish trade.
We showed that LJ001 directly inhibits IHNV infection in rainbow trout (Fig. 3), where 4/5 fish in the 10 μM LJ001 treatment group and 3/5 fish in the 1.0 μM LJ001 treatment group had negative viral titers (complete inhibition). We also suspect that virus inactivated by LJ001 may elicit an innate immune response that could be protective to the host, which would potentially decrease viral loads and shedding or block infection completely. Natural IHNV infection stimulates a robust early innate response followed by a long-term specific immune response (28). The speed and magnitude of the response are dependent on a number of variables, including water temperature, virus dose, strain, and other host/virus factors. The innate immune response to IHNV is mediated largely by type I interferon and interferon-induced proteins (including Mx-1) (29), which can be induced by the G protein alone (30). Studies have shown that the G protein elicits a strong early and nonspecific/cross-protective innate immune response (31, 32). Later, a specific immune response is associated with detectable serum neutralizing antibodies (33, 34). Wild-type IHNV (no treatment) stimulates the upregulation of Mx-1, as shown in Fig. 6. Similarly, IHNV treated with 0.01 μM LJ001 also significantly upregulates Mx-1 gene expression due to a lack of virus inhibition at this dose. The 10 μM LJ001 treatment group was the most noteworthy because Mx-1 gene expression was upregulated albeit not significantly due to the variability of the sampled fish in this group. Virus in this treatment group was interpreted as being inactivated by LJ001 (as judged by the extremely low titer), and our data for Mx-1 suggest that inactivated virus may upregulate innate immune gene expression.
The G protein has been shown to be the only IHNV protein necessary to induce long-term specific immunity (35, 36), which is the basis for the DNA vaccine. However, the exact mechanism and pathway by which the G protein induces interferon are not known. Hypothetically, the G protein could induce interferon at the cell surface (binding), within the endosome (endocytosis), within the cytoplasm (translation), when inserted into the surface membrane for assembly (recognized by immune function cells), or at a combination of these locations (29, 37). Additionally, degraded RNA within the endosome may activate Toll-like receptors 7 and 8 (38), with subsequent induction of interferon. LJ001-inactivated virus should induce a protective (innate and adaptive) immune response based on evidence that the glycoproteins are left unaltered (20), which theoretically still allows binding and endocytosis (internalization) of the virus by host cells. Conformation, glycosylation, and epitopes of G must be preserved to induce a protective immune response, and altered/modified viral proteins have been a pitfall for previously explored inactivated IHNV vaccines (39). The advantages of using LJ001 to inactivate viruses may include the possibility for ease of immersion administration (versus injection), lower costs and less labor associated with dosing mass numbers of fish/shellfish at one time, and the broad range of viruses and hosts that could be targeted.
The use of LJ001 as a therapeutic for IHNV at the tested concentrations may be limited, but LJ001 could be useful as a therapeutic against other enveloped viruses with lower shedding rates and higher MOIs and those transmitted under static-water conditions (e.g., ponds). Future studies will be focused on using LJ001-inactivated IHNV as a preventative measure by determining its ability to induce an immune response and its potential as a vaccine. An additional focus will be the study of the properties of enhanced derivatives of LJ001 as both aquaculture therapeutic inhibitors as well as inactivating compounds for vaccine development.
MATERIALS AND METHODS
LJ001.
LJ001 and a negative-control molecule, LJ025 (20, 21), were produced at the University of California, Los Angeles (UCLA), by Michael Jung's group. LJ001 was reconstituted in 100% DMSO, protected from light, stored at room temperature, and used within 6 months of reconstitution.
Cells and cell viability assay (cytotoxicity).
EPC cells (40, 41) are an IHNV-permissive fish cell line obtained from the Washington Animal Disease Diagnostic Laboratory (WADDL) aquaculture section (ATCC CRL 2872). Cells were seeded into a 96-well plate and incubated at 22°C until they reached confluence. Concentrations of up to 50 μM LJ001 or up to 0.5% DMSO were added to wells in triplicate for 1, 3, and 7 days. Cytotoxicity was assessed by using a cell counting kit (CCK-8; Dojindo Molecular Technologies) according to the manufacturer's instructions. Reaction plates were incubated for 90 min, and the absorbance was read at 450 nm by using a Tecan microplate reader (Infinite M-1000). The quantity of formazan dye produced, when [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] (WST-8) is reduced by dehydrogenases, is directly proportional to the number of living cells (i.e., cell viability).
IHNV propagation.
For in vitro studies, stock concentrations of IHNV (ATCC VR1392 [039-82 {WRAC strain}]; American Type Culture Collection, Rockville, MD) were obtained from the WADDL aquaculture laboratory. The virus was amplified by using Chinook salmon embryo 214 (CHSE-214) cells.
For in vivo studies, IHNV (strain 220-90) was propagated in EPC cells as previously described (42, 43).
In vitro inhibition.
For preincubation, EPC cells were seeded into a 24-well plate at 1 × 106 cells/ml in Roswell Park Memorial Institute medium with 10% fetal bovine serum (RPMI-10). Cells were 95 to 100% confluent in 24 h. IHNV (final viral titer of 1 × 104 PFU/ml) and LJ001 (0 to 10 μM) or LJ025 (10 μM) were preincubated together in RPMI-0 medium (without fetal bovine serum) in the presence of white light for 30 min. Mock negative controls (0.1% DMSO in RPMI-0) were used. The viral titer was determined by a plaque assay as previously described (42).
For time exposure, IHNV (final titer, 1 × 104 PFU/ml) was preincubated with 0.1 and 1.0 μM LJ001 or 10 μM LJ025 for 15, 30, and 60 min, followed by a plaque assay with mock controls.
For direct application, LJ001 (0 to 10 μM) or LJ025 (10 μM) was directly applied to EPC cells, followed by the addition of untreated virus at a final viral titer of 3 × 103 PFU/ml (plaque assay with mock controls).
Stability experiments.
Hatchery water was obtained from the end of the final raceway at a rainbow trout farm (Troutlodge, Inc., Sumner, WA). River water was obtained from a stream in Pullman, WA (downtown area). LJ001 (10 μM final concentration) was added to sterilized deionized (DI), hatchery, or river water samples in the presence of light; LJ001 was added to a new water sample each day and placed at either 4°C or 15°C (LJ001 remained in the water sample for 0 to 8 days). On day 8 (final day), 1 × 104 PFU/ml IHNV (final concentration) were preincubated with each LJ001-treated water sample for 30 min, followed by a plaque assay (described above) to determine the IHNV titer. To prevent osmotic lysis, 1 part LJ001 in water was added to 2 parts virus in medium to attain the final concentration/titer.
Fish experiments.
Live rainbow trout exposure studies were performed at the Western Fisheries Research Center (WFRC); all experiments were approved under WFRC IACUC protocol 2008-28. Fish were donated as fry by Troutlodge, Inc. (Sumner, WA).
Immersion.
IHNV was preincubated with LJ001 to a final viral titer of 1 × 104 PFU/ml and up to 10 μM LJ001 or the vehicle control (0.01% DMSO, final concentration) in challenge containers (500 ml of water and a continuous air supply) in the presence of light. After 15 min, naive rainbow trout fry were added to the challenge container. The fish remained together for 12 h, at which time the fish were separated into individual beakers (to prevent fish-to-fish transmission) containing 400 ml of laboratory water until 72 h postinfection. Mock negative controls (0.01% DMSO in minimal essential medium [MEM]) were used. The entire exposure was conducted in static water with aeration. The fish were humanely euthanized with tricaine methanesulfonate (MS-222) at a final concentration of 240 mg/liter. Fish were frozen at −80°C until processing.
Cohabitation.
Donor fish were immersed in 2 × 105 PFU/ml IHNV or MEM only (mock infected). At 24 h postexposure, three donor fish were placed into each challenge container (700 ml of water and a continuous air supply), and LJ001 (5 μM final concentration) or a carrier reagent (0.005% DMSO, final concentration) was added to challenge containers in the presence of light. After 15 min, nine naive recipient fish were added to each challenge container. Every 24 h, the donor and recipient fish were netted into a new challenge container with fresh water (700 ml) and a new dose of LJ001 (5 μM) or DMSO (0.005%). Following 72 h of cohabitation, fish were euthanized with MS-222 and frozen at −80°C.
Histopathology.
Three fish were exposed to 10 μM LJ001 (or no treatment [negative control]) for 72 h and euthanized as described above. Fish were fixed whole in Davidson's fixative. Seven transverse sections of the head and abdominal cavity and a single longitudinal section of the tail were processed, embedded in paraffin, sectioned at 4 μm, and examined by hematoxylin and eosin (H&E) staining using standard methods (44). Scores defined prior to evaluation were as follows: 0 for no histological abnormalities; 1 for mild, multifocal epithelial hypertrophy and/or hyperplasia in the gills and/or skin; 2 for moderate epithelial hypertrophy and hyperplasia with or without mild inflammation; 3 for moderate epithelial hyperplasia, evident epithelial degeneration or individual-cell necrosis, and/or mild to moderate inflammation; 4 for severe epithelial hyperplasia, individual-cell necrosis, and/or moderate to severe inflammation; and 5 for significant necrosis in any organs or skin ulceration. Histopathologic evaluation and scoring were performed by two veterinary pathologists who were blind to the treatment groups.
Whole-fish processing.
Fish were processed and analyzed as previously described (43). Briefly, fish were thawed and homogenized in MEM by using a stomacher or manual homogenization, and the homogenate was subjected to low-speed centrifugation (1,000 × g). The supernatant was serially diluted for plaque assays as described above. Total RNA was also extracted from the homogenized supernatant (1:4 dilution) by using the Qiagen RNeasy minikit according to the manufacturer's instructions, as previously described (43). Total RNA was eluted in 60 μl of nuclease-free water and stored at −80°C until use. Total RNA was quantified by using a NanoDrop ND-1000 instrument (Thermo Scientific). cDNA was constructed by reverse transcription using random primers from the High Capacity cDNA reverse transcription kit (Applied Biosystems). IHNV N gene quantification was performed via RT-rPCR (43).
Mx-1 expression.
Pectoral fin clips were taken from each fish in the immersion experiment (described above) and stabilized in RNAlater (Qiagen). RNAlater was removed, and magnetic lysing matrix beads (MP Biomedicals) were added to the samples. Tissues were bead homogenized (FastPrep-24 instrument; MP Biomedicals) for 20 s. The homogenate was spun (centrifugation for 10 min at 8,000 rpm), and the supernatant was used for RNA extraction (RNeasy minikit; Qiagen). Total RNA was quantified by using a NanoDrop ND-1000 instrument (Thermo Scientific). cDNA was constructed by reverse transcription as mentioned above. Gene expression levels were measured via qPCR, using probes for the Mx-1 gene, and normalized to the expression levels of the ARP (acidic ribosomal phosphoprotein P0) housekeeping gene (45). Quantification of IHNV N gene copy numbers in fin tissue samples was conducted by RT-rPCR as described above.
Statistical analyses.
Statistical analyses were performed by using GraphPad PRISM 5. One-way analysis of variance (ANOVA) and Dunnett's posttest with comparison to the negative control were used for the cell viability assay (in triplicate). Virus titers and N gene copy numbers were log10 transformed prior to statistical analyses for the remaining assays. ANOVA and Dunnett's posttest with comparison to the positive control were used for the in vitro (n = 3) preincubation and direct application data and for data from the in vivo (5 fish per group) immersion inhibition studies. For time exposure experiments, ANOVA and Tukey's multiple-comparison posttest were used to compare time points for LJ001-treated columns (n = 4). Best-of-fit quadratic curves were applied to data from stability experiments, from which the inhibitory half-life was calculated via the quadratic formula and coordinate plots, and R2 values were generated. Student's t test was applied to data from studies of the inhibition of horizontal transmission (12 donors and 36 recipients per treatment). A nonparametric Kruskal-Wallis test and Dunn's multiple-comparison post hoc test for the negative control were used on data for the upregulation of Mx-1 gene expression (5 fish per group).
ACKNOWLEDGMENTS
We thank Kyle Martin, Doug Dixon, and David Rockefeller (Troutlodge, Inc.) for providing rainbow trout and hatchery water. We are grateful to the WADDL aquaculture section, particularly Katie McMenamin-Snekvik and Andrew Vo, for providing EPC cells and media for in vitro experiments. We also thank Bhadra Murthy Vemulapati from Washington State University (now at Koneru Lakshmaiah University) for his technical assistance in the early stages of the project.
This work was supported by the McCleary Endowment (WSU-VMP, to K.S.) and National Institutes of Health/NIAID grant RO1 AI109022 (to H.C.A.). Use of any trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
We declare that we have no conflicts of interest to disclose.
REFERENCES
- 1.Food and Agriculture Organization of the United Nations. 2014. FAO Yearbook 2012, fishery and aquaculture statistics. Food and Agriculture Organization of the United Nations, Rome, Italy. [Google Scholar]
- 2.Alonso M, Leong JA. 2013. Licensed DNA vaccines against infectious hematopoietic necrosis virus (IHNV). Recent Pat DNA Gene Seq 7:62–65. doi: 10.2174/1872215611307010009. [DOI] [PubMed] [Google Scholar]
- 3.Crane M, Hyatt A. 2011. Viruses of fish: an overview of significant pathogens. Viruses 3:2025–2046. doi: 10.3390/v3112025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amend DF. 1975. Detection and transmission of infectious hematopoietic necrosis virus in rainbow trout. J Wildl Dis 11:471–478. doi: 10.7589/0090-3558-11.4.471. [DOI] [PubMed] [Google Scholar]
- 5.Woo PTK, Leatherland JF, Bruno DW. 2011. Fish diseases and disorders. CABI Publishing, Oxford, United Kingdom. [Google Scholar]
- 6.Phelps NB, Armien AG, Mor SK, Goyal SM, Warg JV, Bhagyam R, Monahan T. 2012. Spring viremia of carp virus in Minnehaha Creek, Minnesota. J Aquat Anim Health 24:232–237. doi: 10.1080/08997659.2012.711267. [DOI] [PubMed] [Google Scholar]
- 7.Dutta S, Chakrabarty U, Mallik A, Mandal N. 2015. White spot syndrome virus (WSSV) prevalence associated with disease resistance among wild populations of black tiger shrimp, Penaeus monodon (Fabricius). Aquacult Res 46:453–461. doi: 10.1111/are.12193. [DOI] [Google Scholar]
- 8.Lo CF, Ho CH, Peng SE, Chen CH, Hsu HC, Chiu YL, Chang CF, Liu KF, Su MS, Wang CH, Kou GH. 1996. White spot syndrome baculovirus (WSBV) detected in cultured and captured shrimp, crabs and other arthropods. Dis Aquat Organ 27:215–225. doi: 10.3354/dao027215. [DOI] [Google Scholar]
- 9.Wagner R. 1987. Rhabdovirus biology and infection, p 9–74. In Wagner R. (ed), The rhabdoviruses. Springer, New York, NY. doi: 10.1007/978-1-4684-7032-1_2. [DOI] [Google Scholar]
- 10.Lapatra SE, Jones GR, Lauda KA, McDowell TS, Schneider R, Hedrick RP. 1995. White sturgeon as a potential vector of infectious hematopoietic necrosis virus. J Aquat Anim Health 7:225–230. doi:. [DOI] [Google Scholar]
- 11.Ludwig M, Palha N, Torhy C, Briolat V, Colucci-Guyon E, Bremont M, Herbomel P, Boudinot P, Levraud JP. 2011. Whole-body analysis of a viral infection: vascular endothelium is a primary target of infectious hematopoietic necrosis virus in zebrafish larvae. PLoS Pathog 7:e1001269. doi: 10.1371/journal.ppat.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winton JR. 1991. Recent advances in detection and control of infectious hematopoietic necrosis virus in aquaculture. Annu Rev Fish Dis 1:83–93. doi: 10.1016/0959-8030(91)90024-E. [DOI] [Google Scholar]
- 13.Wolf K. 1988. Fish viruses and fish viral diseases. Comstock Publishing Associates, Ithaca, NY. [Google Scholar]
- 14.Wedemeyer GA, Nelson NC, Smith CA. 1978. Survival of the salmonid viruses infectious hematopoietic necrosis (IHNV) and infectious pancreatic necrosis (IPNV) in ozonated, chlorinated, and untreated waters. J Fish Res Board Can 35:875–879. doi: 10.1139/f78-140. [DOI] [Google Scholar]
- 15.Anderson ED, Mourich DV, Fahrenkrug SC, LaPatra S, Shepherd J, Leong JA. 1996. Genetic immunization of rainbow trout (Oncorhynchus mykiss) against infectious hematopoietic necrosis virus. Mol Mar Biol Biotechnol 5:114–122. [PubMed] [Google Scholar]
- 16.Heppell J, Davis HL. 2000. Intramuscular injection of DNA vaccines in fish. Methods Mol Med 29:99–103. [DOI] [PubMed] [Google Scholar]
- 17.Kim CH, Johnson MC, Drennan JD, Simon BE, Thomann E, Leong JA. 2000. DNA vaccines encoding viral glycoproteins induce nonspecific immunity and Mx protein synthesis in fish. J Virol 74:7048–7054. doi: 10.1128/JVI.74.15.7048-7054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurath G, Garver KA, Corbeil S, Elliott DG, Anderson ED, LaPatra SE. 2006. Protective immunity and lack of histopathological damage two years after DNA vaccination against infectious hematopoietic necrosis virus in trout. Vaccine 24:345–354. doi: 10.1016/j.vaccine.2005.07.068. [DOI] [PubMed] [Google Scholar]
- 19.Plant KP, LaPatra SE. 2011. Advances in fish vaccine delivery. Dev Comp Immunol 35:1256–1262. doi: 10.1016/j.dci.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Wolf MC, Freiberg AN, Zhang T, Akyol-Ataman Z, Grock A, Hong PW, Li J, Watson NF, Fang AQ, Aguilar HC, Porotto M, Honko AN, Damoiseaux R, Miller JP, Woodson SE, Chantasirivisal S, Fontanes V, Negrete OA, Krogstad P, Dasgupta A, Moscona A, Hensley LE, Whelan SP, Faull KF, Holbrook MR, Jung ME, Lee B. 2010. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc Natl Acad Sci U S A 107:3157–3162. doi: 10.1073/pnas.0909587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vigant F, Lee J, Hollmann A, Tanner LB, Akyol Ataman Z, Yun T, Shui G, Aguilar HC, Zhang D, Meriwether D, Roman-Sosa G, Robinson LR, Juelich TL, Buczkowski H, Chou S, Castanho MA, Wolf MC, Smith JK, Banyard A, Kielian M, Reddy S, Wenk MR, Selke M, Santos NC, Freiberg AN, Jung ME, Lee B. 2013. A mechanistic paradigm for broad-spectrum antivirals that target virus-cell fusion. PLoS Pathog 9:e1003297. doi: 10.1371/journal.ppat.1003297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holthuis JC, Levine TP. 2005. Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol 6:209–220. doi: 10.1038/nrm1591. [DOI] [PubMed] [Google Scholar]
- 23.Mulcahy D, Pascho R, Jenes CK. 1984. Comparison of in vitro growth characteristics of ten isolates of infectious haematopoietic necrosis virus. J Gen Virol 65(Part 12):2199–2207. doi: 10.1099/0022-1317-65-12-2199. [DOI] [PubMed] [Google Scholar]
- 24.Hollmann A, Castanho MA, Lee B, Santos NC. 2014. Singlet oxygen effects on lipid membranes: implications for the mechanism of action of broad-spectrum viral fusion inhibitors. Biochem J 459:161–170. doi: 10.1042/BJ20131058. [DOI] [PubMed] [Google Scholar]
- 25.Garver KA, Batts WN, Kurath G. 2006. Virulence comparisons of infectious hematopoietic necrosis virus U and M genogroups in sockeye salmon and rainbow trout. J Aquat Anim Health 18:232–243. doi: 10.1577/H05-038.1. [DOI] [PubMed] [Google Scholar]
- 26.Hershberger PK, Gregg JL, Grady CA, Hart LM, Roon SR, Winton JR. 2011. Factors controlling the early stages of viral haemorrhagic septicaemia epizootics: low exposure levels, virus amplification and fish-to-fish transmission. J Fish Dis 34:893–899. doi: 10.1111/j.1365-2761.2011.01305.x. [DOI] [PubMed] [Google Scholar]
- 27.Garver KA, Mahony AA, Stucchi D, Richard J, Van Woensel C, Foreman M. 2013. Estimation of parameters influencing waterborne transmission of infectious hematopoietic necrosis virus (IHNV) in Atlantic salmon (Salmo salar). PLoS One 8:e82296. doi: 10.1371/journal.pone.0082296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purcell MK, Laing KJ, Winton JR. 2012. Immunity to fish rhabdoviruses. Viruses 4:140–166. doi: 10.3390/v4010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collet B. 2014. Innate immune responses of salmonid fish to viral infections. Dev Comp Immunol 43:160–173. doi: 10.1016/j.dci.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Verjan N, Ooi EL, Nochi T, Kondo H, Hirono I, Aoki T, Kiyono H, Yuki Y. 2008. A soluble nonglycosylated recombinant infectious hematopoietic necrosis virus (IHNV) G-protein induces IFNs in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 25:170–180. doi: 10.1016/j.fsi.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 31.LaPatra SE, Corbeil S, Jones GR, Shewmaker WD, Lorenzen N, Anderson ED, Kurath G. 2001. Protection of rainbow trout against infectious hematopoietic necrosis virus four days after specific or semi-specific DNA vaccination. Vaccine 19:4011–4019. doi: 10.1016/S0264-410X(01)00113-X. [DOI] [PubMed] [Google Scholar]
- 32.Lorenzen N, Lorenzen E, Einer-Jensen K, LaPatra SE. 2002. Immunity induced shortly after DNA vaccination of rainbow trout against rhabdoviruses protects against heterologous virus but not against bacterial pathogens. Dev Comp Immunol 26:173–179. doi: 10.1016/S0145-305X(01)00059-3. [DOI] [PubMed] [Google Scholar]
- 33.LaPatra SE, Corbeil S, Jones GR, Shewmaker WD, Kurath G. 2000. The dose-dependent effect on protection and humoral response to a DNA vaccine against infectious hematopoietic necrosis (IHN) virus in subyearling rainbow trout. J Aquat Anim Health 12:181–188. doi:. [DOI] [Google Scholar]
- 34.Lorenzen N, LaPatra SE. 2005. DNA vaccines for aquacultured fish. Rev Sci Tech 24:201–213. [PubMed] [Google Scholar]
- 35.Engelking HM, Leong J-AC. 1989. The glycoprotein of infectious hematopoietic necrosis virus elicits neutralizing antibody and protective responses. Virus Res 13:213–230. doi: 10.1016/0168-1702(89)90017-8. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Mourich DV, Engelking HM, Ristow S, Arnzen J, Leong JC. 1991. Epitope mapping and characterization of the infectious hematopoietic necrosis virus glycoprotein, using fusion proteins synthesized in Escherichia coli. J Virol 65:1611–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albertini AA, Baquero E, Ferlin A, Gaudin Y. 2012. Molecular and cellular aspects of rhabdovirus entry. Viruses 4:117–139. doi: 10.3390/v4010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palti Y, Gahr SA, Purcell MK, Hadidi S, Rexroad CE III, Wiens GD. 2010. Identification, characterization and genetic mapping of TLR7, TLR8a1 and TLR8a2 genes in rainbow trout (Oncorhynchus mykiss). Dev Comp Immunol 34:219–233. doi: 10.1016/j.dci.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Anderson E, Clouthier S, Shewmaker W, Weighall A, LaPatra S. 2008. Inactivated infectious haematopoietic necrosis virus (IHNV) vaccines. J Fish Dis 31:729–745. doi: 10.1111/j.1365-2761.2008.00960.x. [DOI] [PubMed] [Google Scholar]
- 40.Fijan N, Sulimanović D, Bearzotti M, Muzinić D, Zwillenberg LO, Chilmonczyk S, Vautherot JF, de Kinkelin P. 1983. Some properties of the epithelioma papulosum cyprini (EPC) cell line from carp Cyprinus carpio. Ann Inst Pasteur Virol 134:207–220. doi: 10.1016/S0769-2617(83)80060-4. [DOI] [Google Scholar]
- 41.Winton J, Batts W, deKinkelin P, LeBerre M, Bremont M, Fijan N. 2010. Current lineages of the epithelioma papulosum cyprini (EPC) cell line are contaminated with fathead minnow, Pimephales promelas, cells. J Fish Dis 33:701–704. doi: 10.1111/j.1365-2761.2010.01165.x. [DOI] [PubMed] [Google Scholar]
- 42.Batts WN, Winton JR. 1989. Enhanced detection of infectious hematopoietic necrosis virus and other fish viruses by pretreatment of cell monolayers with polyethylene glycol. J Aquat Anim Health 1:284–290. doi:. [DOI] [Google Scholar]
- 43.Purcell MK, Thompson RL, Garver KA, Hawley LM, Batts WN, Sprague L, Sampson C, Winton JR. 2013. Universal reverse-transcriptase real-time PCR for infectious hematopoietic necrosis virus (IHNV). Dis Aquat Organ 106:103–115. doi: 10.3354/dao02644. [DOI] [PubMed] [Google Scholar]
- 44.Carson FL. 2015. Histotechnology: a self instructional text. American Society of Clinical Pathology, Chicago, IL. [Google Scholar]
- 45.Purcell MK, Kurath G, Garver KA, Herwig RP, Winton JR. 2004. Quantitative expression profiling of immune response genes in rainbow trout following infectious haematopoietic necrosis virus (IHNV) infection or DNA vaccination. Fish Shellfish Immunol 17:447–462. doi: 10.1016/j.fsi.2004.04.017. [DOI] [PubMed] [Google Scholar]