Abstract
Heart failure with reduced ejection fraction (HFrEF) develops when cardiac output is reduced as a result of cardiac injury. The most well-recognized of the compensatory homeostatic responses to a fall in cardiac output are activation of the sympathetic nervous system and the renin–angiotensin–aldosterone system (RAAS). In the short-term, these ‘neurohormonal’ systems induce a number of changes in the heart, kidneys, and vasculature that are designed to maintain cardiovascular homeostasis. However, with chronic activation, these responses result in haemodynamic stress and exert deleterious effects on the heart and the circulation. Neurohormonal activation is now known to be one of the most important mechanisms underlying the progression of heart failure, and therapeutic antagonism of neurohormonal systems has become the cornerstone of contemporary pharmacotherapy for heart failure. In this Review, we discuss the effects of neurohormonal activation in HFrEF and highlight the mechanisms by which these systems contribute to disease progression.
Heart failure with reduced ejection fraction (HFrEF) classically develops after an ‘index event’ that reduces cardiac pump function. The index event could be an acute injury to the heart, such as a myocardial infarction; or might develop slowly, as with long-standing haemodynamic overload; or occur in response to genetic variations that disrupt contractile function or lead to sarcolemmal fragility and myocyte death. The circulatory changes that arise from impaired myocardial pump function are sensed by peripheral arterial baroreceptors as ‘underfilling’ of the circulation. More recent studies have suggested an important role for peripheral chemoreceptors and ergoreceptors. These sensory receptors activate a series of compensatory mechanisms that lead to changes in heart rate and cardiac contractility, salt and water retention, and constriction of the peripheral blood vessels. These alterations work collectively to maintain cardiovascular homeostasis (FIGURE 1). The compensatory mechanisms that have been described thus far include: activation of the sympathetic (adrenergic) nervous system (SNS) and renin–angiotensin–aldosterone system (RAAS), which maintain cardiac output through increased retention of salt and water, peripheral arterial vasoconstriction and increased contractility; and inflammatory mediators1 that are involved in cardiac repair and remodelling. In addition, HFrEF is characterized by important changes in several systems that normally antagonize SNS and RAAS activation, such as loss of parasympathetic tone and increased resistance to natriuretic peptides. Collectively, these responses are referred to as ‘neurohormonal activation’2. The term ‘neurohormone’ reflects the original observation that many of the molecules that are elaborated in HFrEF are produced by the neuroendocrine system and affect the heart in an endocrine manner. However, many of classical neurohormones such as norepinephrine and angiotensin II are now known to be synthesized directly within the myocardium and, therefore, act in an autocrine or paracrine manner. The important unifying concept that arises is that the overexpression of biologically active molecules contributes to disease progression through deleterious effects on the heart and circulation. These concepts were formalized by Packer in 1992 as the ‘neurohormonal hypothesis’, which states that disease progression is caused by the deleterious effects of sustained neurohormonal activation on the heart and the circulation3. In this Review, we discuss the mechanisms by which neurohormonal activation drives the progression of HFrEF.
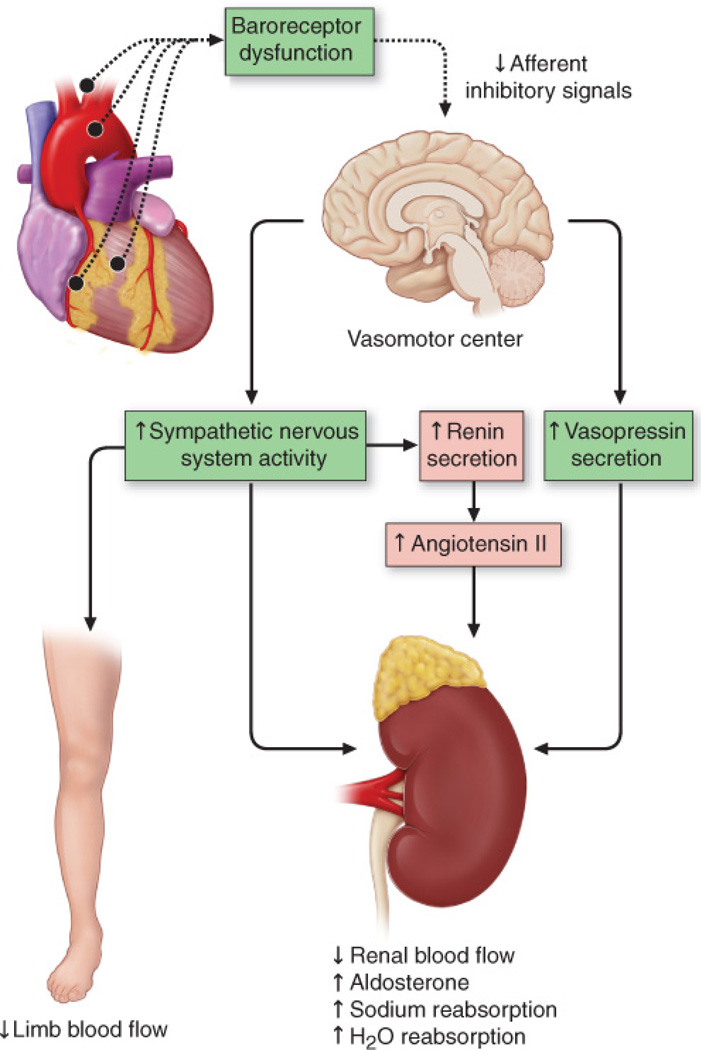
Figure 1. Activation of neurohormonal systems in heart failure.
Decreased cardiac output in patients with heart failure with reduced EF results in the unloading of high-pressure baroceptors (black circles) in the left ventricle, carotid sinus, and aortic arch. This unloading leads to generation of afferent signals to the central nervous system (CNS) that, in turn, lead to activation of efferent sympathetic nervous system pathways that innervate the heart, kidney, peripheral vasculature, and skeletal muscles. This unloading also leads to afferent signals to the CNS that stimulate cardioregulatory centers in the brain that stimulate the release of arginine vasopression from the posterior pituitary. (Modified from Mann, D.L. & Chakinala, M. Heart failure and cor pulmonale in Harrison's Principles of Internal Medicine (Kasper, D.L., Fauci, A.S., Hauser, S.L., Longo, D.L., Jameson, J.L. & Loscalzo, J,) 1500–1506 (McGraw Hill Medical, 2015).
Neurohormonal activation
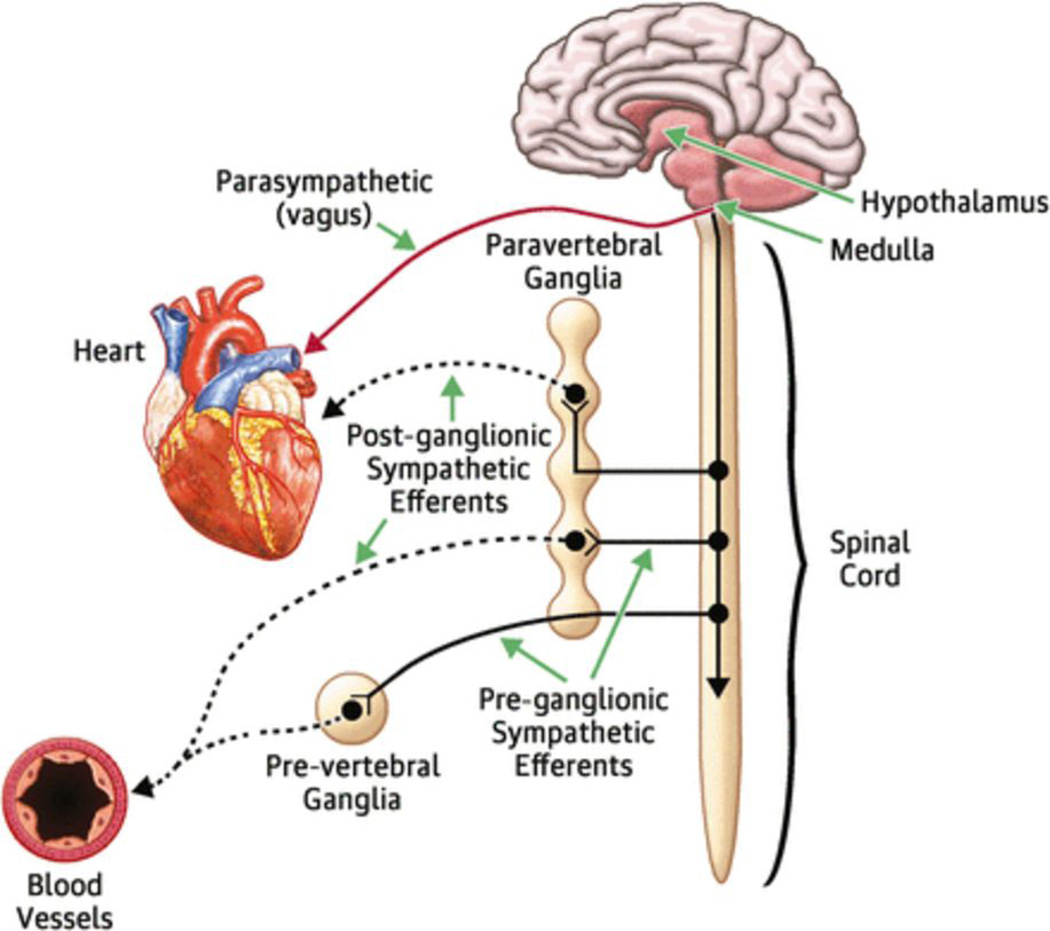
Control of circulating blood volume is a tightly regulated physiological variable, and is critical for maintaining cardiovascular homeostasis. Changes in the ‘effective’ arterial blood volume of the peripheral circulation are sensed by baroreceptors located in the aorta and carotid sinus. In healthy individuals, ‘high pressure’ carotid sinus and aortic arch baroreceptors and ‘low pressure’ cardiopulmonary mechanoreceptors provide inhibitory signals to the central nervous system that repress the sympathetic outflow to the heart and peripheral circulation. Baroreceptor activity decreases in response to alterations in the pumping capacity of the heart, the effective circulating arterial volume, or both. The result is a withdrawal of parasympathetic tone and a reflex increase in sympathetic tone that leads to increased heart rate and contractility, as well as peripheral vasoconstriction.
Additional neurogenic inputs are now known to be involved in the development of the imbalance between the sympathetic and parasympathetic nervous systems in heart failure. Patients with heart failure have increased chemosensitivity to hypoxia and hypercapnia and enhanced ergoreflex activity (that is, enhanced sensitivity to the metabolic effects of muscle work)4,5. Increased chemosensitivity correlates with increased neurohormonal activation, worsened functional capacity, and decreased survival5. Augmented ergoreceptor reflexes are associated with worsened symptoms and reduced exercise tolerance (peak VO2)6. All of these neurogenic systems are likely to have a role in sustained neurohormonal activation, although the relative contribution of each system is unclear. We refer the reader to the Review by Floras and Ponikowski for a more detailed discussion of imbalance between the sympathetic and parasympathetic nervous systems in HFrEF7. Although the activation of these ‘neurohomonal’ systems evolved to maintain cardiovascular homeostasis in the short-term, the extant literature suggests that these compensatory mechanisms can cause additional damage to the heartand circulation when they are sustained8,9. Further, the degree of neurohormonal activation correlates with disease severity and clinical prognosis in heart failure10–12.
Two separate lines of evidence support the point of view that neurohormonal activation is not simply a marker of disease severity, but also mediates worsening of heart failure. First, a number of animal models have demonstrated that the concentrations of circulating neurohormones that are detected in patients with heart failure are sufficient to promote left ventricular (LV) dysfunction and remodeling13–18. Second, pharmacological antagonism of neurohormonal systems leads to improved outcomes in patients with HFrEF19–24. Indeed, antagonism of neurohormonal systems forms the basis for contemporary treatment of heart failure25.
Effects of neurohormonal activation
One of the most important neurohormonal adaptations in heart failure is activation of the SNS, which occurs very early in the course of the disease. There are increased circulating levels of the adrenergic neurotransmitter NE secondary to increased SNS signaling and NE release from adrenergic nerves with subsequent ‘spillover’ into the plasma, as well as reduced uptake by adrenergic nerve endings. As discussed below, sustained activation of the SNS exerts deleterious effects on the heart, kidneys, and peripheral vasculature (FIGURE 2). Increased SNS activation also has important effects on skeletal muscle, which are beyond the scope of this Review (please see the Review by Notarius et al.26 for a discussion of these effects).
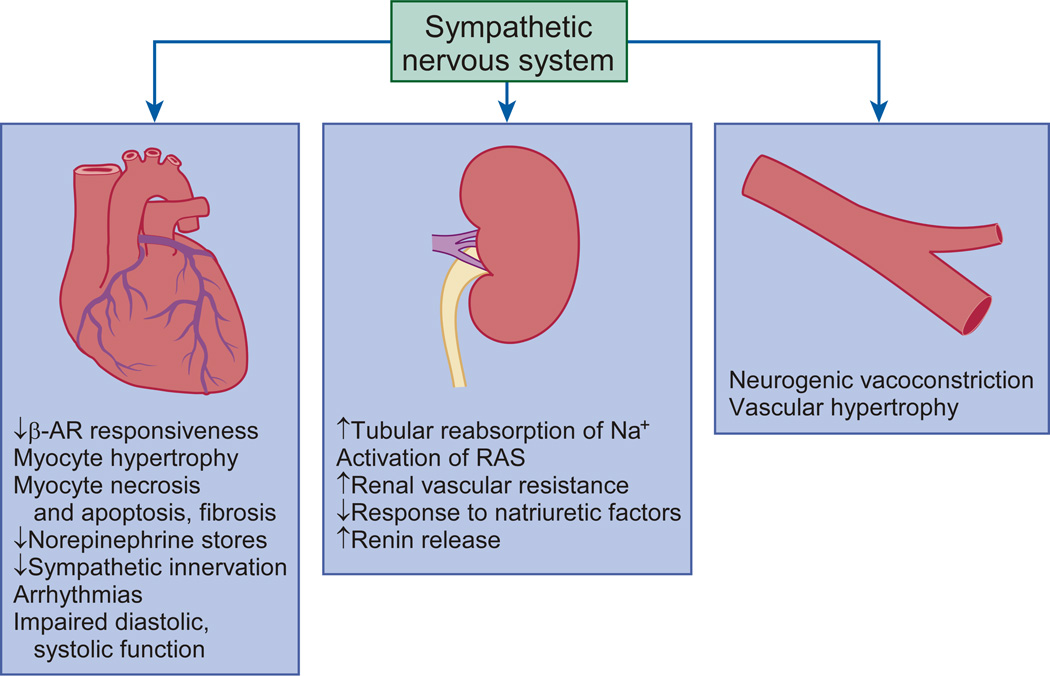
Figure 2. Effects of sympathetic nervous system activation.
Increased sympathetic nervous system (SNS) activity contributes to the pathophysiology of heart failure through multiple mechanisms involving cardiac, renal, and vascular function. In the heart, increased SNS outflow leads to desensitization of the βAR, myocyte hypertrophy, necrosis, apoptosis, and fibrosis. In the kidneys, increased sympathetic activation induces arterial and venous vasoconstriction, activation of the RAAS, increase in salt and water retention, and an attenuated response to natriuretic peptides. In the peripheral vessels, increased SNS activity induces neurogenic vasoconstriction and vascular hypertrophy. (From Hassefuss, G. & Mann, D.L. Pathophysiology of heart failure in Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (Mann, D.L., Zipes, D., Libby P.L. & Bonow, R.L.) 454–472 (Elsevier/Saunders, 2014). βAR, β-adrenergic receptors; RAAS, renin–angiotensin–aldosterone system.
Renal function
Increasing salt and water retention by the kidneys, leading to pulmonary and peripheral oedema, are hallmarks of worsening heart failure. This oedematous state is not the result of intrinsic renal dysfunction, per se, but rather an orchestrated response to increased SNS traffic to the kidney. Increased sympathetic activity leads to peripheral vasoconstriction of the afferent renal artery and decreased blood flow to the juxtaglomerular apparatus in the kidney, with resultant release of renin into the afferent arteriole by the juxtaglomerular apparatus. Increased SNS traffic to the kidney also activates β1-adrenergic receptors on the juxtaglomerular apparatus, which further stimulates the release of renin27. Angiotensinogen formed in the liver is converted by renin to angiotensin I (decapeptide). Angiotensin-converting enzyme (ACE) then cleaves two peptides from angiotensin I to form the octapeptide angiotensin II, a potent vasoconstrictor. Angiotensin-II-mediated stimulation of the angiotensin II receptor type 1 (AT1) in the zona glomerulosa of the adrenal glands leads to the release of aldosterone, which has important effects in the pathogenesis of heart failure. Collectively, upregulation of the components of the RAAS is referred to as ‘RAAS activation’.
Activation of the RAAS leads to increased salt and water retention through multiple mechanisms. Angiotensin II directly causes sodium retention at the proximal tubule, whereas aldosterone leads to increased sodium resorption in the distal tubule. Angiotensin II also stimulates the thirst center of the brain and provokes the release of arginine vasopressin (AVP), which plays a critical role in the determination of free water clearance in the kidney. Normally in the setting of increased osmolality AVP is released resulting in increased water retention, which returns osmolality to its normal physiological set point. However, AVP levels are inappropriately elevated in many patients with heart failure28. This so-called nonosmotic release of AVP contributes to the development of hyponatremia in patients with heart failure, as well increases in peripheral vasoconstriction and endothelin production28.
Evidence suggests that additional short-chain peptides derived from angiotensin II—including the heptapeptide angiotensin III, and the hexapeptides angiotensin IV and angiotensin1–7—serve as effectors in the RAAS. Whereas angiotensin III binds to AT1, angiotensin 1–7 and angiotensin IV have specific receptors of their own. Although the exact role these short-chain peptides in clinical heart failure is not known, in experimental models angiotensin 1–7 seems to counteract the effects of angiotensin II, and attenuates LV remodelling29. Angiotensin III directly stimulates the zona glomerulosa of the adrenal glands to produce aldosterone30, which promotes sodium resorption in the distal collecting duct of the kidney. Angiotensin III also has an important role in vasopressin release in the brain, which controls water retention in the distal collecting duct of the kidney. Angiotensin III in the brain can also modulate cardiac nervous sympathetic hyperactivity, and LV remodelling after myocardial infarction31. Angiotensin IV is a metabolite of angiotensin III and might be involved in regulating vascular smooth muscle cell growth or regulating genes involved in atherogenesis and thrombosis32.
Interestingly, despite the use of ACE inhibitors and angiotensin-receptor blockers (ARBs), aldosterone levels increase in ~30–40% patients with heart failure33. This phenomenon is often referred to as ‘aldosterone escape’, but more accurately should be referred to as ‘aldosterone breakthrough’34. Although circulating levels of aldosterone initially decrease in patients who are treated with ACE inhibitors and ARBs, in some patients aldosterone levels will increase to greater than pretreatment levels. Although the mechanism of aldosterone breakthrough is thought to be secondary to an increased level of renin, the exact pathophysiology is not yet known. Indeed, aldosterone breakthrough might explain the effectiveness of mineralocorticoid receptor antagonists in the RALES and EPHESUS trials21,35.
A variety of feedback mechanisms are normally activated to offset the effects of the RAAS on sodium and water retention. Atrial natriuretic peptide and brain natriuretic peptide are among the most important RAAS counter-regulatory hormones36. These peptides, which are produced in response to myocardial or atrial stretch, lead to increased cGMP production in target cells and functionally unload the heart through peripheral vasodilation, as well as by inducing renal excretion of sodium and water. However, peripheral resistance to the natriuretic peptides occurs in the setting of heart failure. The RAAS, therefore, remains unopposed, contributing to the development of vasoconstriction and volume overload in these patients28,37–39. The importance of natriuretic peptide resistance is highlighted by the development of the new class of angiotensin receptor–neprilysin inhibitors for the treatment of heart failure36,40. Neprilysin is a membrane bound enzyme (peptidase) that catalyzes the degradation of a number of endogenous peptides, most notably the natriuretic peptides, as well as amyloid β peptide, angiotensin II, bradykinin, and substance P. In the PARADIGM trial40, the use of an angiotensin receptor–neprilysin inhibitor (LCZ696, which is a fixed-dose combination of valsartan and sacubitril (AHU377)) resulted in striking reductions in all-cause mortality, cardiovascular mortality, and heart failure hospitalizations when compared with the use of an ACE inhibitor (enalapril) alone.
Peripheral vasculature
Peripheral vascular resistance and the relative distribution of blood flow to various organs are controlled by a combination of systemic factors, including the SNS and RAAS, as well as local autoregulatory mechanisms. Heightened activity of the adrenergic nervous system leads to stimulation of α1-adrenergic receptors, which causes peripheral arterial vasoconstriction (FIGURE 2). Other potent vasoconstrictors relevant in heart failure include angiotensin II, AVP, endothelin, neuropeptide Y, and urotensin II. Arteriolar vasoconstriction helps maintain blood pressure, whereas local autoregulatory mechanisms ensure adequate tissue perfusion to vital organs. In heart failure, these homeostatic mechanisms preserve blood flow to the brain and heart, while shunting blood flow away from the skeletal muscles, skin, splanchnic organs, and kidneys. In addition, neurohormonal activation results in increased venous tone, which is intended to augment cardiac output through increased preload to the heart (Frank-Starling law). However, the increase in venous return can lead to an unwanted increase in LV filling pressure, whereas the increased peripheral vasoconstriction leads to increased afterload. These haemodynamic changes contribute to adverse LV remodelling, worsening LV pump performance, and increased neurohormonal activation. Importantly, many of the counter-regulatory vasodilator responses provided by apelin, bradykinin, natriuretic peptides, nitric oxide, and vasodilatory prostaglandins become blunted in the setting of heart failure, resulting in sustained vasoconstriction.
LV remodelling and myocyte biology
The term ’LV remodelling’ refers to the changes in LV mass, volume, and shape, and in the composition of the heart that occur after cardiac injury or abnormal haemodynamic loading conditions. The mechanical burdens engendered by the changes in LV geometry contribute independently to the progression of heart failure. As will be discussed below, sustained neurohormonal activation leads to a number of cellular and molecular changes in the heart that directly contribute to LV remodelling (BOX 1). These changes include changes in myocyte biology, energetics, and metabolism; and progressive loss of myocytes through apoptosis, autophagic cell death, and necrosis. In addition, reorganization of the extracellular matrix occurs, with dissolution of the organized structural collagen weave surrounding the myocytes and subsequent replacement by an interstitial collagen matrix that does not provide structural support to the myocytes.
Box 1. Myocardial changes in LV remodelling.
Alterations in myocyte biology
Hypertrophy
Myosin heavy chain (fetal) gene expression
Myocytolysis
Changes in cytoskeletal proteins
β-Adrenergic desensitization
Excitation–contraction coupling
Myocardial changes
Myocyte loss
Necrosis
Apoptosis
Autophagy
Alterations in the extracellular matrix
Matrix degradation
Myocardial fibrosis
Alterations in LV chamber geometry
Increased size
Increased sphericity
Wall thinning
Mitral valve incompetence
LV, left ventricular.
Numerous studies have shown that failing human cardiac myocytes undergo a number of important biological changes that might lead to a progressive loss of contractile function. These changes are discussed in detail below and include myocyte hypertrophy; desensitization of β-adrenergic signalling; decreased α-myosin heavy chain gene expression with a concomitant increase in β-myosin heavy chain gene expression; changes in excitation–contraction coupling and energy metabolism; as well as progressive loss of myofilaments in cardiac myocytes (myocytolysis); and alterations in cytoskeletal proteins (BOX 1). A full description of these changes is beyond the scope of this Review, and the reader is referred to two published articles on the subject41,42.
Cardiac myocyte hypertrophy
As shown in FIGURE 3, two basic patterns of cardiac hypertrophy occur in response to haemodynamic overloading. In HFrEF, the failing heart progressively dilates, referred to as ‘eccentric hypertrophy’ because of the lateral (that is, eccentric) location of the heart in the thorax. Eccentric hypertrophy develops in response to volume overload. Individual myocytes develop an elongated appearance and are characterized by the addition of sarcomeres in series43. By contrast, ‘concentric hypertrophy’, which develops in response to pressure overload, leads to the addition of sarcomeres in parallel. The signalling pathways responsible for pathologic cardiac hypertrophy have been extensively studied and reviewed44.
Figure 3. Cardiac and cellular remodelling in response to haemodynamic overloading.
The pattern of remodelling depends on the nature of the inciting stimulus. a | When overload is predominantly caused by an increase in pressure (e.g. with systemic hypertension or aortic stenosis), the increase in systolic wall stress leads to the parallel addition of sarcomeres and widening of the cardiac myocytes, resulting in ‘concentric hypertrophy’. When the overload is predominantly caused by an increase in ventricular volume, the increase in diastolic wall stress leads to the series addition of sarcomeres, lengthening of cardiac myocytes, and left ventricular dilatation, which is referred to as ‘eccentric hypertrophy’. b | Phenotypically distinct changes occur in the morphology of the myocyte in response to the type of haemodynamic overload. When the overload is predominantly caused by an increase in pressure the increase in systolic wall stress leads to the parallel addition of sarcomeres and widening of the cardiac myocytes. When the overload is predominantly caused by an increase in ventricular volume, the increase in diastolic wall stress leads to the series addition of sarcomeres, and thus lengthening of cardiac myocytes. The expression of maladaptive embryonic genes is increased in both eccentric and concentric hypertrophy, but not in physiologic myocyte hypertrophy that occurs with exercise. (From Hassefuss, G. & Mann, D.L. Pathophysiology of heart failure in Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (Mann, D.L., Zipes, D., Libby P.L. & Bonow, R.L.) 454–472 (Elsevier/Saunders, 2014).
Myocyte hypertrophy is also associated with changes in sarcomere composition and architecture. Some of the more pathophysiologically relevant changes include a decrease in α-myosin heavy chain gene (MYH6) expression with an increase in β-myosin heavy chain gene (MYH7)45 expression, which might contribute to decreased contractility. Alterations in the expression or activity of myofilament regulatory proteins has also been proposed as a potential mechanism for the decrease in cardiac contractile function in heart failure, including changes in the myosin light chains, the troponin–tropomyosin complex, and titin. In patients with end-stage heart failure, a loss contractile proteins and functional sarcomeres, termed myocytolysis, occurs46. To what extent this phenomenon occurs at earlier stages of the disease is unclear. However, sarcomere disarray is routinely observed in many disease models, and probably contributes to contractile dysfunction. Moreover, changes to the cytoskeleton, which include increased changes in protein expression and/or disorganization, might also contribute to disease progression47.
β-Adrenergic receptor desensitization
Adrenergic stimulation, primarily through activation of β-adrenergic receptors, is a critical determinant of myocardial performance that enables acute changes in cardiac output to be made. In the healthy human heart, the β1-adrenergic receptor is the predominant subtype (70–80%); the remaining 20–30% of adrenergic receptors are the β2 subtype. These receptors are members of the G-protein-coupled receptor family48,49. β1-Receptor stimulation leads to activation of Gs-protein signalling, which increases adenylate cyclase activity resulting in accumulation of cAMP. β2-Receptors drive a unique set of responses as these receptors link not only to Gs-proteins, but also Gi-proteins, which inhibit adenylate cyclase.
The chronic adrenergic stimulation that occurs in heart failure results in important changes in β-adrenergic receptor signalling49–51. The failing heart exhibits decreased β-receptor-mediated adenylate cyclase stimulation and contractile responses52. This decreased responsiveness is related to changes in receptor expression and function. In the failing heart, expression of the β1-receptor is decreased, with the ratio of β1-receptors to β2-receptors being close to 50:5053. Moreover, adrenergic receptors of both subtypes undergo functional desensitization, leading to uncoupling from downstream G-protein activation48,51.
These changes in receptor biology are caused by members of the G-protein-coupled receptor kinase (GRK) and β-arrestin gene families54. GRK2, also known as β-adrenergic receptor kinase 1 (βARK1), phosphorylates the cytoplasmic loops of the β1-adrenergic and β2-adrenergic receptors. Phosphorylation increases the affinity of the receptors for β-arrestin. β-Arrestin binding both uncouples the receptor from downstream signalling events and leads to internalization of the receptor. The internalized receptor can then either be degraded or recycled back to the cell membrane. Myocardial levels of βARK1 have been shown to be increased in the failing heart, and these increases are blocked by treatment with β-blockers55,56. Experimental studies have shown that inhibition of βARK1 not only restores adrenergic sensitivity, but also improves contractile function of the heart and survival57–60.
Transcriptional reprogramming of cardiac myocytes
Changes in gene expression in failing cardiac myocytes can be caused by several factors including neurohormonal activation and exposure to mechanical stress, proinflammatory cytokines, and reactive oxygen species, which collectively lead to impaired myocyte contractility61. One of the most prominent changes is the switch in myosin isoforms from MYH6 to MYH7, as discussed above. Change in expression of genes involved in excitation–contraction coupling (discussed in the next section) and in cardiac metabolism also occur. The metabolic changes result in glucose being used as the primary fuel in the heart instead of fatty acids, which are the usual substrate in the healthy adult heart. Importantly, treatment with β-blockers, ACE inhibitors, and ARBs results in a partial normalization of transcriptional programme in the failing heart62–64.
Excitation–contraction coupling
Excitation–contraction coupling refers to the series of events that begins with an action potential and ends with myocyte contraction and relaxation65. Calcium cycling is critical to this process, and the magnitude of the calcium transient is a major determinant of contractility. The action potential depolarizes the membrane causing opening of voltage-gated calcium channels. Calcium ions (Ca2+) entering the myocyte through the L-type calcium channel activate the ryanodine receptor 2 (RyR2), which serves as a calcium-gated channel that releases Ca2+ from the sarcoplasmic reticulum. The resultant rise in cytosolic Ca2+ concentration allows actin–myosin cross-bridging. Ca2+ is then primarily pumped back into the sarcoplasmic reticulum by the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a). In addition, a small amount of Ca2+ is pumped into the extracellular space by the sodium–calcium exchanger.
Depletion of Ca2+ in the sarcoplasmic reticulum and impaired calcium transit contribute to impaired ventricular function in heart failure65. Activation of protein kinase A downstream from β-adrenergic receptor signaling results in the phosphorylation of a number of proteins involved in excitation–contraction coupling, including RyR266. Increased phosphorylation of RyR2 results in dissociation of calstabin2 (also known as FK506 binding protein 12.6), which destabilizes the closed state of the RyR2 resulting in diastolic Ca2+ leak66. Depletion of Ca2+ stores in the sarcoplasmic reticulum is caused not only by diastolic leak, but also by impaired reuptake of Ca2+ into the sarcoplasmic reticulum. In the failing heart, SERCA2a expression and function are decreased67,68. The decease in function is due in part to decreased phosphorylation of phospholamban, resulting in increased phospholamban-dependent inhibition of SERCA2a activity. Treatment with β-blockers restores SERCA2a expression and RyR2 function62,69,70.
Changes in the myocardium
The failing myocardium undergoes changes in cellular composition and in the extracellular matrix that, coupled with alterations in myocyte biology, result in adverse LV remodelling—chamber dilation, wall thinning, and increased sphericity. These changes in chamber geometry contribute to LV dysfunction and disease progression71. The role of neurohormonal activation in the pathophysiology of LV remodelling and heart failure is increasingly being recognized.
Cellular composition of the failing heart
The adult mammalian heart has minimal capacity to regenerate; therefore progressive loss of myocytes leads to impaired cardiac function. Myocyte death by various mechanisms, including necrosis, apoptosis, and autophagic cell death, has been observed in end-stage human heart failure72,73. The rate of cell death is increased in patients with heart failure compared with healthy individuals, but is much lower than in those with acute myocardial infarction74. The relative importance of myocyte death in the failing heart could, therefore, be questioned. However, several lines of evidence indicate that progressive myocyte cell loss is likely to contribute to disease progression in patients with heart failure74.
Necrosis was originally thought to be a passive mode of cell death, but is now known to occur in a regulated manner75. Necrotic cell death is characterized by cytoplasmic swelling with eventual rupture of the plasma membrane, as well as swelling of organelles including the mitochondria. Necrosis in the heart is accompanied by an increase in cytosolic Ca2+ levels, resulting in excessive activation of the crossbridges and the formation of stereotypical contraction bands. Neurohormonal activation leads to necrotic myocyte cell death. Pathophysiologically relevant concentrations of norepinephrine and angiotensin II are sufficient to induce cardiac myocyte necrosis13,76,77. Necrotic cell death not only results in myocyte loss, but can also contribute to inflammation in the heart through the release of intracellular molecules termed ‘damage-associated molecular patterns’. These molecules are recognized by the innate immune system, resulting in a sterile inflammatory reaction that can cause further tissue damage and additional myocyte death78,79.
Although increased myocyte apoptosis has been consistently observed in failing hearts, the absolute rate of this type of cell death varies considerably72,73,80,81. Much of the existing data comes from studies of advanced heart failure, and the rate of apoptosis at early stages of the disease is less clear. In transgenic mice overexpressing caspase-8, a chronic low rate of apoptosis (similar to that seen in end-stage human heart failure) was sufficient for the development of dilated cardiomyopathy.82 This finding suggests that ongoing apoptosis, even at a low level, could contribute to the progression of heart failure. Several studies have demonstrated that neurohormonal activation is sufficient to drive apoptosis. The β1-adrenergic receptor mediates an increase in apoptosis, whereas the β2-adrenergic receptor actually protects against apoptosis83. Angiotensin II and tumor necrosis factor have also been shown to induce cardiac myocyte apoptosis84,85.
Autophagic cell death is morphologically defined as cell death occurring with massive autophagic vacuolization of the cytoplasm. Although observed in human heart failure, this mode of cell death is less well-understood than necrosis and apoptosis73. Autophagy is generally thought of as a cell survival pathway activated by a variety of stresses that catalyzes the breakdown of intracellular organelles and proteins86. The process is important in quality control of organelles and long-lived proteins and can be used to generate fuel at times of stress. However, whether autophagy occurs with cell death or actively contributes to the death of the cell, is unclear75. In the setting of cardiovascular biology, the role of autophagy in response to various insults is still being clarified87. In response to ischaemia/reperfusion injury impaired autophagic flux has been linked to cardiac myocyte cell death88.
Extracellular matrix
The organized structure of the heart is determined by a network of extracellular matrix proteins. The extracellular matrix provides a scaffold for the orderly arrangement of myocytes and myofibrils and is critical in the transmission of contractile force89. Several changes to the extracellular matrix occur in heart failure—including alterations in collagen synthesis, degradation, and crosslinking—all of which have been linked to neurohormonal activation and contribute to LV remodelling. Any disturbance in the balance between extracellular matrix breakdown and production can have a detrimental effect on myocardial function. Increased fibrosis results in a stiffened ventricle and can distort tissue architecture. Conversely, increased extracellular matrix breakdown can impair force transduction, and loss of scaffolding contributes to ventricular dilation.
In the healthy heart, the extracellular matrix is maintained by resident fibroblasts. Tissue injury leads to accumulation of myofibroblasts, which share characteristics of both fibroblasts and smooth muscle cells, and are involved in collagen production and fibrosis. Myofibroblast accumulation and activation are controlled by a complex interaction of neurohormonal, mechanical, and inflammatory signals90,91. RAAS activation is a potent stimulus for myocardial fibrosis. Angiotensin II acts directly through the AT1 receptor to drive fibroblast division and collagen production92,93. Treatment with ACE inhibitors or aldosterone antagonists has been shown to decrease cardiac fibrosis94–96.
The fibrillar collagen matrix was initially thought to form a fairly static complex, but these structural proteins are now recognized to undergo rapid turnover89. A family of collagenolytic enzymes collectively referred to as matrix metalloproteinases (MMPs) have been shown to be activated in the failing myocardium, a discovery that has provided new insight into the pathogenesis of LV remodelling89. Conceptually, disruption of the extracellular matrix would be expected to lead to LV dilation and wall thinning as a result of rearrangement of myofibrillar bundles within the LV wall. Although the precise biochemical triggers of MMP activation are not known, many neurohormones expressed within the failing myocardium can activate MMPs. The overall rate of extracellular matrix degradation is controlled by the balance between the expression of MMPs and of their inhibitors—tissue inhibitors of metalloproteinases (TIMPs). In heart failure, MMP-9 expression is increased and expression of TIMP-1 (also known as metalloproteinase inhibitor 1) and TIMP-3 (also known as metalloproteinase inhibitor 3) is decreased97. In experimental models, increased expression of MMPs and decreased expression TIMPs have both been shown to independently contribute to myocardial dilation and heart failure progression98–101.
Left ventricular structure
The aforementioned changes in myocyte biology and in the myocardium of the failing heart are the primary causes of the progressive LV dilation and increased sphericity that occur during cardiac remodelling. Indeed, many of the structural changes in LV remodelling can contribute to worsening heart failure. A full description of these changes in LV geometry is beyond the scope of this Review, and the reader is referred to two published articles on the subject42,102.
Conclusions
In this Review, we have summarized clinical and experimental observations indicating that the progression of heart failure is driven, at least in part, by the sustained activation of the SNS and RAAS. Although the activation of these ‘neurohormonal’ systems maintains cardiovascular homeostasis in the short-term, numerous experimental studies have demonstrated that the biologically active molecules that are produced by the adrenergic nervous system and RAAS (such as norepinephrine, angiotensin II, and aldosterone) are toxic when expressed at the levels observed in the failing heart. These important observations have led to the formulation of the ‘neurohormonal model’ for heart failure, which forms the cornerstone of current heart failure therapy. The deleterious effects of sustained SNS and RAAS activation on the heart are counteracted by β-adrenergic blocking agents and by ACE inhibitors, ARBs, and aldosterone antagonists, respectively. Moreover, the striking success with the use of angiotensin receptor–neprilysin inhibitors40, can also be viewed within the context of our understanding of neurohormonal activation. These drugs are contemporary, second-generation neurohormonal antagonists that block the RAAS and also correct the imbalance between endogenous vasoconstrictors (such as angiotensin II) and vasodilators (such as the natriuretic peptides) that results from sustained neurohormonal activation. However, although neurohormonal antagonists help to stabilize the symptoms of heart failure and promote reverse LV remodelling, the ‘remission’ from heart failure is self-limiting and will eventually recur103. Therefore, although the neurohormonal hypothesis has greatly expanded our understanding of heart failure, this model does not fully explain disease progression. Indeed, focusing directly on the individual biological mechanisms involved in LV remodelling could provide new insights into the pathophysiology of heart failure progression and inform attempts to develop new therapeutics.
Key points.
Heart failure with reduced ejection fraction (HFrEF) is initiated when an ‘index event’ causes the pumping capacity of the heart to be impaired
Reduced pumping capacity of the heart results in compensatory activation of the sympathetic nervous system and the renin–angiotensin–aldosterone system, which together are referred to as ‘neurohormonal activation’
Neurohormonal activation results in a series of coordinated responses that collectively work to restore cardiovascular homeostasis in the short-term
Sustained neurohormonal activation drives the progression of HFrEF through the deleterious effects exerted on the circulation and the myocardium
Antagonism of neurohormonal systems forms the basis of modern therapy for HFrEF
Acknowledgments
The authors’ research is supported by research funds from the N.I.H. (R01 HL58081, RO1 111094, T32 HL007081).
Footnotes
Competing interests statement
D.L.M. is a consultant to Novartis and is on the steering committee for the PARADISE trial with LCZ696. J.H. declares no competing interests.
References
- 1.Mann DL. Innate Immunity and the Failing Heart: The Cytokine Hypothesis Revisited. Circ. Res. 2015;116:1254–1268. doi: 10.1161/CIRCRESAHA.116.302317. doi:CIRCRESAHA.116.302317 [pii];10.1161/CIRCRESAHA.116.302317 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rouleau JL, et al. Activation of neurohumoral systems following acute myocardial infarction. Am. J. Cardiol. 1991;68:80D–86D. doi: 10.1016/0002-9149(91)90264-l. [DOI] [PubMed] [Google Scholar]
- 3.Packer M. The Neurohormonal Hypothesis: a theory to explain the mechanism of disease progression in heart failure. J. Am. Coll. Cardiol. 1992;20:248–254. doi: 10.1016/0735-1097(92)90167-l. [DOI] [PubMed] [Google Scholar]
- 4.Piepoli M, et al. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93:940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- 5.Giannoni A, et al. Combined increased chemosensitivity to hypoxia and hypercapnia as a prognosticator in heart failure. J Am Coll Cardiol. 2009;53:1975–1980. doi: 10.1016/j.jacc.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Ponikowski PP, et al. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation. 2001;104:2324–2330. doi: 10.1161/hc4401.098491. [DOI] [PubMed] [Google Scholar]
- 7.Floras JS, Ponikowski P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J. 2015;36:1974–1982b. doi: 10.1093/eurheartj/ehv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ. Res. 2014;114:1815–1826. doi: 10.1161/CIRCRESAHA.114.302589. doi:CIRCRESAHA.114.302589 [pii];10.1161/CIRCRESAHA.114.302589 [doi] [DOI] [PubMed] [Google Scholar]
- 9.Sigurdsson A, Swedberg K. The role of neurohormonal activation in chronic heart failure and postmyocardial infarction. Am Heart J. 1996;132:229–234. [PubMed] [Google Scholar]
- 10.Cohn JN, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 11.Dzau VJ, Colucci WS, Hollenberg NK, Williams GH. Relation of the renin-angiotensin-aldosterone system to clinical state in congestive heart failure. Circulation. 1981;63:645–651. doi: 10.1161/01.cir.63.3.645. [DOI] [PubMed] [Google Scholar]
- 12.Francis GS, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. Circulation. 1990;82:1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 13.Mann DL, Kent RL, Parsons B, Cooper G., IV Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992;85:790–804. doi: 10.1161/01.cir.85.2.790. [DOI] [PubMed] [Google Scholar]
- 14.Adams JW, et al. Enhanced Gαq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bisognano JD, et al. Myocardial-directed overexpression of the human beta(1)-adrenergic receptor in transgenic mice. J Mol. Cell Cardiol. 2000;32:817–830. doi: 10.1006/jmcc.2000.1123. [DOI] [PubMed] [Google Scholar]
- 16.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bozkurt B, et al. Pathophysiologically relevant concentrations of tumor necrosis factor-α promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97:1382–1391. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- 18.Teerlink JR, Pfeffer JM, Pfeffer MA. Progressive ventricular remodeling in response to diffuse isoproterenol-induced myocardial necrosis in rats. Circ. Res. 1994;75:105–113. doi: 10.1161/01.res.75.1.105. [DOI] [PubMed] [Google Scholar]
- 19.Cohn JN, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N. Engl. J. Med. 1991;325:303–310. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 20.Packer M, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure U.S. Carvedilol Heart Failure Study Group. N. Engl. J. Med. 1996;334:1350–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 21.Pitt B, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 22.SOLVD.Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med. 1991;325:293. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 23.MERIT-HF.Study.Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 24.Bristow MR, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation. 1996;94:2807–2816. doi: 10.1161/01.cir.94.11.2807. [DOI] [PubMed] [Google Scholar]
- 25.Yancy CW, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. doi:CIR.0b013e31829e8776 [pii];10.1161/CIR.0b013e31829e8776 [doi] [DOI] [PubMed] [Google Scholar]
- 26.Notarius CF, Millar PJ, Floras JS. Muscle sympathetic activity in resting and exercising humans with and without heart failure. Appl Physiol Nutr Metab. 2015;40:1107–1115. doi: 10.1139/apnm-2015-0289. [DOI] [PubMed] [Google Scholar]
- 27.Weinberger MH, Aoi W, Henry DP. Direct effect of beta-adrenergic stimulation on renin release by the rat kidney slice in vitro. Circ Res. 1975;37:318–324. doi: 10.1161/01.res.37.3.318. [DOI] [PubMed] [Google Scholar]
- 28.Bekheirnia MR, Schrier RW. Pathophysiology of water and sodium retention: edematous states with normal kidney function. Curr. Opin. Pharmacol. 2006;6:202–207. doi: 10.1016/j.coph.2005.09.008. doi:S1471-4892(06)00025-7 [pii];10.1016/j.coph.2005.09.008 [doi] [DOI] [PubMed] [Google Scholar]
- 29.McCollum LT, Gallagher PE, Ann Tallant E. Angiotensin-(1–7) attenuates angiotensin II-induced cardiac remodeling associated with upregulation of dual-specificity phosphatase 1. Am J Physiol Heart Circ Physiol. 2012;302:H801–H810. doi: 10.1152/ajpheart.00908.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wamberg C, Plovsing RR, Sandgaard NC, Bie P. Effects of different angiotensins during acute, double blockade of the renin system in conscious dogs. Am J Physiol Regul Integr Comp Physiol. 2003;285:R971–980. doi: 10.1152/ajpregu.00262.2003. [DOI] [PubMed] [Google Scholar]
- 31.Huang BS, et al. Inhibition of brain angiotensin III attenuates sympathetic hyperactivity and cardiac dysfunction in rats post-myocardial infarction. Cardiovasc Res. 2013;97:424–431. doi: 10.1093/cvr/cvs420. [DOI] [PubMed] [Google Scholar]
- 32.Esteban V, et al. Angiotensin IV activates the nuclear transcription factor-kappaB and related proinflammatory genes in vascular smooth muscle cells. Circ Res. 2005;96:965–973. doi: 10.1161/01.RES.0000166326.91395.74. [DOI] [PubMed] [Google Scholar]
- 33.Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486–492. doi: 10.1038/ncpneph0575. [DOI] [PubMed] [Google Scholar]
- 34.Schrier RW. Aldosterone 'escape' vs 'breakthrough'. Nat Rev Nephrol. 2010;6:61. doi: 10.1038/nrneph.2009.228. [DOI] [PubMed] [Google Scholar]
- 35.Pitt B, Remme W, Zannad F. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 36.Braunwald E. The Path to an Angiotensin Receptor Antagonist-Neprilysin Inhibitor in the Treatment of Heart Failure. J. Am. Coll. Cardiol. 2015;65:1029–1041. doi: 10.1016/j.jacc.2015.01.033. doi:S0735-1097(15)00292-2 [pii];10.1016/j.jacc.2015.01.033 [doi] [DOI] [PubMed] [Google Scholar]
- 37.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 1999;341:577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 38.Clerico A, Recchia FA, Passino C, Emdin M. Cardiac endocrine function is an essential component of the homeostatic regulation network: physiological and clinical implications. Am J Physiol Heart Circ Physiol. 2006;290:H17–H29. doi: 10.1152/ajpheart.00684.2005. [DOI] [PubMed] [Google Scholar]
- 39.Volpe M, Carnovali M, Mastromarino V. The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin Sci (Lond) 2016;130:57–77. doi: 10.1042/CS20150469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMurray JJ, et al. Angiotensin-Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014;317:993–1004. doi: 10.1056/NEJMoa1409077. [doi] [DOI] [PubMed] [Google Scholar]
- 41.Shah AM, Mann DL. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet. 2011;378:704–712. doi: 10.1016/S0140-6736(11)60894-5. doi:S0140-6736(11)60894-5 [pii];10.1016/S0140-6736(11)60894-5 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 43.Toischer K, et al. Differential cardiac remodeling in preload versus afterload. Circulation. 2010;122:993–1003. doi: 10.1161/CIRCULATIONAHA.110.943431. doi:CIRCULATIONAHA.110.943431 [pii];10.1161/CIRCULATIONAHA.110.943431 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest. 2013;123:37–45. doi: 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowes BD, et al. Changes in gene expression in the intact human heart. Downregulation of α-Myosin heavy chain in hypertrophied, failing ventricular myocardium. J. Clin. Invest. 1997;100:2315–2324. doi: 10.1172/JCI119770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kostin S, Hein S, Arnon E, Scholz D, Schaper J. The cytoskeleton and related proteins in the human failing heart. Heart Fail. Rev. 2000;5:271–280. doi: 10.1023/A:1009813621103. [DOI] [PubMed] [Google Scholar]
- 47.Hein S, Kostin S, Heling A, Maeno Y, Schaper J. The role of the cytoskeleton in heart failure. Cardiovasc Res. 2000;45:273–278. doi: 10.1016/s0008-6363(99)00268-0. [DOI] [PubMed] [Google Scholar]
- 48.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 49.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ. Res. 2013;113:739–753. doi: 10.1161/CIRCRESAHA.113.300308. doi:CIRCRESAHA.113.300308 [pii];10.1161/CIRCRESAHA.113.300308 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feldman DS, Carnes CA, Abraham WT, Bristow MR. Mechanisms of disease: beta-adrenergic receptors--alterations in signal transduction and pharmacogenomics in heart failure. Nat. Clin. Pract. Cardiovasc. Med. 2005;2:475–483. doi: 10.1038/ncpcardio0309. [DOI] [PubMed] [Google Scholar]
- 51.Port JD, Bristow MR. Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. J. Mol. Cell Cardiol. 2001;33:887–905. doi: 10.1006/jmcc.2001.1358. [DOI] [PubMed] [Google Scholar]
- 52.Bristow MR, et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 53.Bristow MR, et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 54.Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of β-adrenergic receptor kinase and β1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 56.Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by beta-adrenergic receptor stimulation and blockade. Circulation. 1998;98:1783–1789. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- 57.Rockman HA, et al. Expression of a beta-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rengo G, et al. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raake PW, et al. AAV6.betaARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur Heart J. 2013;34:1437–1447. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harding VB, Jones LR, Lefkowitz RJ, Koch WJ, Rockman HA. Cardiac beta ARK1 inhibition prolongs survival and augments beta blocker therapy in a mouse model of severe heart failure. Proc Natl Acad Sci U S A. 2001;98:5809–5814. doi: 10.1073/pnas.091102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12:331–343. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 62.Lowes BD, et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N. Engl. J Med. 2002;346:1357–1365. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- 63.Brooks WW, et al. Captopril modifies gene expression in hypertrophied and failing hearts of aged spontaneously hypertensive rats. Hypertension. 1997;30:1362–1368. doi: 10.1161/01.hyp.30.6.1362. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, Guo X, Dhalla NS. Modification of myosin protein and gene expression in failing hearts due to myocardial infarction by enalapril or losartan. Biochim. Biophys. Acta. 2004;1690:177–184. doi: 10.1016/j.bbadis.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 65.Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest. 2013;123:46–52. doi: 10.1172/JCI62834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 67.Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. Circ. Res. 1993;72:463–469. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]
- 68.Hasenfuss G, et al. Relation between myocardial function and expression of sarcoplasmic reticulum ca2+-ATPase in failing and nonfailing human myocardium. Circ. Res. 1994;75:434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- 69.Reiken S, et al. beta-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation. 2001;104:2843–2848. doi: 10.1161/hc4701.099578. [DOI] [PubMed] [Google Scholar]
- 70.Reiken S, et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003;107:2459–2466. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- 71.Mann DL. Left ventricular size and shape: determinants of mechanical signal transduction pathways. Heart Fail. Rev. 2005;10:95–100. doi: 10.1007/s10741-005-4636-y. [DOI] [PubMed] [Google Scholar]
- 72.Guerra S, et al. Myocyte death in the failing human heart is gender dependent. Circ. Res. 1999;85:856–866. doi: 10.1161/01.res.85.9.856. [DOI] [PubMed] [Google Scholar]
- 73.Kostin S, et al. Myocytes die by multiple mechanisms in failing human hearts. Circ. Res. 2003;92:715–724. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- 74.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu. Rev. Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [doi] [DOI] [PubMed] [Google Scholar]
- 75.Kroemer G, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tan LB, Jalil JE, Pick R, Janicki JS, Weber KT. Cardiac myocyte necrosis induced by angiotensin II. Circ. Res. 1991;69:1185–1195. doi: 10.1161/01.res.69.5.1185. [DOI] [PubMed] [Google Scholar]
- 77.Todd GL, Baroldi G, Pieper GM, Clayton FC, Eliot RS. Experimental catecholamine-induced myocardial ncrosis I. Morphology, quantification and regional distribution of acute contraction band lesions. J. Mol. Cell. Cardiol. 1985;17:317–338. doi: 10.1016/s0022-2828(85)80132-2. [DOI] [PubMed] [Google Scholar]
- 78.Zhang W, et al. Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. J. Am. Heart Assoc. 2015;4:e001993. doi: 10.1161/JAHA.115.001993. doi:JAHA.115.001993 [pii];10.1161/JAHA.115.001993 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 2015;15:117–129. doi: 10.1038/nri3800. doi:nri3800 [pii];10.1038/nri3800 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olivetti G, et al. Apoptosis in the failing human heart. N. Engl. J. Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 81.Saraste A, et al. Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur. J. Clin. Invest. 1999;29:380–386. doi: 10.1046/j.1365-2362.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 82.Wencker D, et al. A mechanistic role for cardiac myocyte apoptosis in heart failure. J. Clin. Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis : role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 84.Haudek SB, Taffet GE, Schneider MD, Mann DL. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J. Clin. Invest. 2007;117:2692–2701. doi: 10.1172/JCI29134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kajstura J, et al. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J. Mol. Cell Cardiol. 1997;29:859–870. doi: 10.1006/jmcc.1996.0333. [DOI] [PubMed] [Google Scholar]
- 86.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lavandero S, Chiong M, Rothermel BA, Hill JA. Autophagy in cardiovascular biology. J Clin Invest. 2015;125:55–64. doi: 10.1172/JCI73943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma X, et al. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation. 2012;125:3170–3181. doi: 10.1161/CIRCULATIONAHA.111.041814. doi:CIRCULATIONAHA.111.041814 [pii];10.1161/CIRCULATIONAHA.111.041814 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davis J, Molkentin JD. Myofibroblasts: trust your heart and let fate decide. J. Mol. Cell Cardiol. 2014;70:9–18. doi: 10.1016/j.yjmcc.2013.10.019. doi:S0022-2828(13)00318-0 [pii];10.1016/j.yjmcc.2013.10.019 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hartupee J, Mann DL. Role of inflammatory cells in fibroblast activation. J. Mol. Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.11.016. doi:S0022-2828(15)30122-X [pii];10.1016/j.yjmcc.2015.11.016 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schorb W, et al. Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circ. Res. 1993;72:1245–1254. doi: 10.1161/01.res.72.6.1245. [DOI] [PubMed] [Google Scholar]
- 93.Sadoshima JI, Izumo S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ. Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 94.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–1393. doi: 10.1161/01.cir.102.12.1388. [DOI] [PubMed] [Google Scholar]
- 95.Izawa H, et al. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation. 2005;112:2940–2945. doi: 10.1161/CIRCULATIONAHA.105.571653. [DOI] [PubMed] [Google Scholar]
- 96.Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation. 2000;102:2700–2706. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 97.Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98:1728–1734. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 98.Creemers EE, et al. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am. J. Physiol Heart Circ. Physiol. 2003;284:H364–H371. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 99.Ducharme A, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J. Clin. Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim HE, et al. Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. J Clin. Invest. 2000;106:857–866. doi: 10.1172/JCI8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peterson JT, et al. Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation. 2001;103:2303–2309. doi: 10.1161/01.cir.103.18.2303. [DOI] [PubMed] [Google Scholar]
- 102.Koitabashi N, Kass DA. Reverse remodeling in heart failure--mechanisms and therapeutic opportunities. Nat. Rev. Cardiol. 2012;9:147–157. doi: 10.1038/nrcardio.2011.172. doi:nrcardio.2011.172 [pii];10.1038/nrcardio.2011.172 [doi] [DOI] [PubMed] [Google Scholar]
- 103.Mann DL, Barger PM, Burkhoff D. Myocardial recovery: myth, magic or molecular target? J. Amer. Coll. Cardiol. 2012;60:2465–2472. doi: 10.1016/j.jacc.2012.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]