Abstract
Background
This study was done to verify whether propofol could inhibit esophageal squamous cell carcinoma (ESCC) cell line EC9706 cell migration and invasion by targeting SOX4.
Material/Methods
Different concentrations of propofol were co-incubated with EC9706 cells. The pcDNA-SOX4 or SOX4 siRNA plasmid was transfected into cells before the treatment with propofol 5 μg/L. The migratory and invasion ability of EC9706 cells were tested by wound-healing assay and Transwell chambers. Western blotting was used to investigate the expressions of MMP-2, MMP-9, TIMP-1, TIMP-2, and SOX4. Gelatin zymography was employed to detect the activity of MMP2 and MMP-9.
Results
Compared with the control, the migration and invasion activity of EC9706 cells were decreased after incubation with different concentrations of propofol (P<0.01). The expression of MMP-2, MMP-9, and SOX4 was decreased and that of TIMP-1 was increased in the propofol-treated EC9706 cells (P<0.01). Down-regulation of SOX4 by SOX4-siRNA had the same effect as propofol on EC9706 cells, including suppressing cell migration and invasion, inhibiting the expression and activity of MMP-2/9, and increasing the expression TIMP-1. Over-expression of SOX4 could partly abrogated propofol-mediated inhibition of EC9706 cell migration and invasion.
Conclusions
Propofol inhibits EC9706 cell migration and invasion by down-regulation of SOX4.
MeSH Keywords: Barrett Esophagus, Propofol, SOXC Transcription Factors
Background
As one of the most common malignant tumors derived from the gastrointestinal tract, esophageal squamous cell carcinoma (ESCC) has a high incidence and mortality, and more than 300,000 people died from this cancer, making it the 6th leading cause of cancer-related death worldwide [1,2]. Benefiting from the advances in the early diagnosis and adjuvant treatment of ESCC, the rate of three-year disease-free survival in patients has been increasing in these decades. However, many patients who were treated with such therapy still experience disease progression [3]. Therefore, new treatment choices are critically required.
Metastasis is the first reason for the mortality of patients with most cancers including ESCC. Tumor metastasis is a complex process that includes migratory tumor cells leaving the primary position by invasion, disseminating throughout the body via the circulation, and eventually colonizing at distant organs [4]. It has already been proved that acquiring the ability to migrate from the original locality and invade is the prerequisite for tumor cell metastasis. Therefore, it is important to find an effective anti-ESCC cell migration and invasion treatment in order to improve the prognosis of patients with ESCC.
Propofol (2,6-diisopropylphenol), the most commonly used intravenous anesthetic agent, which provides smooth induction and rapid recovery from anesthesia, has gained wide acceptance since 1980s. In addition to its multiple anesthetic advantages, propofol also demonstrated anti-tumor effects on breast cancer, gallbladder cancer, hepatocellular carcinoma, and cervical cancer in recent years [5–8]. However, whether propofol has anti-tumor effects on ESCC is still unknown. Considering the wide use of propofol in ESCC surgery, it is of great importance to investigate the effects of propofol on ESCC.
SOX4 (sex-determining region Y-box 4), one of the sex-determining region Y (SRY)-related high-mobility-group (HMG) box family that affect multiple developmental processes, is important in the development and fate decisions of cells. In tumor genesis, lots of studies showed that SOX4 was significantly elevated in multiple human cancers like esophageal cancer, breast cancer, hepatocellular carcinoma, colorectal cancer, and lung cancer [9–12]. In addition, studies demonstrated that down-regulation of SOX4 could inhibit the metastasis of cancers [13–15]. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) together keep the balance of extracellular matrix (ECM), whose degradation and remodeling provide space for the migration and invasion of cancer cells [16,17]. In the present study, we investigated the effect of propofol on ESCC migration and invasion, and demonstrated the underlying mechanisms associated with SOX4, MMP-2, and MMP-9.
Material and Methods
Cell culture and transfection
The human ESCC cell line (EC9706) was obtained from the American Type Culture Collection (ATCC, Rockville, Maryland, USA) and kept in RMPI 1640 medium with 10% fetal bovine serum (FBS) (Gibco, California, USA), 1% of 100 U/mL penicillin, and 1% of 100 mg/mL streptomycin sulfate. The cells were incubated in humidified incubators with 5% CO2 at 37°C. The cells were first treated with different concentrations of propofol to test the effect of propofol on EC9706 cells. Then, EC9706 cells were transfected with SOX4-siRNA or DNA3.1+HA-SOX4 to decrease or increase the expression of SOX4 before propofol was added for testing whether SOX4 plays the key role in propofol’s effect on EC9706 cells.
NC-siRNA and SOX4-siRNA were synthesized chemically at Suzhou GenePharma Co. Ltd. (Suzhou, China). Human SOX4 gene was constructed into pcDNA3.1+HA vector by Life Technologies (Invitrogen, California, USA), and the empty vector served as the negative control. For transfection, after the cells were cultured to 70–80% confluence, NC-siRNA or SOX4-siRNA was transfected by using Lipofectamine 2000 (Invitrogen, California, USA) according to the manufacturer’s instructions for inhibition of the expression of SOX4 in the cells. Meanwhile, pcDNA3.1+HA-SOX4 or pcDNA3.1+HA empty vector was transfected by using Lipofectamine 2000 for over-expression of SOX4 in the cells.
Wound-healing assay
Normal EC9706 cells or EC9706 cells transfected with SOX4-siRNA or DNA3.1+HA-SOX4 were used in this experiment. When cells reached approximately 90% confluence, the cell scratch spatula was used to scratch the cell layer. After being washed three times with warm phosphate-buffered saline (PBS), propofol (5 μg/L) was added to each group and the cells continued to be incubated at 37°C for 18 h. A digital camera system (Olympus Corp., Tokyo, Japan) was used to acquire images of the scratches on the cells after incubating for 0 and 18 h.
Transwell migration assay
Normal EC9706 cells or EC9706 cells transfected with SOX4-siRNA or DNA3.1+HA-SOX4 were used in this experiment. After the cells were trypsinized and re-suspended, 2.0×104 cells in 200 μL of RPMI 1640 medium (containing propofol 5 μg/L) were placed into the upper chambers (8-mm pore size; Corning Inc., Lowell, Massachusetts, USA). The lower chambers were filled with 600 μL of complete medium with 10% FBS. After incubation for 12 h at 37°C, the cells on the upper side of the inserts were softly scraped off. Cells that migrated to the lower side of the inserts were fixed with 4% paraformaldehyde and stained with 4′,6-diamidino-2-phenylindole (DAPI) (2 μg/mL), and then the cells from five independent, randomly chosen visual fields were counted under an immunofluorescence microscope (×200 magnification) for quantification of cells.
Quantitative real-time polymerase chain reaction (qRT-PCR)
EC9706 cells were transfected with SOX4-siRNA or DNA3.1+HA-SOX4 following to the manufacturer’s instructions. Total RNA was extracted from cells by using TRIzol reagent (Invitrogen, USA), and then miRNA was reverse transcribed to cDNA by using a reverse transcription kit (Takara, Japan). qRT-PCR was performed by using the SYBR Green PCR Kit on the ABI 7500 Fast Real-Time PCR system according to the manufacturer’s recommendation. The expression of SOX4 mRNA was normalized to U6. All experiments were done triplicate. The 2−ΔΔCt method was used to calculate the relative expression of genes.
Western blotting
Protein from cells was extracted with radio immunoprecipitation assay (RIPA) lysis buffer (Biyuntian, China). Protein lysates were then separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes (PVDF, Millipore, Massachusetts, USA). After blocking with 5% non-fat milk for 2 h at room temperature, the membranes were incubated with the primary antibodies: MMP-2 (1:1000, Abcam, USA), MMP-9 (1:1000, Abcam, USA), TIMP-1 (1:1000, Abcam, USA), TIMP-2 (1:2000, Abcam, USA), SOX4 (11000, Abcam, USA), and β-actin (1:1000, Abcam, USA) overnight at 4°C. Then the membranes were incubated in horseradish peroxidase (HRP)-linked secondary antibodies (Santa Cruz Biotechnology, USA) for 2 h. Western blotting signals were detected using the ECL Plus Kit (Biyuntian, China). Each experiment was repeated three times independently.
Gelatin zymography analysis
Zymography was used to measure the activity of MMP-2 and MMP-9 in the cells. The supernatant was collected and mixed with 5×SDS sample buffer without a reducing agent. Then, equal amounts (30 mg) of the sample were loaded onto the SDS-PAGE gel (8% polyacrylamide gel containing 0.1% gelatin) for electrophoresis. After that, the gels were washed for 30 min twice in 2.5% Triton X-100 at room temperature to remove the SDS and incubated in the renaturation buffer (pH 7.5, 50 mM Tris-HCl, 10 mM CaCl2, 0.02% NaN3) for 24 h at 37°C. To stain the gels, 0.1% Coomassie blue R-250 in 10% glacial acetic acid/45% methanol was used. The gels were destained (50% methanol, 10% acetic acid, and 40% water solution) until clear bands of gelatinolysis appeared on a dark background, and then were transferred to water for rehydration before acquiring images. Afterward, the images of the bands were analyzed by Quantity One 4.4 (Bio-Rad, Hercules, California, USA).
Statistical analysis
All experiments were repeated three times. All statistical analyses were performed using SPSS 20.0. Student’s t-test was used for the comparisons between two different groups. One-way analysis of variance (ANOVA), followed by Tukey’s post hoc analysis, was performed to compare between multiple experimental groups. The results were considered statistically significant at a P value of <0.05.
Results
Propofol inhibited EC9706 migration and invasion
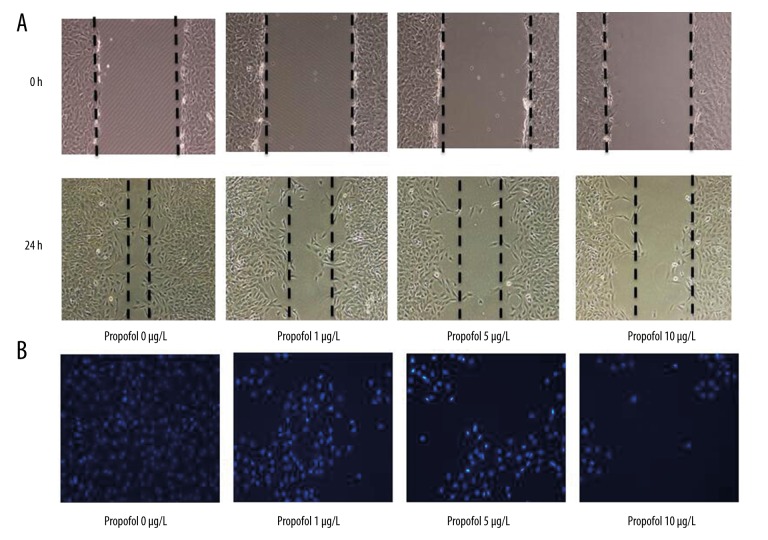
We first investigated the effects of propofol on cell migration and invasion. The EC9706 cell lines were cultured in the presence of various concentrations of propofol (0–10 μg/L), and wound-healing assay was used to test the migration ability of cells. Images of the scratches were captured at 0 and 18 h after propofol was added. We found that propofol markedly inhibited EC9706s migration after 18 h in a dose-dependent manner (Figure 1A, P<0.01). Transwell assay were used to test the invasion ability of EC9706 cells; in agreement, the results showed that propofol could also decrease cell invasion ability after 12 h in a dose-dependent manner (Figure 1B).
Figure 1.
Propofol inhibits EC9706 migration and invasion. (A) Wound-healing assay demonstrated that propofol inhibited EC9706 migration in a dose-dependent manner. (B) Transwell chambers revealed that propofol inhibited EC9706 invasion in a dose-dependent manner.
Propofol decreased the expression and activity of MMP-2 and MMP-9 in EC9706
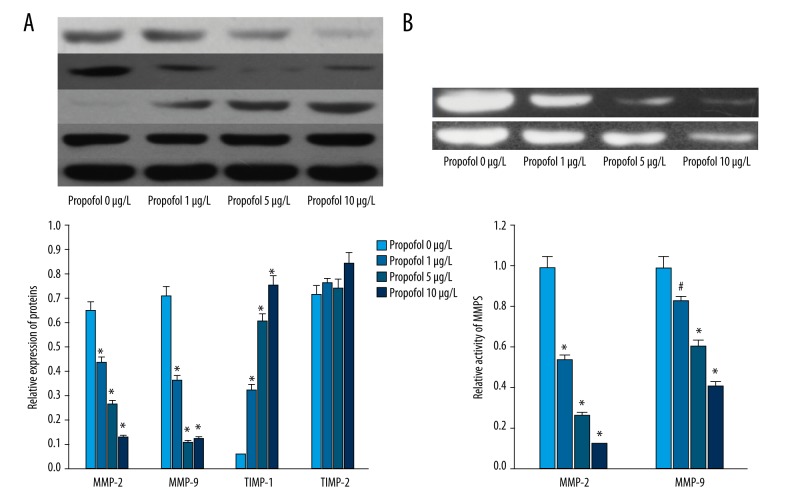
MMPs and TIMPs played critical roles in cell migration and invasion. As shown in Figure 2A, propofol could decreased the expression of MMP-2 and MMP-9 in EC9706 cells in a dose-dependent manner (P<0.01). Besides, the expression of TIMP-1 was increased by propofol (P<0.01). However, there was no significant difference in the expression of TIPM-2 in the EC9706 cells of each group (Figure 2A, P<0.01).
Figure 2.
Propofol inhibited the expression and activity of MMP-2, MMP-9 in EC9706. (A) Western blot analysis showed that propofol decreased the expression of MMP-2 and MMP-9, and increased the expression of TIMP-1 in a dose-dependent manner. (B) Gelatin zymography analysis showed that propofol inhibited the activity of MMP-2 and MMP-9 in a dose-dependent manner. # P<0.05 compared with control; * P<0.01 compared with control.
Gelatin zymography analysis showed that propofol could significantly decrease the activities of MMP-2 and MMP-9 in a dose-dependent manner (Figure 2B, P<0.01).
Propofol decreased the expression of SOX4 in EC9706
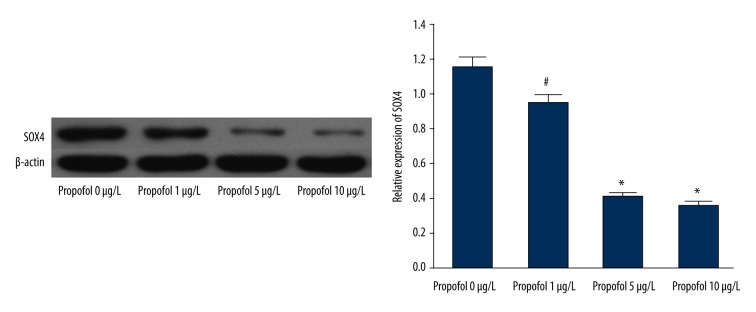
Cells were incubated with increasing concentrations of propofol (0–10 μg/L). Western blot analysis showed that the expression of SOX4 was decreased by propofol in a dose-dependent manner (Figure 3, P<0.01). Considering the critical role of SOX4 in the development of cancer, these results prompted us to focus on SOX4 in the following study.
Figure 3.
Propofol inhibited the expression of SOX4 in EC9706. # P<0.05 compared with control; * P<0.01 compared with control.
Down-regulation of SOX4 had the same effect as propofol on EC9706
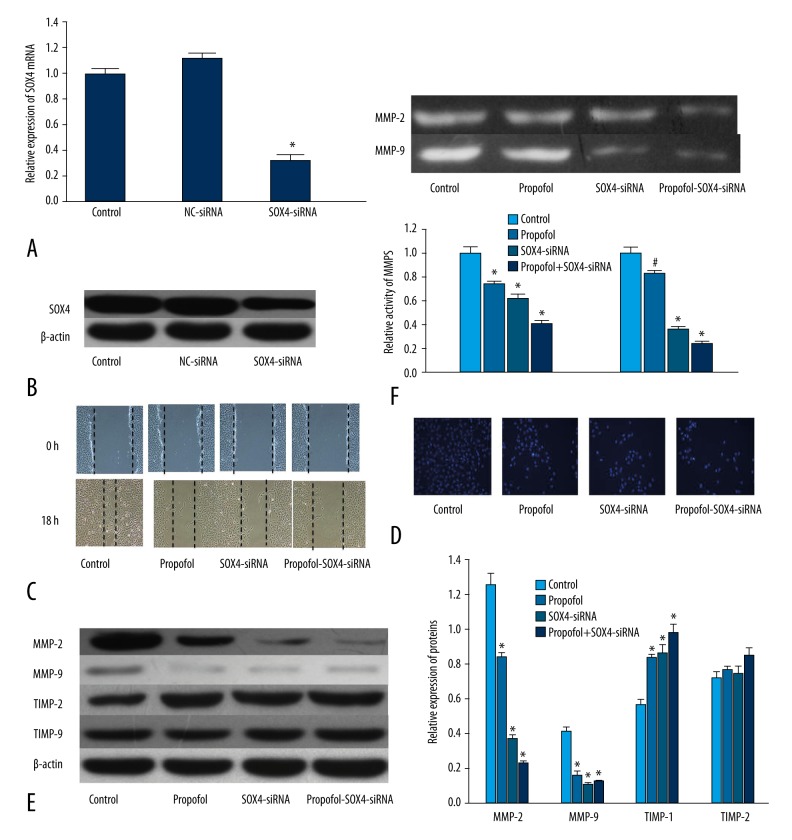
To examine the role of SOX4 in propofol-induced inhibition of EC9706 migration and invasion, SOX4-siRNA was used to inhibit the expression of SOX4. In order to knock down SOX4, we constructed SOX4-siRNA recombinant plasmids and transfected them into EC9706 cells. qRT-PCR showed that the mRNA of SOX4 was decreased in the cells transfected with SOX4-siRNA compared with the NC-siRNA cells (Figure 4A, P<0.01). As show in Figure 4B, Western blot analysis demonstrated the same results according to RT-PCR.
Figure 4.
Down-regulation of SOX4 by SOX4-siRNA had the same effect as propofol on EC9706. (A) PCR showed that SOX4-siRNA decreased the expression of SOX4 in EC9706. (B) Western blot analysis showed that SOX4-siRNA decreased the expression of SOX4 in EC9706. (C) Wound-healing assay demonstrated that, the same as propofol, down-regulation of SOX4 could also inhibit EC9706 migration. (D) Transwell chambers revealed that, the same as propofol, down-regulation of SOX4 could also inhibit EC9706 invasion. (E) The same as propofol, down-regulation of SOX4 decreased the expression of MMP-2 and MMP-9, and increased the expression of TIMP-1. (F) Down-regulation of SOX4 decreased the activity of MMP-2 and MMP-9. * P<0.01 compared with control
Identical to the effect of propofol (5 μg/L), inhibition of SOX4 by SOX4-siRNA could also decrease EC9706 migration and invasion (Figure 4C and 4D, P<0.05). In agreement, Western blot analysis revealed that, the same as propofol (5 μg/L), SOX4-siRNA suppressed the expression of MMP-2 and MMP-9, and increased the expression of TIMP-1 (Figure 4E, P<0.05). Besides, the activity of MMP-2/9 was also decreased in the cells transfected with SOX4-siRNA (Figure 4F, P<0.05). These data showed that down-regulation of SOX4 had the same effect as propofol on EC9706.
Over-expression of SOX4 reversed the propofol-induced inhibition of EC9706 migration and invasion
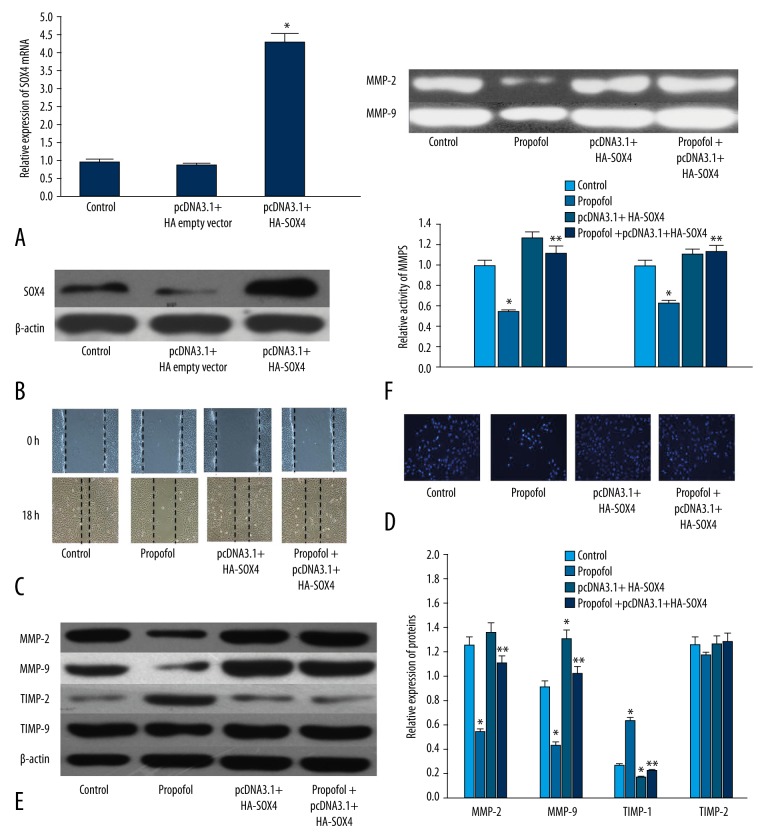
To further verify the role of the SOX4 in propofol-induced inhibition of EC9706 migration and invasion, pcDNA3.1+HA-SOX4 was transfected to increase the expression of SOX4. qRT-PCR showed that the mRNA of SOX4 was increased in the cells transfected with pcDNA3.1+HA-SOX4 compared with the pcDNA3.1+HA-empty plasmid (Figure 5A, P<0.01). Western blot analysis demonstrated the same results according to RT-PCR (Figure 5B, P<0.01).
Figure 5.
Over-expression of SOX4 reversed propofol-induced inhibition of EC9706 cells migration and invasion. (A) PCR showed that pcDNA3.1+HA SOX4 increased the expression of SOX4 in EC9706. (B) Western blot analysis showed that pcDNA3.1+HA SOX4 increased the expression of SOX4 in EC9706. (C) Wound-healing assay demonstrated that over-expression of SOX4 reversed propofol-induced inhibition of EC9706 migration. (D) Transwell chambers revealed that over-expression of SOX4 reversed propofol-induced inhibition of EC9707 invasion. (E) Over-expression of SOX4 increased the expression of MMP-2 and MMP-9, and decreased the expression of TIMP-1. (F) Over-expression of SOX4 increased the activity of MMP-2 and MMP-9. * P<0.01 compared with control; ** P<0.01 compared with propofol.
After being transfected by pcDNA3.1+HA-SOX4 or pcDNA3.1+HA-empty plasmid, EC9706 cells were incubated with propofol (5 μg/L) or vehicle. We found that over-expression of SOX4 suppressed the propofol-induced inhibition of EC9706 migration and invasion (Figure 5C, 5D, P<0.01). In addition, over-expression of SOX4 could also reverse the propofol-induced decreased expression and activity of MMP-2/9 (Figure 5E, 5F, P<0.01). These results further verified that SOX4 was involved in propofol-induced inhibition of EC9706 migration and invasion.
Discussion
In the present study, we investigated the effects of propofol on ESCC cell line EC9706. We found that propofol significantly decreased the migration and invasion of EC9706 cells. What’s more, our data showed that SOX4 participated in the promoting cell migration and invasion via regulation of the activity and expression of MMP-2/9 in EC9706 cells.
Propofol has been used to induce or maintain anesthesia during multiple procedures, tests, or surgeries [18,19]. In addition, in the past decade, propofol has been revealed to have an antitumor effect, including reducing tumor size and weight in vivo [7,20]; inhibiting cancer cell adhesion, migration, and invasion; and inducing apoptosis of cancer cells in vitro [21–24]. Ye et al. showed that propofol could inhibit cell proliferation and invasion by regulation of microRNA-143 expression in osteosarcoma cells [25]. Later, Zhang et al. found that propofol suppressed cervical cancer cell growth via inhibiting the HOTAIR-mediated mTOR pathway [7]. Chen et al. demonstrated that perioperative propofol-paravertebral anesthesia decreased the metastasis and progression of breast cancer compared with other anesthetic agents [26]. Besides, Li et al. revealed that propofol could reduce MMP expression through inhibiting NF-kappaB activity in breast cancer cells [8]. Consistent with the results of these research studies, the present study demonstrated that the migration and invasion ability of EC9706 cells was inhibited by propofol in a dose-dependent manner. In addition, the expression and activity of MMP-2/9 were decreased in the EC9706 cells treated with propofol. We also found that propofol could inhibit the expression of SOX4 in EC9706 cells.
SOX4 belongs to the C subgroup of the SOX transcription factor family, and the SOX family plays a key role in many developmental processes by controlling terminal differentiation of a wide variety of cell types and cell fate decisions [27]. Alterations in transcriptional activities that result in imbalance between oncogenes and tumor suppressor genes finally cause initiation and progression of cancer [28,29]. The relationship between high expression of SOX4 and tumor development or progression has been observed in breast cancer, gastric cancer, lung cancer, colon cancer, prostate cancer, and endometrial cancer [9,10,12,14,28,30]. Li et al. demonstrated that down-regulation of SOX4 by siRNA decreased the migration and invasion ability of esophageal cancer cells [10]. In addition, Wang et al. revealed that over-expression of SOX4 was associated with colorectal cancer progression [12]. What’s more, a lot of studies found that miRNAs like miR-133a, miR-132, and miR-211 played that anti-cancer role by targeting SOX4 [9,10,28]. Therefore, we supposed that propofol inhibited EC9706 cell migration and invasion by down-regulation of SOX4. In this study, we found that knock down of SOX4 by siRNA had the same effect as propofol on EC9706, including suppressing cell migration and invasion, inhibiting the expression and activity of MMP-2/9, and increasing the expression TIMP-1, which indicated that SOX4 played an important role in the regulation of EC9706 cell migration and invasion. Additionally, we also found that over-expression of SOX4 increased the expression of MMP-2/9 and promoted the migration and invasion ability of EC9706 cells. What’s more, our results showed that over-expression of SOX4 reversed propofol-induced inhibition of EC9706 migration and invasion. These results indicated that besides the NF-kappaB pathway, mTOR pathway, and miR-143 [7,8,25], SOX4 also played a key role in propofol-induced inhibition of EC9706 cell migration and invasion.
Conclusions
The present study provides new insights into the effect of propofol on migration and invasion of EC9706 cells and the related mechanism. This study suggests that propofol inhibits migration and invasion of EC9706 cells through, at least partly, down-regulation of SOX4. Therefore, it might be assumed that propofol is the appropriate anesthetic drug in surgery on ESCC patients. However, this must be verified in further studies, including animal trials and prospective clinical studies.
Footnotes
Competing interest statement
All authors: no conflicts.
Source of support: Departmental sources
References
- 1.Wen SW, Zhang YF, Li Y, et al. Association of miR-21 with esophageal cancer prognosis: A meta-analysis. Genet Mol Res. 2015;14(2):6578–82. doi: 10.4238/2015.June.12.12. [DOI] [PubMed] [Google Scholar]
- 2.Zhao K, Chen BJ, Chen ZG, et al. Effect of miR-503 down-regulation on growth and invasion of esophagus carcinoma and related immune function. Med Sci Monit. 2015;21:3564–69. doi: 10.12659/MSM.895518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang T, Chen T, Niu H, et al. MicroRNA-218 inhibits the proliferation and metastasis of esophageal squamous cell carcinoma cells by targeting BMI1. Int J Mol Med. 2015;36(1):93–102. doi: 10.3892/ijmm.2015.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han TS, Hur K, Xu G, et al. MicroRNA-29c mediates initiation of gastric carcinogenesis by directly targeting ITGB1. Gut. 2015;64(2):203–14. doi: 10.1136/gutjnl-2013-306640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Wang N, Zhou S, et al. Propofol induces proliferation and invasion of gallbladder cancer cells through activation of Nrf2. J Exp Clin Cancer Res. 2012;31:66. doi: 10.1186/1756-9966-31-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Shan WF, Jin TT, et al. Propofol exerts anti-hepatocellular carcinoma by microvesicle-mediated transfer of miR-142-3p from macrophage to cancer cells. J Transl Med. 2014;12:279. doi: 10.1186/s12967-014-0279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang D, Zhou XH, Zhang J, et al. Propofol promotes cell apoptosis via inhibiting HOTAIR mediated mTOR pathway in cervical cancer. Biochem Biophys Res Commun. 2015;468(4):561–67. doi: 10.1016/j.bbrc.2015.10.129. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Zhang L, Han Y, et al. Propofol reduces MMPs expression by inhibiting NF-kappaB activity in human MDA-MB-231 cells. Biomed Pharmacother. 2012;66(1):52–56. doi: 10.1016/j.biopha.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Zu L, Wang Y, et al. miR-132 inhibits lung cancer cell migration and invasion by targeting SOX4. J Thorac Dis. 2015;7(9):1563–69. doi: 10.3978/j.issn.2072-1439.2015.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Qin X, Li Y, et al. MiR-133a suppresses the migration and invasion of esophageal cancer cells by targeting the EMT regulator SOX4. Am J Transl Res. 2015;7(8):1390–403. [PMC free article] [PubMed] [Google Scholar]
- 11.Hanieh H. Aryl hydrocarbon receptor-microRNA-212/132 axis in human breast cancer suppresses metastasis by targeting SOX4. Mol Cancer. 2015;14:172. doi: 10.1186/s12943-015-0443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, Li Y, Tan F, Xiao Z. Increased expression of SOX4 is associated with colorectal cancer progression. Tumour Biol. 2016 doi: 10.1007/s13277-015-4756-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Shen H, Blijlevens M, Yang N, et al. Sox4 expression confers bladder cancer stem cell properties and predicts for poor patient outcome. Int J Biol Sci. 2015;11(12):1363–75. doi: 10.7150/ijbs.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Y, Zhao M, Xie Q, et al. MicroRNA-338-3p functions as tumor suppressor in breast cancer by targeting SOX4. Int J Oncol. 2015;47(4):1594–602. doi: 10.3892/ijo.2015.3114. [DOI] [PubMed] [Google Scholar]
- 15.Wu D, Pan H, Zhou Y, et al. Upregulation of microRNA-204 inhibits cell proliferation, migration and invasion in human renal cell carcinoma cells by downregulating SOX4. Mol Med Rep. 2015;12(5):7059–64. doi: 10.3892/mmr.2015.4259. [DOI] [PubMed] [Google Scholar]
- 16.Liang L, Li X, Zhang X, et al. MicroRNA-137, an HMGA1 target, suppresses colorectal cancer cell invasion and metastasis in mice by directly targeting FMNL2. Gastroenterology. 2013;144(3):624–35.e4. doi: 10.1053/j.gastro.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 17.Tabouret E, Bertucci F, Pierga JY, et al. MMP2 and MMP9 serum levels are associated with favorable outcome in patients with inflammatory breast cancer treated with bevacizumab-based neoadjuvant chemotherapy in the BEVERLY-2 study. Oncotarget. 2016 doi: 10.18632/oncotarget.7612. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habre C, Tramer MR, Popping DM, Elia N. Ability of a meta-analysis to prevent redundant research: systematic review of studies on pain from propofol injection. BMJ. 2014;348:g5219. doi: 10.1136/bmj.g5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cote GA. Patient-controlled propofol for sedation in endoscopic retrograde cholangiopancreatography: An alternative to anesthesia-administered sedation? Gastroenterology. 2014;146(7):1818–19. doi: 10.1053/j.gastro.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Song J, Shen Y, Zhang J, Lian Q. Mini profile of potential anticancer properties of propofol. PLoS One. 2014;9(12):e114440. doi: 10.1371/journal.pone.0114440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang P, Chen J, Mu LH, et al. Propofol inhibits invasion and enhances paclitaxel-induced apoptosis in ovarian cancer cells through the suppression of the transcription factor slug. Eur Rev Med Pharmacol Sci. 2013;17(13):1722–29. [PubMed] [Google Scholar]
- 22.Wang ZT, Gong HY, Zheng F, et al. Propofol suppresses proliferation and invasion of gastric cancer cells via downregulation of microRNA-221 expression. Genet Mol Res. 2015;14(3):8117–24. doi: 10.4238/2015.July.17.20. [DOI] [PubMed] [Google Scholar]
- 23.Wang ZT, Gong HY, Zheng F, et al. Propofol suppresses proliferation and invasion of pancreatic cancer cells by upregulating microRNA-133a expression. Genet Mol Res. 2015;14(3):7529–37. doi: 10.4238/2015.July.3.28. [DOI] [PubMed] [Google Scholar]
- 24.Su Z, Hou XK, Wen QP. Propofol induces apoptosis of epithelial ovarian cancer cells by upregulation of microRNA let-7i expression. Eur J Gynaecol Oncol. 2014;35(6):688–91. [PubMed] [Google Scholar]
- 25.Ye Z, Jingzhong L, Yangbo L, et al. Propofol inhibits proliferation and invasion of osteosarcoma cells by regulation of microRNA-143 expression. Oncol Res. 2013;21(4):201–7. doi: 10.3727/096504014X13890370410203. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Lu P, Chen L, et al. Perioperative propofol-paravertebral anesthesia decreases the metastasis and progression of breast cancer. Tumour Biol. 2015;36(11):8259–66. doi: 10.1007/s13277-015-4027-5. [DOI] [PubMed] [Google Scholar]
- 27.Yoon TM, Kim SA, Cho WS, et al. SOX4 expression is associated with treatment failure and chemoradioresistance in oral squamous cell carcinoma. BMC Cancer. 2015;15:888. doi: 10.1186/s12885-015-1875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CY, Hua L, Sun J, et al. MiR-211 inhibits cell proliferation and invasion of gastric cancer by down-regulating SOX4. Int J Clin Exp Pathol. 2015;8(11):14013–20. [PMC free article] [PubMed] [Google Scholar]
- 29.Foronda M, Morgado-Palacin L, Gomez-Lopez G, et al. Profiling of Sox4-dependent transcriptome in skin links tumour suppression and adult stem cell activation. Genom Data. 2015;6:21–24. doi: 10.1016/j.gdata.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilir B, Osunkoya AO, Wiles WGt, et al. SOX4 is essential for prostate tumorigenesis initiated by PTEN ablation. Cancer Res. 2016;76(5):1112–21. doi: 10.1158/0008-5472.CAN-15-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]