ABSTRACT
Relapse in cancer patients following an apparent cure and a prolonged latency period, known as tumor dormancy, remains an unrelenting clinical crisis. Here, I expand on our recent findings that potentially link cancer cell cannibalism of bone marrow-derived mesenchymal stem/stromal cells (BM-MSCs) to the senescence-associated secretory phenotype (SASP) and tumor dormancy.
KEYWORDS: Cancer, cell cannibalism, inflammation, MSC, SASP, senescence, tumor dormancy
One mysterious feature of breast cancer is that it can reemerge abruptly many years after apparent eradication of the primary tumor. The asymptomatic latency period that precedes clinical relapse is best explained by the phenomenon known as tumor dormancy, a divergent stage of tumor progression in which residual growth-arrested solitary cancer cells or small cell clusters linger somewhere in the body for an unpredictable amount of time.1 Deciphering the causal basis of tumor dormancy therefore has important therapeutic significance.
It is well established that non-malignant cell types of the tumor-associated stroma profoundly influence the behavior of breast cancer cells (BCCs).2 Given that breast cancer preferentially relapses in bone, recent emphasis has been placed on uncovering the role of bone marrow-derived mesenchymal stem/stromal cells (BM-MSCs) in tumor dormancy.2 BM-MSCs and BCCs readily interact once disseminated cancer cells enter the bone marrow or after MSCs are recruited into primary tumors.2 We recently focused on these cellular interactions using a 3-dimensional (3D) tumor niche model that, due to the high cell-to-medium ratio, creates hostile conditions such as low pH and nutritional deficits characteristic of overcrowded developing tumors with vascular limitations. We discovered that human BCCs under duress in 3D cultures enter dormancy after internalizing and degrading (i.e., cannibalizing) human BM-MSCs (Fig. 1).3 This conclusion was drawn from compelling data demonstrating that, after consuming BM-MSCs, cannibalistic BCCs became highly resistant to stresses imposed by nutrient deprivation and chemotherapy but, strikingly, their ability to form tumors in mice was greatly diminished.3
Figure 1.
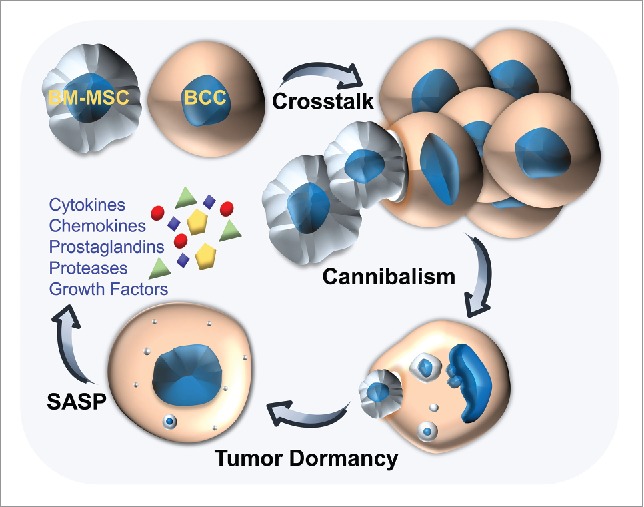
Interactions between bone marrow-derived mesenchymal stem/stromal cells (BM-MSCs) and breast cancer cells (BCCs) that lead to cannibalism of BM-MSCs and tumor dormancy. Following cannibalization of the BM-MSCs, BCC tumorigenicity is reduced; however, the cells become more resilient against stresses imposed by nutrient deprivation. As a result, the BCCs enter dormancy. Our findings indicate that dormant tumor cells are constantly evolving and can adopt a phenotype rich in factors secreted by metabolically active senescent cells as part of the senescence-associated secretory phenotype (SASP). Components of the SASP include inflammatory mediators (cytokines, chemokines, and prostaglandins), proteases, and select growth factors.
In general, cellular cannibalism is a term used to describe a distinct phagocytic-like process in which one cell engulfs and often eliminates neighboring intact cells.4 Although the phenomenon was first demonstrated over a century ago and has been observed frequently in cancer tissue,4 its impact on tumor progression has only recently received substantial attention. Initially, it was considered a way for malignant cells to scavenge critical cellular resources or eliminate antitumor immunity.5 More recently, cell cannibalism or related processes have been reported to promote aneuploidy through non-genetic means,6 and therefore could perhaps provide a conduit for transfer of beneficial cellular traits.
Notably, in our study transcriptome profiles revealed that after consuming BM-MSCs the BCCs acquired a unique molecular signature enriched in factors involved in cytoprotection, cell chemotaxis, and inflammatory response (Fig. 1).3 Interestingly, the resulting cannibalistic BCCs produced an array of cytokines/chemokines and proteases commonly secreted by senescent cells as part of the senescence-associated secretory phenotype (SASP).7,8 Historically, cellular senescence, which by definition is linked to permanent cell cycle arrest, was considered a mechanism of tumor suppression and aging, although it is now acknowledged to be important for embryogenesis and injury repair.7,8 Absurdly, senescence can also encourage tumor progression probably in part due to the SASP, in which the secreted cytokines and proteases potentiate survival and mobility of malignant cells.7 Moreover, these secreted SASP factors could allow dormant BCCs to communicate with various components of the microenvironment and perhaps equip them with the tools to construct a personalized pro-tumorigenic niche. Our data therefore indicated that, similar to senescent cells, dormant tumor cells are functionally dynamic and exhibit a high level of metabolic activity. This is in contrast to the current murky picture that portrays dormant tumor cells as essentially being asleep. Of course, further studies are needed to determine if the secretory activities and metabolic features acquired by dormant BCCs in our study are context-dependent and/or if our findings are predominantly a product of cannibalizing BM-MSCs.
It is important to note that senescence-associated growth arrest is not only caused by predetermined replicative limits, but is also a product of various acute stressors such as reactive oxygen species, chemotherapy, and cytokines.7,8 Likewise, acute senescence can be triggered by autophagy, also known as self-cannibalism, which likely shares many features with cell cannibalism.8,9 It is also important to consider that senescence-associated growth arrest is now recognized to be reversible, particularly when one of its drivers, tumor protein 53 (TP53, commonly known as p53), becomes inactive.10 Therefore BCCs, which often have mutations that functionally impair p53, could escape the permanent growth restrictions associated with senescence and in parallel exploit the tumorigenic properties of the SASP.7 Overall, this might explain why the cannibalistic BCCs in our study recovered their ability to proliferate after just a few days in culture but continued to express SASP factors such as interleukin-1 α (IL1A), C-X-C motif chemokine ligand 8 (CXCL8), CXCL1, C-C motif chemokine ligand 20 (CCL20), and SERPINE1, among other molecules, for at least 5 weeks in vivo.3
Taken together, our results indicated that BCC cannibalism of BM-MSCs supports certain facets of tumor dormancy (Fig. 1); although a direct causal relationship in patients has been challenging to elucidate as unique MSC markers in vivo are unknown. However, our findings suggest that the presence of cell cannibalism in primary tumors, which is discernible using simple imaging tools, could have prognostic value, particularly when these cannibalistic cells simultaneously express factors of the SASP. Moreover, the aggressive nature of cell cannibalism when coupled with the SASP could provide unique pharmacologic targets that improve the clinical management of cancer recurrence. Our findings also support a conceptual framework in which tumor dormancy is considered a dynamic and metabolically active stage of tumor progression that allows dormant cells to acquire diverse phenotypic states and co-evolve with their microenvironment, similar to senescent cells. The SASP would seemingly provide cannibalistic dormant cancer cells with this diverse functionality. Ultimately, our investigation has opened a new road in research on cell cannibalism and tumor dormancy that, if traveled, could lead to novel cancer therapies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Cancer Prevention and Research Institute of Texas Award RP150637, and by NIH grant P40RR17447.
References
- 1.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 2014; (9):611-22; PMID:25118602; http://dx.doi.org/ 10.1038/nrc3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker ND, Patel J, Munoz JL, Hu M, Guiro K, Sinha G, Rameshwar P. The bone marrow niche in support of breast cancer dormancy. Cancer Lett 2016; 380(1):263-71; PMID:26546045; http://dx.doi.org/ 10.1016/j.canlet.2015.10.033 [DOI] [PubMed] [Google Scholar]
- 3.Bartosh TJ, Ullah M, Zeitouni S, Beaver J, Prockop DJ. Cancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs). Proc Natl Acad Sci U S A 2016; 113(42):E6447-56; PMID:27698134; http://dx.doi.org/ 10.1073/pnas.1612290113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma N, Dey P. Cell cannibalism and cancer. Diagn Cytopathol 2011; 39(3):229-33; PMID:21319327; http://dx.doi.org/ 10.1002/dc.21402 [DOI] [PubMed] [Google Scholar]
- 5.Lugini L, Matarrese P, Tinari A, Lozupone F, Federici C, Iessi E, Gentile M, Luciani F, Parmiani G, Rivoltini L, et al.. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res 2006; 66(7):3629-38; PMID:16585188; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-3204 [DOI] [PubMed] [Google Scholar]
- 6.Krajcovic M, Johnson NB, Sun Q, Normand G, Hoover N, Yao E, Richardson AL, King RW, Cibas ES, Schnitt SJ, et al.. A non-genetic route to aneuploidy in human cancers. Nat Cell Biol 2011; 13(3):324-30; PMID:21336303; http://dx.doi.org/ 10.1038/ncb2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.JP Coppé, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010; 5:99-118. Review; PMID:20078217; http://dx.doi.org/ 10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez-Mancera PA, Young AR, Narita M. Inside and out: the activities of senescence in cancer. Nat Rev Cancer 2014; 14(8):547-58; PMID:25030953; http://dx.doi.org/ 10.1038/nrc3773 [DOI] [PubMed] [Google Scholar]
- 9.Goehe RW, Di X, Sharma K, Bristol ML, Henderson SC, Valerie K, Rodier F, Davalos AR, Gewirtz DA. The autophagy-senescence connection in chemotherapy: must tumor cells (self) eat before they sleep? J Pharmacol Exp Ther 2012; 343(3):763-78; PMID:22927544; http://dx.doi.org/ 10.1124/jpet.112.197590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beauséjour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J 2003; 22(16):4212-22; PMID:12912919; http://dx.doi.org/ 10.1093/emboj/cdg417 [DOI] [PMC free article] [PubMed] [Google Scholar]


