ABSTRACT
The relevance of epithelial-to-mesenchymal transition (EMT) in cancer is still under debate. Recently, we reported that EMT bestows key pericyte properties on cancer cells and may thus represent epithelial-to-pericyte transition (EPT). Carcinoma cells undergo EPT to stabilize blood vessels and fuel primary tumor growth. Association of EPT cancer cells with vascular niches may also promote resistance to therapy.
KEYWORDS: Blood vessel, EMT, endothelial cell, EPT, metastasis, N-cadherin, PDGFR-β, pericyte
Epithelial-to-mesenchymal transition (EMT) is a cellular reprogramming process by which well-knit epithelial cells lose intercellular adhesion and acquire the increased migratory capacity of mesenchymal cells.1 The cardinal features of EMT have led to the popular hypothesis that EMT is a prerequisite for carcinoma metastasis.1 However, recent studies have shown that EMT is dispensable for spontaneous metastasis in various genetically engineered mouse models of breast and pancreatic cancers.2,3 Therefore, the significance of EMT in cancer progression remains to be determined, and the fates and roles of epithelial tumor cells naturally transitioning to a mesenchymal state in vivo are largely elusive.
In our study4 we tracked epithelial cancer cells that underwent inducible or spontaneous EMT in tumor transplantation models. Unlike epithelial cells, the majority of EMT cancer cells preferentially occupy the perivascular space and are closely associated with blood vessels, thus simulating pericytes.4 This intriguing observation prompted us to examine EMT cancer cells in tumor vascularization. EMT markedly upregulates various pericyte markers in carcinoma cells, including platelet-derived growth factor receptor (PDGFR)-β and N-cadherin. In tumor xenografts, induction of EMT in cancer cells increases pericyte coverage of tumor vasculature. In tumors that are generated from EMT-prone carcinoma cells, a substantial fraction of tumor pericytes are derived from naturally occurring EMT cancer cells. Depletion of these EMT cells reduces the number of pericytes, destabilizes blood vessels, and attenuates tumor growth.4 Therefore, EMT cancer cells phenotypically and functionally resemble pericytes and are indispensable for vascular stabilization and sustained tumor growth. We propose that in the primary tumor, a small subset of epithelial cancer cells undergo EMT and the resulting enhanced mobility enables EMT cancer cells to migrate within the tumor mass. Moreover, acquisition of PDGFR-β expression by EMT cells allows their chemotaxis toward the endothelium, and homodimerization of N-cadherin on the plasma surface of EMT cells and endothelial cells (ECs) establishes their intercellular adhesion. Like pericytes, EMT cancer cells improve vascular support for growth of the bulk tumor. These findings suggest that EMT confers key pericyte attributes on cancer cells and may often represent epithelial-to-pericyte transition (EPT) (Fig. 1).
Figure 1.
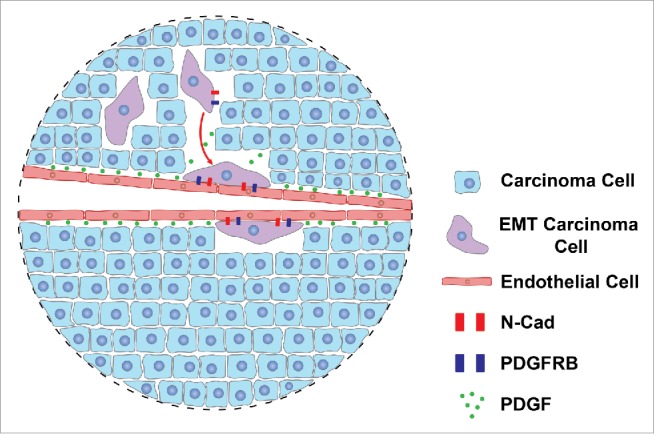
Epithelial-to-pericyte transition (EPT) in tumor vascularization. A small subset of carcinoma cells undergo epithelial-to-mesenchymal transition (EMT) to become mobile and migrate within the primary tumor. Through the EMT process, these cells also acquire expression of platelet-derived growth factor receptor (PDGFR)-β and N-cadherin, which enables their chemotaxis toward vasculature in response to an endothelial cell (EC)-secreted PDGF gradient and subsequent adhesion to ECs via N-cadherin homodimerization. EMT cancer cells thus function like pericytes to stabilize the blood vessels and support growth of the bulk tumor. Such EMT represents EPT.
EMT consists of a broad spectrum of intermediate phenotypes between the completely epithelial state and the completely mesenchymal state. Given the mesenchymal nature of pericytes, it is likely that EPT cells exhibit a complete EMT phenotype. Our study also reinforces the importance of tumor vascularization. Avascular tumors are severely restricted in their growth due to the lack of a blood supply. Cancer cells are well known to be able to induce angiogenesis, the formation of new blood vessels, for expansion of the tumor mass. Furthermore, certain cancer cells may mimic ECs to form de novo perfusable vascular-like networks by themselves, a phenomenon termed vascular mimicry.5 Pericyte coverage is critical for the maturation of nascent vasculature. Glioblastoma stem cells can indeed differentiate into functional pericytes.6 Our study further suggests that EPT may be a general mechanism by which cancer cells perform pericyte functions.
Our findings uncover a key role of EPT cancer cells in tumor growth, but the potential implication of EPT in metastasis remains to be elucidated. In our study we did not observe evident distant metastasis by labeled EMT cancer cells. This might be attributed to the metastatic incompetence of the chosen cancer cells and/or the relatively short experimental duration that may be insufficient for the development of metastasis. It has been suggested that deficient pericyte coverage of tumor vasculature increases interstitial fluid pressure and facilitates cancer cell intravasation; thus, pericytes may stabilize the vasculature to limit metastasis.7 As EPT cancer cells function like pericytes to stabilize blood vessels, this special EMT program may potentially suppress blood-borne metastasis. On the other hand, migration of cancer cells toward blood vessels in the primary tumor is a natural part of the intravasation process. Association of EPT cancer cells with ECs may expedite their entry into the circulation. It is possible that the EMT process may generally enable cancer cells to be chemo-attracted to and associated with blood vessels, but whether such cells stabilize the vasculature or intravasate for metastasis might be determined by their intrinsic malignant properties.
A key contribution of EMT to malignancy is therapy resistance. Recent studies showed that, although it is dispensable for metastasis, EMT promotes chemoresistance in vivo.2,3 While the EMT process per se confers resistance to cell death induced by various cancer therapies,1 the vascular association of EPT cancer cells observed in our study may further contribute to therapy resistance. It has been recognized that capillary ECs are not just passive conduits for delivering blood but also form vascular niches that produce a variety of growth factors and cytokines (e.g., platelet-derived growth factor [PDGF], hepatocyte growth factor [HGF], stromal cell-derived factor 1 [SDF1]), Wnts, Notch ligands, and adhesion molecules. These factors are defined collectively as “angiocrine factors,”8 and act in a paracrine or juxtacrine manner to promote the survival of cancer cells in the vicinity. It is well established that vascular niches in the bone marrow provide a sanctuary for subpopulations of leukemic cells to resist chemotherapy-induced death.9 EMT rewires signaling pathways in carcinoma cells; for instance, through the EMT process mouse mammary epithelial tumor cells downregulate epidermal growth factor receptor (Egfr), Her2, and Her3, but upregulate Pdgfrs, Axl, nerve growth factor receptor (Ngfr), HGF receptor Met, and C-X-C chemokine receptor type 4 (Cxcr4).10 Many of these newly acquired receptors can recognize EC-derived angiocrine factors. EPT cancer cells that reside in proximity to capillary ECs and express cognate receptors for angiocrine factors are primed to respond to perivascularly enriched angiocrine signals. In contrast, non-EMT carcinoma cells do not share the same receptor repertoire and/or close distance to blood vessels. We thus envision that under chemotherapy EPT cancer cells display a selective survival advantage, in part due to protection by vascular niches, and are able to withstand the cytotoxic effects of the treatment. Therefore, the functional interactions of EPT cancer cells with capillary ECs may promote therapy resistance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Joseph Garcia for assistance with figure preparation.
References
- 1.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871-90; PMID:19945376; http://dx.doi.org/ 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 2.Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al.. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015; 527:472-6; PMID:26560033; http://dx.doi.org/ 10.1038/nature15748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015; 527:525-30; PMID:26560028; http://dx.doi.org/ 10.1038/nature16064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shenoy AK, Jin Y, Luo H, Tang M, Pampo C, Shao R, Siemann DW, Wu L, Heldermon CD, Law BK, et al.. Epithelial-to-mesenchymal transition confers pericyte properties on cancer cells. J Clin Invest 2016; 126:4174-86; PMID:27721239; http://dx.doi.org/ 10.1172/JCI86623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer 2003; 3:411-21; PMID:12778131; http://dx.doi.org/ 10.1038/nrc1092 [DOI] [PubMed] [Google Scholar]
- 6.Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, et al.. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 2013; 153:139-52; PMID:23540695; http://dx.doi.org/ 10.1016/j.cell.2013.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerhardt H, Semb H. Pericytes: gatekeepers in tumour cell metastasis? J Mol Med (Berl) 2008; 86:135-44; PMID:17891366; http://dx.doi.org/ 10.1007/s00109-007-0258-2 [DOI] [PubMed] [Google Scholar]
- 8.Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature 2016; 529:316-25; PMID:26791722; http://dx.doi.org/ 10.1038/nature17040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabe Y, Konopleva M. Advances in understanding the leukaemia microenvironment. Br J Haematol 2014; 164:767-78; PMID:24405087; http://dx.doi.org/ 10.1111/bjh.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jahn SC, Law ME, Corsino PE, Parker NN, Pham K, Davis BJ, Lu J, Law BK. An in vivo model of epithelial to mesenchymal transition reveals a mitogenic switch. Cancer Lett 2012; 326:183-90; PMID:22906417; http://dx.doi.org/ 10.1016/j.canlet.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]


