Abstract
Acquired aplastic anemia is an autoimmune-mediated bone marrow failure syndrome. The mechanism by which such an autoimmune reaction is initiated is unknown. Whether and how the genetic lesions detected in patients cause autoimmune bone marrow failure have not yet been determined. We found that mice with spontaneous deletion of the TGFβ-activated kinase-1 gene in a small subset of hematopoietic cells developed bone marrow failure which resembled the clinical manifestations of acquired aplastic anemia patients. Bone marrow failure in such mice could be reversed by depletion of CD4+ T lymphocytes or blocked by knockout of interferon-γ, suggesting a Th1-cell-mediated autoimmune mechanism. The onset and progression of bone marrow failure in such mice were significantly accelerated by the inactivation of tumor necrosis factor-α signaling. Tumor necrosis factor-α restricts autoimmune bone marrow failure by inhibiting type-1 T-cell responses and maintaining the function of myeloid-derived suppressor cells. Furthermore, we determined that necroptosis among a small subset of mutant hematopoietic cells is the cause of autoimmune bone marrow failure because such bone marrow failure can be prevented by deletion of receptor interacting protein kinase-3. Our study suggests a novel mechanism to explain the pathogenesis of autoimmune bone marrow failure.
Introduction
Acquired aplastic anemia (AAA) is a common type of bone marrow failure (BMF) syndrome characterized by significant reductions in bone marrow (BM) cellularity and peripheral blood (PB) pancytopenia.1–3 Activated Th1 and Tc1 lymphocyte-mediated autoimmune responses are known to be the major reasons underlying hematopoietic repression since elimination of such cells results in the successful recovery of hematopoiesis in more than 70% of patients.4–9 Cryptic clonal somatic mutations are detected in BM samples from most AAA patients.5,10–16 As a consequence, AAA patients show an increased risk for the development of paroxysmal nocturnal hemoglobinuria, myelodysplastic syndrome and acute myeloid leukemia. In addition, autoimmune-related inflammatory reactions were detected in almost all AAA patients, as demonstrated by increased levels of both tumor necrosis factor-α (TNFα) and interferon-γ (IFNγ) in patient samples.17–20 However, the mechanism of activation of this autoimmune reaction is still not known. The relationship between the cryptic clonal somatic mutations and the autoimmune pathogenesis of AAA has not been identified, and the roles of TNFα and IFNγ in the development and progression of AAA have not been fully determined.
TGF1β-activated kinase-1 (Tak1) is a member of the MAP3K family which mediates TNFα, IL1β and TLR-stimulated NF-kB, JNK and p38 signaling.21 It is required for cell survival and prevention of inflammatory reactions in many tissues.22 We previously reported that Tak1 is required to protect hematopoietic stem progenitor cells (HSPCs) from inflammatory cytokine-induced apoptosis and necroptosis. Necroptosis is a type of programmed necrosis which is regulated by the Rip1-Rip3-Mlkl pathway. As distinct from apoptotic cells, which are eliminated by macrophages and do not induce inflammatory reactions, necroptotic cells secrete factors which do stimulate immune/inflammatory reactions.23,24 Mx1Cre+Tak1fx/fx mice (Tak1mut) develop severe BMF within 2–3 days of polyI:C injection to induce Tak1 deletion in up to 100% of BM hematopoietic cells.25,26 Interestingly, we found that without polyI:C injection, spontaneous deletion of the Tak1 gene in a low percentage of hematopoietic cells (1%–3%) occurs due to the leakage of Mx1Cre,27 causing a chronic autoimmune BMF in mice after long-term observation. TNFα is thought to be a key mediator of autoimmune BMF.28,29 However, inactivation of TNFα signaling surprisingly accelerates the progression of BMF in Tak1mut mice, an observation which runs counter to generally accepted theory. The BMF phenotypes of Tak1mut and Tak1mutTnfr−/− mice (deletion of both TNF receptor 1 and 2) resemble most of the clinical manifestations seen in chronic and severe AAA patients, respectively.4 Thus these experimental animals provide a better model system by which to study the autoimmune pathogenesis of AAA. Importantly, although necroptosis has been implicated in BMF in hematopoietic-specific Tak1 and receptor interacting protein kinase-1 (Rip1)-knockout mice, these studies suggested that both Tak1 and Rip1 are required for the survival of HSPCs by repressing apoptosis and necroptosis.25,30,31 However, due to the rapid death of the knockout mice, none of these studies was able to detect the activation of immune cells. In addition, although it has been proposed that necroptotic cells can be immunogenic,23,24,32 to our knowledge, we are the first to experimentally demonstrate that necroptotic cells actually do initiate autoimmune diseases.
It was known that some hematopoietic parameters (such as HSPC numbers, red blood counts and hemoglobin concentration), as well as immune responses, differ between male and female mice.33,34 We were concerned that the phenotypic differences in autoimmune reactivity and hematopoiesis between male and female mice might influence our data interpretation. Therefore, in this study, only male mice were used for analysis. In addition, we and others found that Tnfr−/−, Ifnγ−/− and Rip3−/− mice are phenotypically normal and their hematopoietic parameters are comparable to those of WT mice. Thus, to make our data easier to comprehend, we include only data from single-gene knockout mice as controls for studies of compound-gene knockout mice.
Methods
Mice and genotyping
All mice were maintained in a C57BL6/J background and housed under a 12-h light/dark cycle in micro-isolator cages contained within a laminar flow ventilation system. All procedures were conducted in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals for research purposes and were approved by Loyola University Chicago’s Institutional Animal Care and Use Committee (IACUC) (AU#513380). Ifnγ−/− and Tnfr−/− mice (B6.129S-Tnfrsf1aTnfrsf1b, knockout of both Tnfr 1 and 2) were purchased from the Jackson Laboratory. Rip3−/− mice were kindly provided by Dr. Vishva Dixit (Genentech Inc., South San Francisco, CA, USA).35 Mx1cre+Tak1fx/fxTnfr−/− mice (Tak1mutTnfr−/−) are maintained from breeders of Mx1cre+Tak1fx/+Tnfr−/− and Tak1fx/fxTnfr−/− mice. Mx1cre+Tak1fx/+Tnfr−/− and Tak1fx/fxTnfr−/− mice were further crossed with Ifnγ−/− and Rip3−/− mice, respectively, to produce Mx1cre+Tak1fx/fxTnfr−/− Ifnγ−/− (Tak1mutTnfr−/−Ifnγ−/−) and Mx1cre+Tak1fx/fxTnfr−/−Rip3−/− (Tak1mutTnfr−/−Rip3−/−) mice, as well as the heterozygous littermates including Tak1mutTnfr−/− Ifnγ−/+ and Tak1mutTnfr−/−Rip3−/+. The gross phenotypes of these mice are summarized in Table 1. The PCR primers used for genotyping of experimental mice are listed in the Online Supplementary Appendix.
Bone marrow failure monitoring, blood analysis and mouse survival
All mice were monitored for BMF development by dynamic examination of peripheral blood cell counts and observation for symptoms such as hunched body, growth arrest and/or significant weight loss. The death of mice specifically from BMF was confirmed by hematologic analysis using a Hemavet 950 Hematology System (Drew Scientific Inc., FL, USA). Spleens, livers and BM were collected upon animal sacrifice for further validation. All mice were monitored up to two years of age and analyzed by Kaplan-Meier survival graphing (GraphPad Prism v.5.04).
Histological analysis
Bones were decalcified and fixed with Calfor™ decalcifying solution (Cancer Diagnostic Inc., Durham, NC, USA) according to the manufacturer’s instructions. Spleens were fixed in 10% zinc-formalin. Tissues were sectioned and stained with H&E. Photographs were taken using an Olympus BX50 microscope equipped with a digital camera system (DP21).
Flow cytometric analysis
For intracellular staining, cells were pre-treated with PMA (50 ng/mL; St. Louis, MO, USA) for six hours in the presence of 10 μg/mL Brefeldin-A; St. Louis, MO, USA) for the final four hours, followed by a 5-minute fixation in 4% paraformaldehyde, and were permeabilized with saponin (0.1%; St. Louis, MO, USA). Cells were suspended in FACS buffer (1×PBS supplemented with 2% FBS) at a concentration of 1×107 cells mL-1 and aliquotted into flow cytometry tubes for antibody staining. Surface staining was performed without fixation or permeabilization. Stained cells were subjected to multi-color analysis using a BD LSRFortessa™ flow cytometer. Data were analyzed using Flowjo software. During the analysis, cells were first gated on live cells, then further analyzed for specific staining. The antibody resource and clone information are listed in the Online Supplementary Appendix.
Further details on the methods used can be found in the Online Supplementary Appendix.
Statistical analysis
Data are expressed as means±SD. Two-way ANOVA (multiple groups) and Student’s t-test (two groups) were performed to determine the statistical significance of differences among and between experimental groups. P<0.05 was considered significant.
Results
Development of chronic BMF in Tak1mut mice
Due to endogenous Ifnα expression, even without polyI:C injection, Mx1Cre induces the spontaneous deletion of target genes in BM hematopoietic cells which is sufficient to induce the development of T-cell leukemia in Ptenfx/fx mice.27 Using GFP-reporter mice, we found Mx1Cre-induced GFP expression was detected in 1%–3% and 5%–7% of BM hematopoietic cells when analyzed in 3- and 12-month old mice, respectively (Online Supplementary Figure S1). To determine whether deletion of Tak1 in this small subset of hematopoietic cells influences normal hematopoietic homeostasis, we maintained a cohort of Tak1mut mice for long-term observation to dynamically examine the PB cell counts. Mx1Cre− littermates and Cre+ littermates (including Mx1Cre+Tak1fx/+ and Mx1Cre+Tak1+/+) were studied in parallel as controls. We found that Tak1mut mice were generally normal up to eight months of age, with no detectable pathological phenotype. The body size and weight of Tak1mut mice are comparable to their littermate controls (Table 1 and Online Supplementary Figure S2A and B). However, most Tak1mut mice start to develop chronic BMF after eight months, as demonstrated by reduction of white blood cell counts (WBC), red blood cell counts (RBC), hemoglobin concentration (Hb), and platelet numbers (plt) in PB, as well as a reduction in cell counts in BM (Figure 1A–C and Online Supplementary Figure S2H). In addition, all Tak1mut mice developed more pronounced thymic degeneration as indicated by decreased size and cell counts (Online Supplementary Figure S2C and D). Interestingly, despite the splenomegaly observed (increased spleen size and weight) (Online Supplementary Figure S2E and F), significantly lower numbers of cells can be collected from the spleens of Tak1mut mice (Online Supplementary Figure S2G) due to increased fibrosis. These phenotypic changes in Tak1mut mice resemble the clinical manifestations seen in chronic AAA patients.3,4 Further analysis of HSPCs showed that, compared to WT and Cre+ controls, the BM of Tak1mut mice showed a significantly increased percentage of Sca1v cells, including Lin−Sca1+c-kit+ (LSK) and Lin−Sca1+c-kit− (LS) cells, in Lineage− (Lin−) (Figure 1D) or total nucleated cell (TNC) populations (Figure 1E). However, the absolute number of LSK cells was reduced due to the reduction in TNC (Online Supplementary Figure S2I). Such alterations in HSPCs in Tak1mut mice are similar to the HSPC changes observed in mice with chronically enhanced Ifnγ.20,36 HSCs and MPPs in such mice cannot be reliably analyzed using any of the current standard panels of surface markers such as CD150, CD34, or FLT3 (data not shown).
Figure 1.
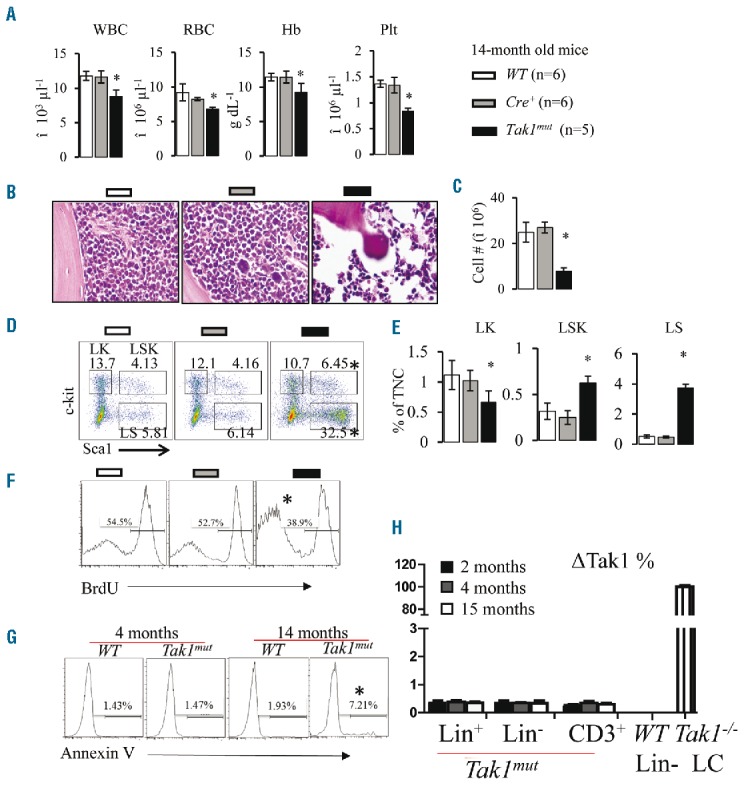
Spontaneous Tak1 deletion in a small subset of hematopoietic stem progenitor cells (HSPCs) results in chronic bone marrow failure (BMF). Peripheral blood (PB) and bone marrow (BM) were collected from Tak1mut mice and their WT and Cre+ littermates at age 14 months. (A) White blood cells (WBC), red blood cells (RBC), hemoglobin (Hb) and platelets (plt) were analyzed using Hemavet 950 Hematology System. (B) H&E-stained bone marrow section (tibia) after decalcification. (C) Number of total nucleated cells (TNCs) in BM from two hind limbs (four bones pooled: 2 tibias and 2 femurs from each mouse) were compared. (D) Representative flow cytometric plots for analysis of BM hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs). BM cells were first gated on Lin− population and then analyzed for LK, LSK and LS populations. (E) Percentages of LK, LSK and LS populations in TNCs from BM. (F) Proliferation of Lin−c-kit+ HSPCs was compared among WT, Cre+ and Tak1mut mice (14 months old) by BrdU pulse-labeling and flow cytometric analysis. (G) Death of Lin-c-kit+ HSPCs was compared among WT, Cre+ and Tak1mut mice at indicated ages by Annexin-V staining followed by flow cytometric analysis. (I) Percentages of cells with ΔTak1 (Tak1 deletion) in Lin− HSPCs, Lin+ differentiated BM cells and CD3+ T lymphocytes from Tak1mut mice were determined by quantitative PCR. Lin− HSPCs from WT mouse BM and Tak1−/− leukemic cells (LCs) were used as negative and positive controls. *P<0.05 compared to WT and Cre+ mice.
Enhanced Th1 and Tc1-immune responses in Tak1mut mice
Theoretically, increased elimination of 1%–3% of HSPCs in BM is insufficient to induce BMF in animals because it has been shown that approximately 5% of normal HSPCs are sufficient to maintain normal hematopoietic homeostasis in mice. One of the possible mechanisms of BMF development in TAK1mut mice is exhaustion of HSPCs due to the constant elimination of the TAK1mut HSPCs. Using Annexin-V staining assays, we found that the percentage of death in HSPCs is comparable between TAK1mut mice and the littermate controls before BMF developed. Reduced proliferation and increased death of HSPCs were only observed in TAK1mut mice after BMF had developed (Figure 1F and G), which did not support such a hypothesis. Another possible explanation is the accumulation of Takmut HSPCs, which have compromised hematopoietic reconstitutive capacity. We excluded such a possibility since fewer than 0.5% of HSPCs with Tak1 deletion (ΔTak1) could be detected in Tak1mut mice of any age before or after the development of BMF (Figure 1H); most Takmut HSPCs died of apoptosis or necroptosis shortly after Tak1 was deleted (Online Supplementary Figure S3). Interestingly, WT mice whose BM cells were chimerized with Mx1Cre+Tak1fx/fx hematopoietic cells but not WT hematopoietic cells also developed a pancytopenic phenotype 1–2 months after 3 polyI:C injections (Online Supplementary Figure S4B–D). Low percentages of Mx1Cre+Tak1fx/fx hematopoietic cells were detected in these recipient mice after pancytopenia developed (Online Supplementary Figure S4A), suggesting that the factors released by dying Takmut hematopoietic cells might be inducing BMF.
The detection of cryptic clonal genetic mutations in AAA patients suggests that a small subset of mutant cells might be the cause of T-cell mediated autoimmune reactions and hematopoietic repression.5,10–15 Thus, we speculated that BMF in Tak1mut mice might be induced by Tak1mut cells through the stimulation of autoimmune responses. To test this hypothesis, we examined T and B lymphocytes in BM, PB, lymph nodes and spleens of Tak1mut mice. While the frequencies of CD3+ T lymphocytes were significantly increased in all tested tissues, B220+ B lymphocytes were remarkably reduced in Tak1mut mice compared to control mice (Figure 2A; only data from BM are shown), suggesting B lymphopoiesis is repressed. In addition, T-cell activation was increased in Tak1mut mice as shown by a decrease in naïve T cells (CD62L+CD44−) and a simultaneous increase in CD62LlowCD44high effector T cells (Figure 2B; only data from BM are shown). Furthermore, we used intracellular staining and FACS analysis to examine the subtypes of the CD4+ and CD8+ T-cell subsets in the BM (Online Supplementary Figure S5). Compared to control mice, Tak1mut mice showed a significant increase in Th1 and Tc1 responses, as indicated by increased frequencies of Tnfα/Ifnγ-expressing Th1 (CD4+) and Tc1 (CD8+) cells (Online Supplementary Figure S2C and D), as well as by an increase in Tnfα and Ifnγ expression (Figure 2E and F). Of note, compared to WT and Cre+ controls, Tak1mut mice displayed a decrease in Foxp3+ Treg cells but no significant difference in the frequencies of IL-17-expressing Th17/Tc17 or IL-4-expressing Th2/Tc2 cells. Taken together, these data suggested that Th1/Tc1 cell-mediated autoimmune responses might be the cause of BMF in Tak1mut mice. TCR repertoire analysis suggested an expansion of oligoclone Th1 cells in Tak1mut BMF mice as demonstrated by a skewed spectratype profile with 1–2 dominant peaks (Online Supplementary Figure S6). We excluded the possibility that accumulation of Tak1 deletion in T cells is the direct cause of T-cell overactivation in Tak1mut mice because: 1) Tak1 is required for the survival of peripheral T cells;37–39 and 2) ΔTak1 is barely detected in T cells in Tak1mut mice at any age before or after BMF development (Figure 1H).
Figure 2.
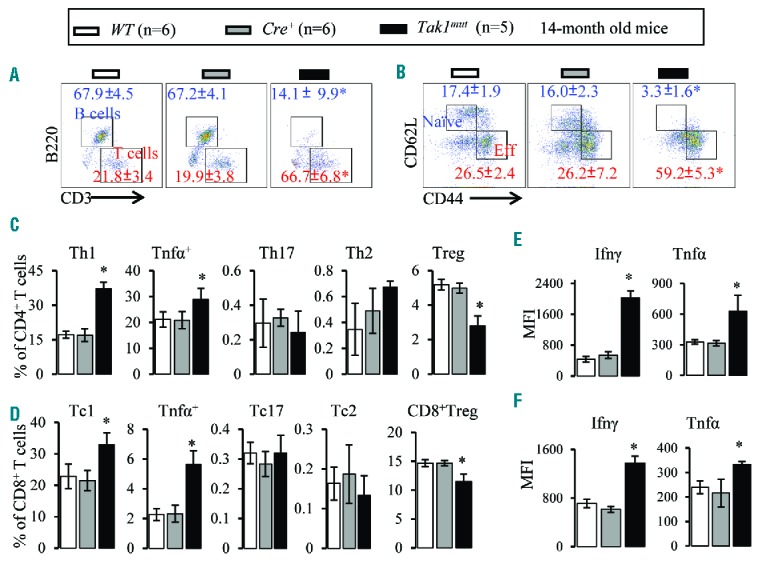
Increased activation of Th1 and Tc1 cells in Takmut mice. Bone marrow (BM) samples were collected from Tak1mut mice and their WT and Cre+ littermates at age 14 months. (A) Flow cytometric analysis of BM CD3+ T cells and B220+ B cells (gated on lymphocytes). (B) Flow cytometric analysis of naïve T cells (CD62L+ CD44−) and effector T cells (CD62LlowCD44hi) in BM (gated on CD3+ cells). (C) Percentages of Th1, Tnfa+, Th17, Th2, and Treg cells in BM (gated on CD4+ T cells). (D) Percentages of Tc1, Tnfa+, Tc17, Tc2, and Treg cells in BM (gated on CD8+ T cells). Ifnγ and Tnfα levels in BM CD4+ (E) and CD8+ (F) T cells were analyzed by mean fluorescence intensity (MFI) of intracellular antibody staining. Data are presented as means±SD. *P<0.05 compared to WT and Cre+ mice.
Tnfα signaling inactivation accelerated disease onset and progression of BMF
Increased levels of TNFα have been detected in many AAA patients, and such levels have been suggested to be contributory to the pathogenesis of AAA.17–19,28,29 We have reported that inactivation of Tnfα signaling by knockout of both Tnfr1 and 2 (Tnfr−/−) can partially prevent the death of Tak1-null HSPCs.25 Thus, we expected that inactivation of Tnfα signaling may attenuate the progression of BMF in Tak1mut mice. However, to our surprise, we found that the progression of BMF in Tak1mut Tnfr−/− mice without polyI:C induction was dramatically exacerbated and much more severe than what was observed in Tak1mut mice. All Tak1mut Tnfr−/− mice were infertile and started to show growth retardation at two months of age compared to Tak1mut and Tnfr−/− mice (Online Supplementary Figure S7A and B). All Tak1mut Tnfr−/− mice developed more pronounced thymic degeneration as indicated by decreased thymic size and cell counts (Online Supplementary Figure S7C and D), as well as splenomegaly but with reduced cellularity (Online Supplementary Figure S7E–G). BMF could be detected in Tak1mut Tnfr−/− mice as early as two months of age. Almost all Tak1mut Tnfr−/− mice become morbid and died by approximately 3–7 months of age (Table 1 and Figure 3A). In contrast, Tak1mut mice of the same age grow normally and breed with no signs of BMF. These mice develop severe pancytopenia by four months of age, characterized by significant reductions in red blood cell (RBC), hemoglobin (Hb), platelet (plt), white blood cell (WBC) and BM cell counts (Figure 3B–D and Online Supplementary Figure S7H). Reduced proliferation and increased death of HSPCs could only be detected after BMF had developed, suggesting they are not the initiators of BMF (Online Supplementary Figure S7K and I). The hematopoietic phenotype in 4-month old Tak1mutTnfr−/− mice resembles the clinical manifestations of severe AAA,3,4,40 and is much more severe than that of 14-month old Tak1mut mice. Analysis of HSPCs showed that among Lin− BM cells, almost all c-Kit+ cells expressed Sca1 (Figure 3E and F and Online Supplementary Figure S7I), a phenomenon commonly observed in mice with increased Ifnγ activity.41–44 These results suggested that Tnfα signaling restricts the development of BMF.
Figure 3.
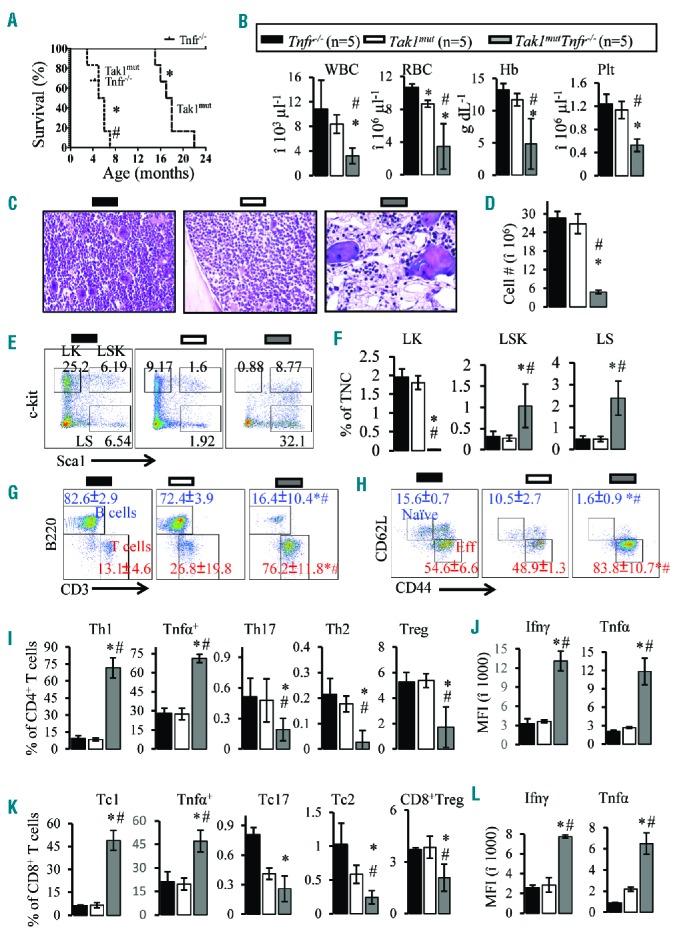
Deficiency of Tnfr accelerates bone marrow failure (BMF) and enhances Th1 cell responses in Tak1mut mice. (A) Survival of Tak1mutTnfr−/− and Tak1mut and Tnfr−/− mice were recorded and compared (*P<0.05, compared to Tnfr−/−mice; #P<0.05 compared to Tak1mut mice). Peripheral blood (PB) and bone marrow (BM) were collected from 4-month old Tak1mutTnfr−/− mice and age-matched Tak1mut and Tnfr−/− mice. (B) White blood cells, (WBC), red blood cells (RBC), hemoglobin (Hb) and platelets (plt) were analyzed using the Hemavet 950 Hematology System. (C) H&E stained bone marrow section (tibia) after decalcification. (D) Number of total nucleated cells (TNCs) in BM from two hind limbs were counted and compared. (E) Representative flow cytometric plots for analysis of BM hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs). BM cells were first gated on the Lin− population and then analyzed for LK, LSK and LS populations. (F) Percentages of LK, LSK and LS populations in total nucleated cells (TNCs) from BM. (G) Flow analysis of BM CD3+ T cells and B220+ B cells (gated on lymphocytes). (H) Flow cytometric analysis of naïve T cells (CD62L+ CD44−) and activated T cells (CD62LlowCD44hi) in BM (gated on CD3+ cells). (I) Percentages of Th1, Tnfα+, Th17, Th2, and Treg cells in BM (gated on CD4+ T cells). Ifnγ and Tnfα levels in BM CD4+ (J) and CD8+ (I) T cells were analyzed by mean fluorescence intensity (MFI) of intracellular antibody staining. (K) Percentages of Tc1, Tnfα+, Tc17, Tc2, and Treg cells in BM (gated on CD8+ T cells). Data are presented as means±SD. *P<0.05 compared to Tnfr−/−; #P<0.05 compared to Tak1mut.
Although inactivation of Tnfα signaling can partially prevent necroptosis of Tak1mut HSPCs,25 most Tnfr−/−Tak1mut HSPCs eventually die of apoptosis. This explains why inactivation of Tnf signaling only slightly extends the lifespan of Tak1−/− mice upon polyI:C injection (maximum of 1 week).25 Consistent with this observation, we did not detect any accumulation of HSPCs in ΔTak1 in Tak1mut Tnfr−/− mice without polyI:C injection (Online Supplementary Figure S7J). We also failed to detect increased HSPC death in Tak1mut Tnfr−/− mice before BMF development when compared to WT and Tak1mut mice (Online Supplementary Figure S7K). Increased apoptosis and reduced proliferation of HSPCs were detected only after BMF had developed (Online Supplementary Figure S7J and K). This suggested that inactivation of Tnfα signaling did not directly alter the survival or proliferation of HSPCs.
Deficiency in Tnfα signaling enhances Th1- and Tc1-cell responses
To determine whether the acceleration of BMF in the absence of Tnfα signaling is due to an enhancement of autoimmune responses, we compared the frequency of lymphocytes and activation of T lymphocytes in hematopoietic and lymphoid tissues among Tak1mutTnfr−/− mice, age-matched Tak1mut mice, and Tnfr−/− and WT control mice. At four months of age, when all the parameters analyzed in Tak1mut mice were still comparable to those of Tnfr−/− and WT mice (data not shown), a consistent decrease in B cells and an increase in T-cell frequency, as well as elevated T-cell activation, were observed in the spleens, PB and BM of Tak1mutTnfr−/− mice (Figure 3G and H; only data from BM were shown). Intracellular staining and FACS analysis (Online Supplementary Figure S8) revealed a significant increase in Th1 and Tc1 cells and a decrease in Th17, Th2 and Treg cells in Tak1mutTnfr−/− mice (Figure 3I and K). The expression of Ifnγ and Tnfα in Th1 cells and Tc1 cells was also significantly elevated (Figure 3J and L). These data were similar to those obtained for Tak1mut BMF mice but to a much greater extent. For example, in BM of Tak1mutTnfr−/− mice, over 80% of T cells displayed an activated phenotype and over 70% of CD4+ T cells were Th1 cells and 45% of CD8+ T cells were Tc1 cells. These data indicate that a Tnfr deficiency prompts Th1/Tc-cell responses, which in turn accelerates BMF. Again, no accumulation of ΔTak1 in T cells was detected (Online Supplementary Figure S7J), suggesting that ΔTak1 in T cells is not the direct cause of T-cell overactivation in Tak1mutTnfr−/− mice.
Deficiency in Tnfα signaling enhances the ability of APCs to prime Type 1 T-cell development and reduces the ability of MDSCs to suppress T-cell proliferation
There are three potential mechanisms which could explain why a Tnfα signaling deficiency enhances Th1/Tc1-cell responses: 1) Tnfr−/− T cells are more prone to develop into Th1 cells than are WT T cells; 2) Tnfr−/− antigen-presenting cells (APCs) have an enhanced ability to support Th1/Tc1 responses compared to WT APCs; 3) a reduced number and/or capacity of Tnfr−/− myeloid-derived suppressor cells (MDSCs). To discriminate among these possibilities, we compared the frequencies of Ifnγ-expressing cells in both CD62Lhigh naïve and CD62Llow effector T cells from WT and Tnfr−/− mice. We found Ifnγ-expressing cells in both populations of T cells from Tnfr−/− mice were reduced compared to those from WT mice (Online Supplementary Figure S9A), suggesting that T-cell responses in Tnfr−/− mice are normally maintained at lower levels in the absence of pathogenic stimuli. To determine whether there are functional changes in APCs in Tnfr−/− mice compared to WT mice, we compared the expression of Ifnγ in WT T cells following co-culturing with either WT or Tnfr−/− APCs under three different conditions: neutral condition (N), Th1-inducing condition (C1), and Th2-inducing condition (C2). Following seven days of co-culturing, the frequency of Ifnγ-expressing T cells is consistently higher when cultured with Tnfr−/− APCs than with WT APCs under all three conditions (Figure 4A). In the presence of Tnfr−/− APCs, the frequency of Ifnγ-expressing T cells increases when compared to co-culturing with WT APCs (Figure 4A). In addition, T cells cultured with Tnfr−/− APCs showed higher levels of Ifnγ expression, as demonstrated by increased mean fluorescence intensity (Figure 4B). The frequency of Ifnγ-expressing T cells and expression levels of Ifnγ are consistent with the three conditions, as C1>N>C2 for both WT and Tnfr−/− groups. Tnfα and IL-17 expression were not obviously different between WT and Tnfr−/− groups (Online Supplementary Figure S9B). Furthermore, we measured the proliferation of T cells cultured with WT or Tnfr−/− APCs in the presence of anti-CD3 and anti-CD28. After three days in culture, the portion of T cells with more than 3 divisions was higher in the Tnfr−/− group than in the WT group (Figure 4C). As IL-12 is the critical cytokine that determines the differentiation of Th1 cells, we measured the expression of IL-12 in WT and Tnfr−/− APCs. We found that both the frequency of IL-12-expressing cells and the levels of IL-12 were higher in Tnfr−/−APCs than in WT APCs (Figure 4D and E). Using the same co-culture system, we determined that the activity of APCs in Tak1mutTnfr−/− mice was also increased compared to APCs isolated from WT littermate controls (Figure 4G). To test whether the number and/or function of MDSCs was altered in Tnfr−/− mice, we measured the frequency of CD11b+Gr1lowLy6Chi (containing granulocytic MDSCs) or CD11b+Gr1highLy6Clow (containing monocytic MDSCs) cells in the BM and conducted an inhibition assay. The frequencies of both populations in the BM were not significantly different when comparing WT and Tnfr−/− mice (Online Supplementary Figure S9C). However, Tnfr−/− MDSCs showed a reduced ability to suppress T-cell proliferation compared to WT MDSCs (Figure 4F and Online Supplementary Figure S9D). We also determined that the MDSCs isolated from Tak1mutTnfr−/− mice showed reduced ability to suppress T-cell proliferation compared to MDSCs isolated from WT littermate controls (Figure 4H). These data suggest that enhanced functions of APCs and reduced functions of MDSCs may concurrently contribute to the T-cell overactivation and high Ifnγ levels seen in Tak1mutTnfr−/− mice.
Figure 4.
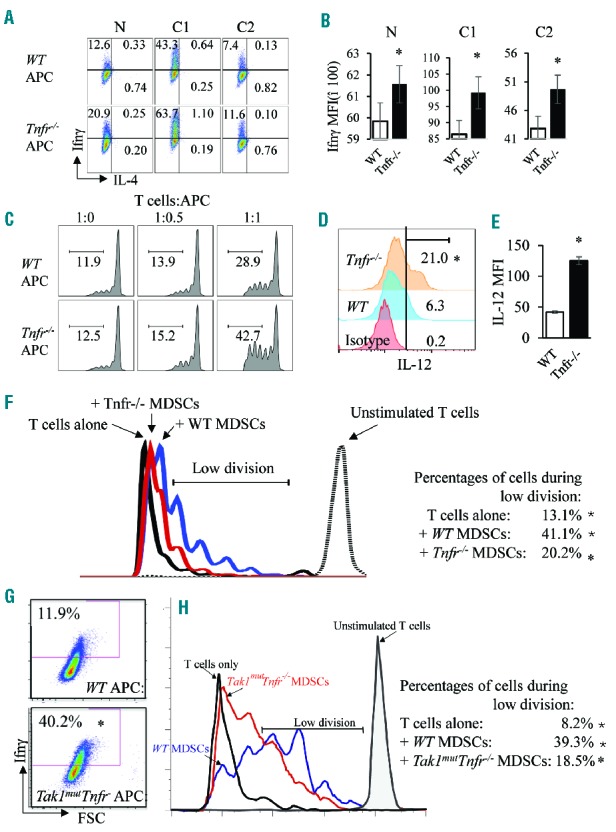
Enhanced function of Tnfr−/− APCs and decreased function of Tnfr−/− MDSCs. (A and B) T cells from wild-type (WT) spleens were first isolated using a pan-T-cell isolation kit, then separated with anti-CD62L. Naïve T cells (CD62L+) were further cultured with WT and Tnfr−/− APCs for seven days under three conditions: 1) neutral condition (N); 2) Th1-priming condition (C1, in the presence of IL-12 and anti-IL-4); and 3) Th2-priming condition (C2, in the presence of IL-4 and anti-IL-12). Cells were collected and subjected to intracellular staining for cytokines (A). The mean fluorescence intensity (MFI) of Ifnγ in all of the groups was calculated and is presented in bar graphs (B). (C–E) CFSE-labeled WT T cells were co-cultured with either WT or Tnfr−/− APCs in the presence of anti-CD3 and anti-CD28. After three days of culturing, cells were collected and analyzed for the percentage of T cells with more than 3 divisions (C). WT and Tnfr−/− APCs were stained for intracellular IL-12 (D). MFI of IL-12 in the two groups in (D) was calculated and presented in bar graphs (E). (F) CD11b+Gr1+ cells were sorted from WT and Tnfr−/− bone marrow and added to anti-CD3-activated, CFSE-labeled WT T cells at a ratio of T:MDSC=1:2 and co-cultured for six days. The CFSE signals were analyzed on day 6. The results were analyzed to show the cells with a low number of divisions (2–6 divisions). (G) T cells from WT spleens were isolated using a pan-T-cell isolation kit and were cultured with WT and Tak1mutTnfr−/− APCs for seven days under Th1-priming condition. Cells were collected and subjected to intracellular staining for Ifnγ. (I) CD11b+Gr1+ cells were sorted from WT and Tak1mutTnfr−/− BM, added to anti-CD3-activated, CFSE-labeled WT T cells at a ratio of T:MDSC=1:2 and co-cultured for six days. The CFSE signals were analyzed on day 6. The results were analyzed to show the cells with a low number of divisions. The samples were analyzed in triplicate and experiments were repeated independently twice. *P<0.05 compared to WT.
CD4+ lymphocyte depletion restores normal hematopoiesis to Tak1mutTnfr−/− mice
T-cell depletion by anti-thymocyte globulin (ATG) has been demonstrated to be the most effective treatment for AAA.4–9 Our data indicate that CD4+ Th1 cells are the major type of cell that is increased in the BM of BMF mice. Therefore, we assessed whether depletion of CD4+ T cells would restore normal hematopoiesis to BMF mice. After the onset of BMF in Tak1mutTnfr−/− mice at the age of two months, we treated them with anti-CD4 antibody (i.p. injection of 250 μg/mouse, weekly). We found that anti-CD4 antibody treatment efficiently depleted CD4+ T cells from BM (Figure 5A) and PB (Online Supplementary Figure 10A). Of note, following the depletion of CD4+ T cells, the frequency of CD8+ T cells also decreased to a normal level (Figure 5A), suggesting that the increased number of activated CD8+ T cells in BMF mice is secondary to the activation of CD4+ T cells. Strikingly, after anti-CD4 treatment for two months (9 injections), all BMF mice regained body weight and became grossly normal (Online Supplementary Figure S10B). In addition, anti-CD4 treatment prevented both degeneration of the thymus and splenomegaly (Online Supplementary Figure S10D-H). While all IgG-treated control mice died by six months of age, anti-CD4 treated mice (weekly treatment) survived for over ten months (Table 1). At the conclusion of the experiments, we examined the hematopoietic tissues of the experimental animals. We found that anti-CD4-treated mice showed normal thymic and splenic sizes and normal cell counts (Online Supplementary Figure S10D–F). RBC/Hb, WBC and plt in PB and BM cellularity values were comparable to WT control mice (Figure 5B and C and Online Supplementary Figure S10). Analysis of HSPCs showed that the expression of Sca1 in Lin− BM cells was also comparable in anti-CD4-treated mice and WT mice (Figure 5D and E). Also, Tnfα and Ifnγ levels in PB returned to normal levels (Online Supplementary Figure S15). Thus, we concluded that the BMF observed in Tak1mutTnfr−/− mice is caused by autoreactive CD4+ T lymphocytes. In addition, the reversible hematopoiesis in Tak1mutTnfr−/− BMF mice suggested that HSPCs were not exhausted in such mice. Th1-mediated hematopoietic repression is the key pathogenic factor for BMF in such mice. Th1 and Tc1 cells did not seem to directly attack the HPSCs (Online Supplementary Figure S11), but induced apoptosis and repression of proliferation (Figure 1F and G), as well as induction of differentiation of HSPCs (Online Supplementary Figure S11) by secreting Ifnγ.
Figure 5.
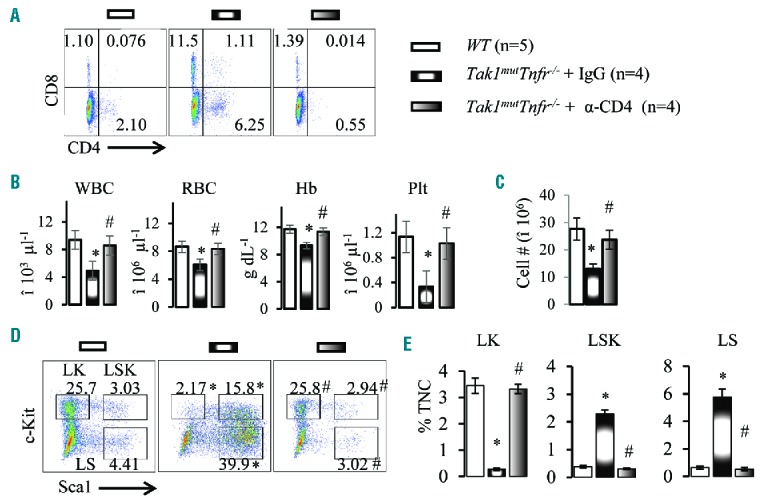
Depletion of CD4+ T cells restores normal hematopoiesis to Tak1mutTnfr−/− mice. (A) Bone marrow (BM) samples were collected from wild-type (WT) mice and Tak1mutTnfr−/− mice treated with IgG and anti-CD4 antibody. Flow cytometric analyses of the frequencies of CD4+ and CD8+ T cells are shown. (B) White blood cells (WBC), red blood cells (RBC), hemoglobin (Hb) and platelets (plt) were analyzed using the Hemavet 950 Hematology System. (C) Number of total nucleated cells (TNCs) in BM from two hind limbs were counted and compared. (D) Representative flow cytometric plots for analysis of BM hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs). BM cells were first gated on the Lin− population and then analyzed for LK, LSK and LS populations. (E) Percentages of LK, LSK and LS populations in TNCs of BM. Data are presented as means±SD. *P<0.05 compared to WT; #P<0.05 compared to IgG treatment.
Ifnγ knockout prevents BMF development in Tak1mutTnfr−/− mice
Increased levels of IFNγ are believed to be important in the pathogenesis of AAA.20,45 Under appropriately clean housing conditions, Ifnγ−/− mice are grossly healthy without detectable hematopoietic defects. To determine whether Ifnγ mediates the hematopoietic repressive activity of activated CD4+ T cells in Tak1mutTnfr−/− mice, we studied whether Ifnγ deletion can prevent the development of AAA. As expected, Ifnγ deletion completely prevented development of the disease in these mice. Tak1mutTnfr−/−Ifnγ−/+ mice showed normal growth and development of both the thymus and bone marrow, bred normally and had the same lifespan as WT controls (Table 1 and Online Supplementary Figure S12A–G). All Tak1mutTnfr−/−Ifnγ−/− mice were comparable to WT and Ifnγ−/− control mice with respect to hematopoietic parameters in PB and BM (Figure 6A–F); B/T cell numbers and T-cell activation (Figure 6G–J) were likewise comparable. In addition, Tak1mutTnfr−/−Ifnγ−/+ mice (Ifnγ heterozygous litter-mates) showed an attenuated phenotype compared to Tak1mutTnfr−/− mice. BMF in Tak1mutTnfr−/−Ifnγ+/− mice was less severe than that seen in Tak1mutTnfr−/− mice but more severe than that seen in Tak1mut mice based on studies of hematopoietic parameters in PB, BM, spleen and thymus (Online Supplementary Figure S12C–G). Tak1mutTnfr−/−Ifnγ−/+ mice could survive up to ten months, a significant extension in lifespan compared to Tak1mutTnfr−/− mice (Table 1). These mice also showed a reduced Th1 responsiveness and Ifnγ expression than did Tak1mutTnfr−/−Ifnγ+/+ mice (Online Supplementary Figure S12I and J). It can be concluded that Ifnγ derived from Th1 cells is responsible for the development of BMF in a gene dose-dependent manner.
Figure 6.
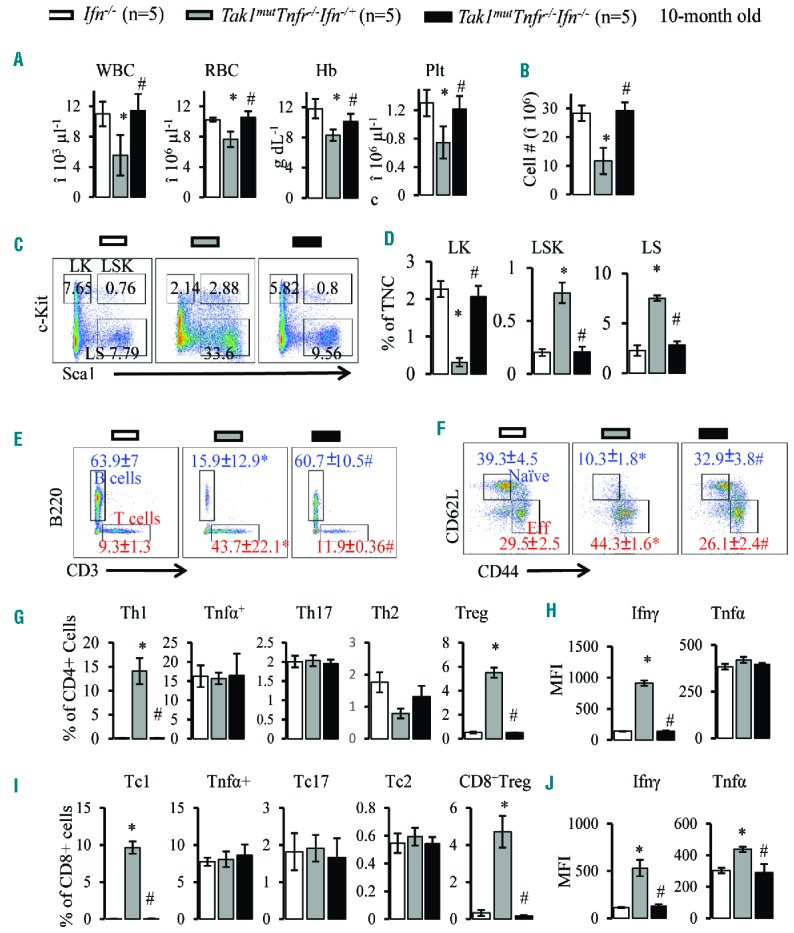
Ifnγ knockout prevents BMF in Tak1mutTnfr−/− mice. Peripheral blood (PB) and bone marrow (BM) samples were collected from Ifnγ−/−, Tak1mutTnfr−/−Ifnγ+/− and Tak1mutTnfr−/−Ifnγ−/− mice at the age of ten months (n=5/group). (A) White blood cells (WBC), red blood cells (RBC), hemoglobin (Hb) and platelets (plt) were analyzed using the Hemavet 950 Hematology System. (B) Number of total nucleated cells (TNCs) in BM from two hind limbs were counted and compared. (C) Representative flow cytometric plots for analysis of BM hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs). BM cells were first gated on the Lin− population and then analyzed for LK, LSK and LS populations. (D) Percentages of LK, LSK and LS populations in total nucleated cells (TNCs) of BM. (E) Flow analysis of BM CD3+ T cells and B220+ B cells (gated on lymphocytes). (F) Flow cytometric analysis of naïve T cells (CD62L+ CD44−) and activated T cells (CD62LlowCD44hi) in BM (gated on CD3+ cells). (G) Percentages of Th1, Tnfα+, Th17, Th2, and Treg cells in BM (gated on CD4+ T cells). (I) Percentages of Tc1, Tnfa+, Tc17, Tc2, and Treg cells in BM (gated on CD8+ T cells). (H and J) Ifnγ and Tnfα levels in BM CD4+ (H) and CD8+ (J) T cells were analyzed by mean fluorescence intensity (MFI) of intracellular antibody staining. Data are presented as means±SD. *P<0.05 compared to Tnfr−/−; #P<0.05 compared to Tak1mutTnfr−/−Ifnγ+/−.
Rip3 deletion prevented BMF development in Tak1mutTnfr−/− mice
HSPCs with Tak1 deletion died from either apoptosis or necroptosis.25,26 Apoptotic cells are usually removed by macrophages, which normally do not induce immune reactions. However, necroptotic cells release intracellular components called DAMPs (damage-associated molecular patterns) which can induce immune responses.23,24,46–48 Thus we speculated that the Th1-mediated autoimmune BMF in Tak1mut mice might be induced by factors released from cells undergoing necroptosis. Rip3 is the key mediator of necroptosis.49,50 To test such a hypothesis, we crossed Rip3−/− mice with Cre+Tak1f/+Tnfr−/− mice to produce mice with Tak1mutTnfr−/−Rip3+/− and Tak1mutTnfr−/−Rip3−/− genotypes. Heterozygous deletion of Rip3 did not influence the disease phenotype of Tak1mutTnfr−/− mice. Interestingly, homozygous Rip3 deletion completely prevented BMF development. Tak1mutTnfr−/−Rip3−/− mice were grossly normal in growth and reproductive capability (Table 1 and Online Supplementary Figure S13A and B). RBC, Hb, plt and WBC in PB (Figure 6A), cellularity in BM, thymuses and spleens (Figure 6B and Online Supplementary Figure S13CG), BM HSPCs (Figure 6C and D), as well as B/T cell numbers and T-cell activation (Figure 6E–J) of Tak1mutTnfr−/−Rip3−/− mice were comparable to WT and Rip3−/− controls at the age of four months, a time point at which all Tak1mutTnfr−/− mice show severe BMF. Again, no accumulation of ΔTak1 HSPCs was detected in Tak1mutTnfr−/−Rip3−/− mice (Online Supplementary Figure S14), suggesting: 1) BMF in Tak1mutTnfr−/− mice is not due to an increase in ΔTak1 HSPCs; 2) inactivation of Rip3 did not prevent the elimination of ΔTak1 HSPCs. To support this notion further, we found that ΔTak1 HSPCs died of apoptosis when Rip3 was inactivated (Online Supplementary Figure S3).
Discussion
Although the autoimmune feature of AAA has been well documented, the pathogenesis of this life-threatening disease is still not clear owing to the lack of an adequately representative animal model which can be used to test hypotheses. Most previous studies used an allogeneic T-cell-transfer model which induces BMF by donor T cells from a different genetic background.51,52 Such a model best resembles a graft-versus-host disease dynamic which does not sufficiently reproduce the disease onset and progression observed with AAA patients. By using genetically-mutant animal models, we developed an autoimmune BMF model. In this model, the Tak1 gene is spontaneously mutated in a small subset of HSPCs, which scenario might mimic the cryptic genetic lesions (clonal somatic mutations in a small subset of HSPCs) which are typically detected in AAA patients.5,10–15 Using such a model, we demonstrated that mutant HSPCs undergoing Rip3-mediated necroptosis might be the cause of autoimmune BMF. The mutant HSPCs release factors such as DNA and intracellular proteins which may serve as autoimmunogenic antigens to induce Th1-related autoimmune responses in mice. Our data suggest that necroptosis-Th1 response-Ifnγ is the axis of development of this disease (Figure 8). It might also explain the pathogenesis of some viral infection-related BMF because viral DNA products also induce necroptosis.53
Figure 8.
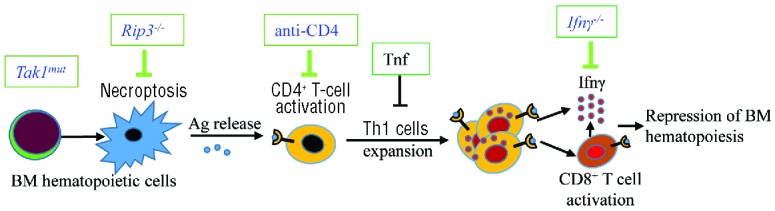
A working model for necroptosis-initiated autoimmune bone marrow failure (BMF). Antigens released from cells undergoing necroptosis owing to genetic mutation of Tak1 in a small subset of hematopoietic stem and progenitor cells (HSPC) stimulate CD4+ T-cell activation and promote the development of Th1/Tc1 cells, which in turn produce a large amount of IFNγ, resulting in bone marrow (BM) exhaustion. Tnf restricts the onset and progression of BMF by repressing Th1 and Tc1 activation. BMF in the current model can be prevented/cured by targeting the key components of the necropotosis-Th1 cell-Ifnγ axis.
Figure 7.
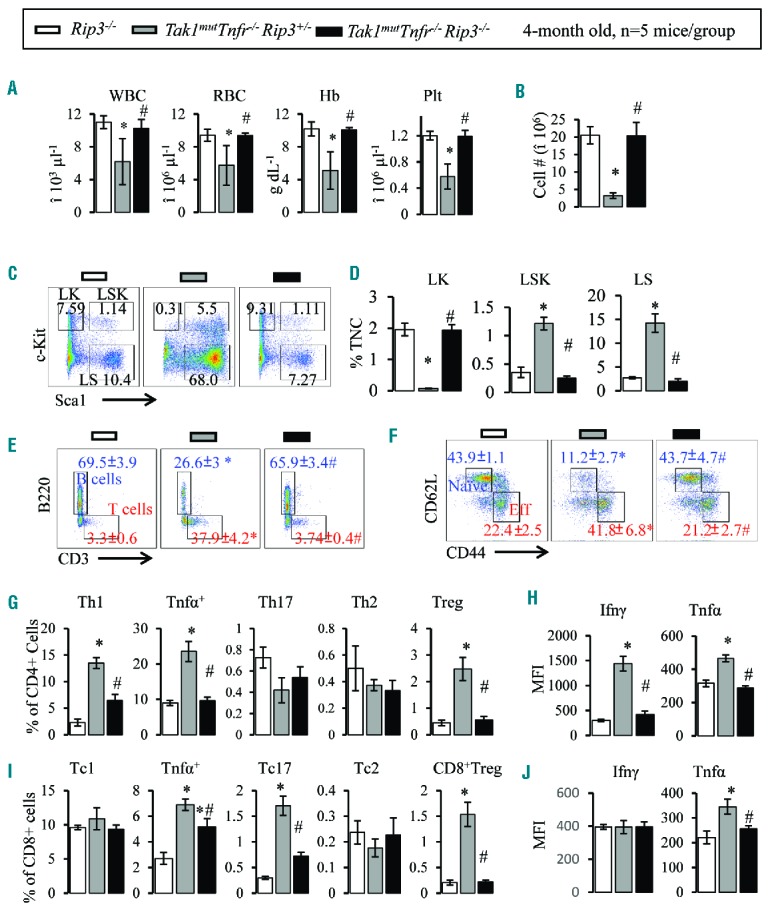
Rip3 knockout prevents BMF in Tak1mutTnfr−/− mice. Peripheral blood (PB) and bone marrow (BM) samples were collected from 4-month old Rip3−/−, Tak1mutTnfr−/−Rip3+/− and Tak1mutTnfr−/−Rip3−/− mice. (A) White blood cells (WBC), red blood cells (RBC), hemoglobin (Hb) and platelets (plt) were analyzed using the Hemavet 950 Hematology System. (B) Number of total nucleated cells (TNCs) in BM from two hind limbs were counted and compared. (C) Representative flow cytometric plots for analysis of BM hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs). BM cells were first gated on the Lin− population and then analyzed for LK, LSK and LS populations. (D) Percentages of LK, LSK and LS populations in TNCs of BM. (E) Flow analysis of BM CD3+ T cells and B220+ B cells (gated on lymphocytes). (F) Flow cytometric analysis of naïve T cells (CD62L+ CD44−) and activated T cells (CD62LlowCD44hi) in BM (gated on CD3+ cells). (G) Percentages of Th1, Tnfα+, Th17, Th2, and Treg cells in BM (gated on CD4+ T cells). (I) Percentages of Tc1, Tnfa+, Tc17, Tc2, and Treg cells in BM (gated on CD8+ T cells). Ifnγ and Tnfα levels in BM CD4+ (H) and CD8+ (J) T cells were analyzed by mean fluorescence intensity (MFI) of intracellular antibody staining. Data are presented as means±SD. *P<0.05 compared to Rip3−/−; #P<0.05 compared to Tak1mutTnfr−/−Rip3+/−.
Elevated levels of both IFNγ and TNFα are commonly detected in AAA patients’ blood. It has been proposed that both IFNγ and TNFα contribute to hematopoietic repression in the pathogenesis of AAA.17–19,28,29 The elevated serum levels of both IFNγ and TNFα are consistent with the occurrence of BMF in our animal model (Online Supplementary Figure S15). However, we determined that these two inflammatory cytokines function in opposite ways in the pathogenesis of AAA. Ifnγ is the key effector of the autoimmune response which represses BM hematopoiesis,20,45 whereas Tnfα is a factor which restricts autoimmune responsiveness and inhibits the progression of AAA. This observation is contrary to the currently accepted dogma for the role of TNFα in the pathogenesis of autoimmune diseases.28,29 Based on the data we have presented in the current studies, we suggest that anti-TNF treatment should be avoided in treating patients with autoimmune-related BMF such as AAA. In fact, TNF blockade-related autoimmune BMF has been reported consistently.54,55
Many potential mechanisms could explain how Tnfα restricts the development of BMF. After experimentally ruling out other mechanisms, we concluded that Tnf signaling restricts autoimmune reactions by: 1) repressing the Th1-stimulating activity of APC by suppressing IL-12 expression; and 2) promoting the T-cell-repressing functions of MDSCs.56 Treg cell changes in our model are more likely to be a result rather than the cause of abnormal immune responsiveness because such changes are not always correlated to disease onset, progression and severity.
The clonal evolution of mutant HSPCs and associated subsequent leukemic transformation is one of the critical problems for AAA patients. Inhibition of apoptosis is always correlated to increased risk of tumor development. However, the role of necroptosis in carcinogenesis and drug resistance has not been well explored. One of the concerns for the inhibition of necroptosis in BMF patients is whether it also increases the development of leukemia by promoting the clonal expansion of mutant cells. Myeloid-specific Tak−/− mice develop chronic myelomonocytic leukemia.57 We predicted that Tak1mut in Tak1mutRip3−/− mice might be more susceptible to the development of leukemia as they age. However, we did not detect an age-related accumulation of Tak1mut HSPCs in BM of Tak1mutRip3−/− mice (Online Supplementary Figure S14). Such mice did not show any sign of hematopoietic abnormalities for up to one year of age. We found that Tak1mut HSPCs died of apoptosis when necroptosis is inhibited because Rip3-mediated necroptotic signaling and caspase 8-mediated apoptotic signaling are mutually repressed and interconvertible. In fact, in another of our studies, we found that Rip3 signaling in required for maintaining the undifferentiated state of leukemic cells by repressing calpain-mediated STAT3α and SOCS1 degradation (J Xin, 2016, unpublished data). Thus, we believe that inhibition of Rip3 signaling might be a reasonable explanation for the repression of the clonal evolution of mutant HSPCs by promoting apoptosis or differentiation in BMF patients.
Supplementary Material
Acknowledgments
The authors thank the staff of the Department of Comparative Medicine of Loyola University Medical Center for their excellent animal care services, as well as Drs. Manuel Diaz, Nancy Zeleznik-Le, Andrew Dingwall and Wei Qiu for their ongoing professional collaboration and scientific suggestions and discussions, which improved the scientific quality of the present studies. We appreciate laboratory support from Patience Oladeinde, Danielle Howard and Emma Yao, and FACS sorting and analysis assistance from Patricia Simms, Ashley Hess, Shwetha Ravichandran and Veronica Volgina. We also appreciate Drs. Xiaoping Du and Aleksandra Stojanovic-Terpo (University of Illinois Chicago) for their assistance with CBC analysis.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/2/295
Funding
This work was supported by US Department of Defense grant (BM120072) and NIH grants (R01HL95896 and R21CA181970) to JZ through Loyola University Chicago, and also by the Leukemia Research Foundation New Investigator Award (8th Annual George Richard Memorial Grant) to JX. JX was also supported in part by a grant from the Muscular Dystrophy Association (MDA202906).
References
- 1.Young NS, Scheinberg P, Calado RT. Aplastic anemia. Curr Opin Hematol. 2008. (3):162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodsky RA, Jones RJ. Aplastic anaemia. Lancet. 2005;365(9471):1647–1656. [DOI] [PubMed] [Google Scholar]
- 3.Alter BP. Diagnosis, genetics, and management of inherited bone marrow failure syndromes. Hematology Am Soc Hematol Educ Program. 2007:29–39. [DOI] [PubMed] [Google Scholar]
- 4.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012; 120(6):1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant. 2010;(1 Suppl):S119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young NS. Pathophysiologic mechanisms in acquired aplastic anemia. Hematology Am Soc Hematol Educ Program. 2006:72–77. [DOI] [PubMed] [Google Scholar]
- 7.Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, Wu CO, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365(5):430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kordasti S, Marsh J, Al-Khan S, et al. Functional characterization of CD4+ T cells in aplastic anemia. Blood. 2011;119(9): 2033–2043. [DOI] [PubMed] [Google Scholar]
- 9.Risitano AM, Maciejewski JP, Green S, et al. In-vivo dominant immune responses in aplastic anaemia: molecular tracking of putatively pathogenetic T-cell clones by TCR beta-CDR3 sequencing. Lancet. 2004; 364(9431):355–364. [DOI] [PubMed] [Google Scholar]
- 10.Afable MG, 2nd, Wlodarski M, Makishima H, et al. SNP array-based karyotyping: differences and similarities between aplastic anemia and hypocellular myelodysplastic syndromes. Blood. 2011;117(25):6876–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiu R, Gondek L, O’Keefe C, Maciejewski JP. Clonality of the stem cell compartment during evolution of myelodysplastic syndromes and other bone marrow failure syndromes. Leukemia. 2007;21(8):1648–1657. [DOI] [PubMed] [Google Scholar]
- 12.Bagby GC, Fleischman AG. The stem cell fitness landscape and pathways of molecular leukemogenesis. Front Biosci (Schol Ed). 2011;3:487–500. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Li X, Ge M, et al. Long-term follow-up of clonal evolutions in 802 aplastic anemia patients: a single-center experience. Ann Hematol. 2011;90(5):529–537. [DOI] [PubMed] [Google Scholar]
- 14.Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic Mutations and Clonal Hematopoiesis in Aplastic Anemia. N Engl J Med. 2015;373(1):35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babushok DV, Olson TS, Bessler M. Somatic Mutations and Clonal Hematopoiesis in Aplastic Anemia. N Engl J Med. 2015;373(17):1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a subgroup of aplastic anemia patients who progress to myelodysplastic syndrome. Blood. 2014;124(17):2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sloand E, Kim S, Maciejewski JP, et al. Intracellular interferon-gamma in circulating and marrow T cells detected by flow cytometry and the response to immunosuppressive therapy in patients with aplastic anemia. Blood. 2002;100(4):1185–1191. [DOI] [PubMed] [Google Scholar]
- 18.Dufour C, Corcione A, Svahn J, et al. TNF-alpha and IFN-gamma are overexpressed in the bone marrow of Fanconi anemia patients and TNF-alpha suppresses erythropoiesis in vitro. Blood. 2003;102(6): 2053–2059. [DOI] [PubMed] [Google Scholar]
- 19.Dybedal I, Bryder D, Fossum A, et al. Tumor necrosis factor (TNF)-mediated activation of the p55 TNF receptor negatively regulates maintenance of cycling reconstituting human hematopoietic stem cells. Blood. 2001;98(6):1782–1791. [DOI] [PubMed] [Google Scholar]
- 20.de Bruin AM, Voermans C, Nolte MA. Impact of interferon-gamma on hematopoiesis. Blood. 2014;124(16):2479–2486. [DOI] [PubMed] [Google Scholar]
- 21.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26(22):3214–3226. [DOI] [PubMed] [Google Scholar]
- 22.Mihaly SR, Ninomiya-Tsuji J, Morioka S. TAK1 control of cell death. Cell Death Differ. 2014;21(11):1667–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015; 517(7534):311–320. [DOI] [PubMed] [Google Scholar]
- 24.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370(5):455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao Y, Li H, Zhang J, et al. TNF-alpha/Fas-RIP-1-induced cell death signaling separates murine hematopoietic stem cells/progenitors into 2 distinct populations. Blood. 2011;118(23):6057–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang M, Wei X, Guo Y, et al. TAK1 is required for the survival of hematopoietic cells and hepatocytes in mice. J Exp Med. 2008;205(7):1611–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Xiao Y, Guo Y, et al. Differential requirements for c-Myc in chronic hematopoietic hyperplasia and acute hematopoietic malignancies in Pten-null mice. Leukemia. 2011;25(12):1857–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Zou Z, Wu Z, et al. TNF-alpha-induced programmed cell death in the pathogenesis of acquired aplastic anemia. Expert Rev Hematol. 2015;8(4):515–526. [DOI] [PubMed] [Google Scholar]
- 29.Dufour C, Ferretti E, Bagnasco F, et al. Changes in cytokine profile pre- and post-immunosuppression in acquired aplastic anemia. Haematologica. 2009;94(12):1743–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roderick JE, Hermance N, Zelic M, et al. Hematopoietic RIPK1 deficiency results in bone marrow failure caused by apoptosis and RIPK3-mediated necroptosis. Proc Natl Acad Sci USA. 2014;111(40):14436–14441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dillon CP, Weinlich R, Rodriguez DA, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157(5):1189–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol. 2015;16(7): 689–697. [DOI] [PubMed] [Google Scholar]
- 33.Nakada D, Oguro H, Levi BP, et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505(7484):555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24(4):1464–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Libregts SF, Gutierrez L, de Bruin AM, et al. Chronic IFN-gamma production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. 2011;118(9):2578–2588. [DOI] [PubMed] [Google Scholar]
- 37.Wan YY, Chi H, Xie M, et al. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol. 2006;7(8):851–858. [DOI] [PubMed] [Google Scholar]
- 38.Sato S, Sanjo H, Tsujimura T, et al. TAK1 is indispensable for development of T cells and prevention of colitis by the generation of regulatory T cells. Int Immunol. 2006;18(10):1405–1411. [DOI] [PubMed] [Google Scholar]
- 39.Liu HH, Xie M, Schneider MD, et al. Essential role of TAK1 in thymocyte development and activation. Proc Natl Acad Sci USA. 2006;103(31):11677–11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young NS. Current concepts in the pathophysiology and treatment of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2013;2013:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2010;32(2):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacNamara KC, Jones M, Martin O, Winslow GM. Transient activation of hematopoietic stem and progenitor cells by IFNgamma during acute bacterial infection. PLoS One. 2011;6(12):e28669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldridge MT, King KY, Boles NC, et al. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010; 465(7299):793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato T, Onai N, Yoshihara H, et al. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med. 2009;15(6):696–700. [DOI] [PubMed] [Google Scholar]
- 45.Lin FC, Karwan M, Saleh B, et al. IFN-gamma causes aplastic anemia by altering hematopoietic stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124(25):3699–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38(2):209–223. [DOI] [PubMed] [Google Scholar]
- 47.Han J, Zhong CQ, Zhang DW. Programmed necrosis: backup to and competitor with apoptosis in the immune system. Nat Immunol. 2011;12(12):1143–1149. [DOI] [PubMed] [Google Scholar]
- 48.Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol. 2014;35:14–23. [DOI] [PubMed] [Google Scholar]
- 49.Moriwaki K, Chan FK. RIP3: a molecular switch for necrosis and inflammation. Genes Dev. 2013;27(15):1640–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang DW, Shao J, Lin J, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. [DOI] [PubMed] [Google Scholar]
- 51.Bloom ML, Wolk AG, Simon-Stoos KL, et al. A mouse model of lymphocyte infusion-induced bone marrow failure. Exp Hematol. 2004;32(12):1163–1172. [DOI] [PubMed] [Google Scholar]
- 52.Chen J, Desierto MJ, Feng X, Biancotto A, Young NS. Immune-mediated bone marrow failure in C57BL/6 mice. Exp Hematol. 2015;43(4):256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaiser WJ, Sridharan H, Huang C, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013; 288(43):31268–31279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuruvilla J, Leitch HA, Vickars LM, et al. Aplastic anemia following administration of a tumor necrosis factor-alpha inhibitor. Eur J Haematol. 2003;71(5):396–398. [DOI] [PubMed] [Google Scholar]
- 55.Kozak N, Friedman J, Schattner A. Etanercept-associated transient bone marrow aplasia: a review of the literature and pathogenetic mechanisms. Drugs R D. 2014;14(2):155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sade-Feldman M, Kanterman J, Ish-Shalom E, et al. Tumor necrosis factor-alpha blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity. 2013; 38(3):541–554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


