Abstract
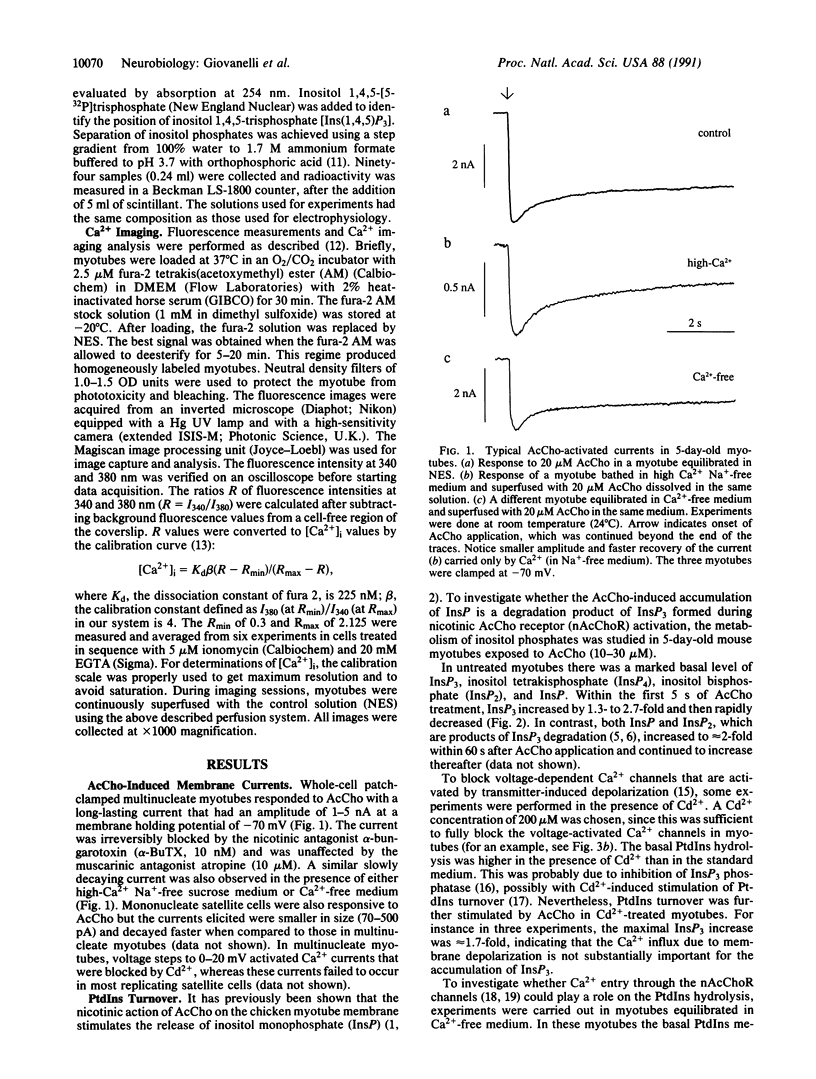
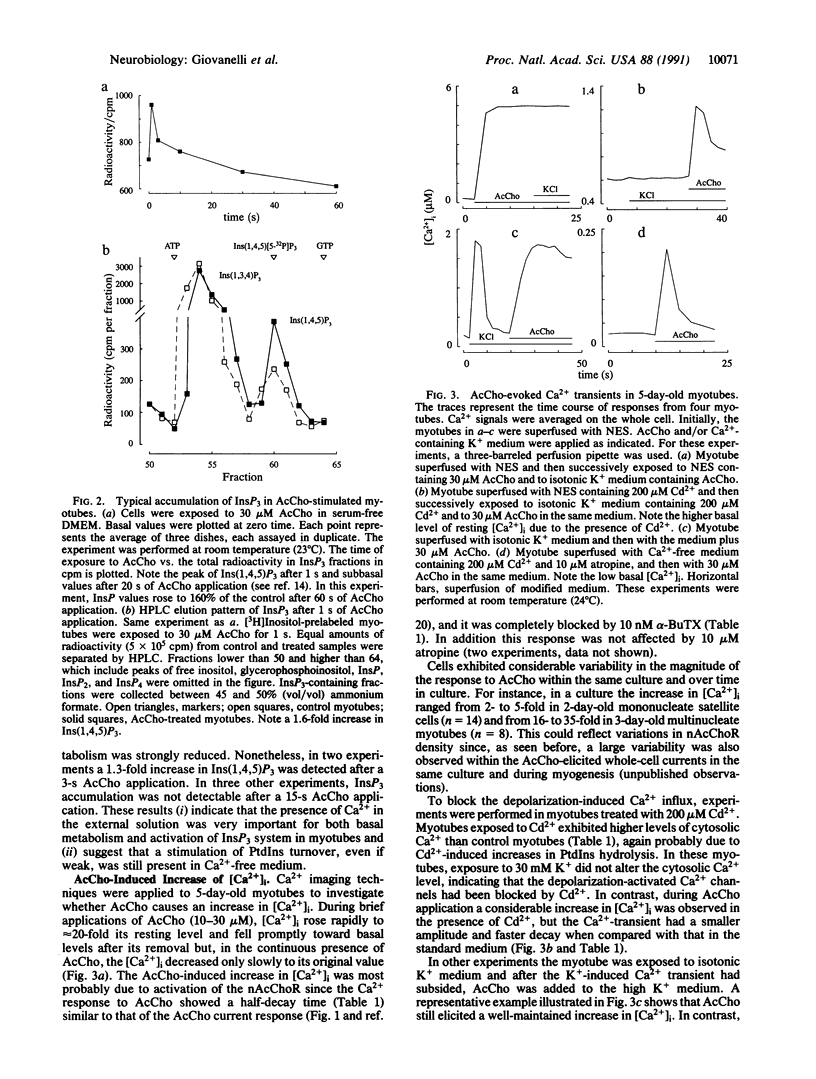
Electrophysiological, biochemical, and Ca2+ imaging studies of cultured mouse myotubes were used to investigate whether the neurotransmitter acetylcholine causes an increase in intracellular Ca2+ concentration ([Ca2+]i) through activation of a second messenger system. Bath applications of acetylcholine to myotubes (i) elicited a significant membrane current even in a Na(+)-free Ca2+ medium, when the current was carried mainly by calcium ions; (ii) caused a rapid and transient cytosolic accumulation of inositol 1,4,5-trisphosphate; (iii) evoked a conspicuous alpha-bungarotoxin-sensitive long-lasting [Ca2+]i enhancement even in the presence of Cd2+; and (iv) transiently increased [Ca2+]i when cells were equilibrated in a Ca(2+)-free atropine-containing medium. We propose that, in addition to opening ion channels, the nicotinic action of acetylcholine on the muscle cell membrane increases [Ca2+]i through activation of the inositol 1,4,5-trisphosphate second messenger system and mobilization of Ca2+ from intracellular stores.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamo S., Zani B. M., Nervi C., Senni M. I., Molinaro M., Eusebi F. Acetylcholine stimulates phosphatidylinositol turnover at nicotinic receptors of cultured myotubes. FEBS Lett. 1985 Oct 7;190(1):161–164. doi: 10.1016/0014-5793(85)80449-x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Chilvers E. R., Challiss R. A., Barnes P. J., Nahorski S. R. Mass changes of inositol(1,4,5)trisphosphate in trachealis muscle following agonist stimulation. Eur J Pharmacol. 1989 May 30;164(3):587–590. doi: 10.1016/0014-2999(89)90269-0. [DOI] [PubMed] [Google Scholar]
- Eberhard D. A., Holz R. W. Intracellular Ca2+ activates phospholipase C. Trends Neurosci. 1988 Dec;11(12):517–520. doi: 10.1016/0166-2236(88)90174-9. [DOI] [PubMed] [Google Scholar]
- Eusebi F., Grassi F., Nervi C., Caporale C., Adamo S., Zani B. M., Molinaro M. Acetylcholine may regulate its own nicotinic receptor-channel through the C-kinase system. Proc R Soc Lond B Biol Sci. 1987 Apr 22;230(1260):355–365. doi: 10.1098/rspb.1987.0024. [DOI] [PubMed] [Google Scholar]
- Eusebi F., Molinaro M., Zani B. M. Agents that activate protein kinase C reduce acetylcholine sensitivity in cultured myotubes. J Cell Biol. 1985 Apr;100(4):1339–1342. doi: 10.1083/jcb.100.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannelli A., Farini D., Gauzzi M. C., Alema S., Eusebi F. Regulation of acetylcholine receptor desensitization in mouse myotubes by cytosolic cyclic AMP. Cell Signal. 1990;2(4):347–352. doi: 10.1016/0898-6568(90)90064-h. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Glutamate and kainate receptors induced by rat brain messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):127–143. doi: 10.1098/rspb.1984.0027. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Houamed K. M., Kuijper J. L., Gilbert T. L., Haldeman B. A., O'Hara P. J., Mulvihill E. R., Almers W., Hagen F. S. Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science. 1991 May 31;252(5010):1318–1321. doi: 10.1126/science.1656524. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Spontaneous and evoked activity of motor nerve endings in calcium Ringer. J Physiol. 1969 Aug;203(3):689–706. doi: 10.1113/jphysiol.1969.sp008887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M., Tanabe Y., Tsuchida K., Shigemoto R., Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991 Feb 28;349(6312):760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- Milani D., Guidolin D., Facci L., Pozzan T., Buso M., Leon A., Skaper S. D. Excitatory amino acid-induced alterations of cytoplasmic free Ca2+ in individual cerebellar granule neurons: role in neurotoxicity. J Neurosci Res. 1991 Mar;28(3):434–441. doi: 10.1002/jnr.490280317. [DOI] [PubMed] [Google Scholar]
- Miledi R. Intracellular calcium and desensitization of acetylcholine receptors. Proc R Soc Lond B Biol Sci. 1980 Sep 26;209(1176):447–452. doi: 10.1098/rspb.1980.0106. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Woodward R. M. Membrane currents elicited by divalent cations in Xenopus oocytes. J Physiol. 1989 Oct;417:173–195. doi: 10.1113/jphysiol.1989.sp017796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Sladeczek F., Pin J. P., Récasens M., Bockaert J., Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985 Oct 24;317(6039):717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- Strange P. G. The structure and mechanism of neurotransmitter receptors. Implications for the structure and function of the central nervous system. Biochem J. 1988 Jan 15;249(2):309–318. doi: 10.1042/bj2490309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescence measurement and photochemical manipulation of cytosolic free calcium. Trends Neurosci. 1988 Oct;11(10):419–424. doi: 10.1016/0166-2236(88)90192-0. [DOI] [PubMed] [Google Scholar]
- Vergara J., Tsien R. Y., Delay M. Inositol 1,4,5-trisphosphate: a possible chemical link in excitation-contraction coupling in muscle. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6352–6356. doi: 10.1073/pnas.82.18.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]



