Abstract
Central nervous system infiltration and relapse are poorly understood in childhood acute lymphoblastic leukemia. We examined the role of zeta-chain-associated protein kinase 70 in preclinical models of central nervous system leukemia and performed correlative studies in patients. Zeta-chain-associated protein kinase 70 expression in acute lymphoblastic leukemia cells was modulated using short hairpin ribonucleic acid-mediated knockdown or ectopic expression. We show that zeta-chain-associated protein kinase 70 regulates CCR7/CXCR4 via activation of extracellular signal-regulated kinases. High expression of zeta-chain-associated protein kinase 70 in acute lymphoblastic leukemia cells resulted in a higher proportion of central nervous system leukemia in xenografts as compared to zeta-chain-associated protein kinase 70 low expressing counterparts. High zeta-chain-associated protein kinase 70 also enhanced the migration potential towards CCL19/CXCL12 gradients in vitro. CCR7 blockade almost abrogated homing of acute lymphoblastic leukemia cells to the central nervous system in xenografts. In 130 B-cell precursor acute lymphoblastic leukemia and 117 T-cell acute lymphoblastic leukemia patients, zeta-chain-associated protein kinase 70 and CCR7/CXCR4 expression levels were significantly correlated. Zeta-chain-associated protein kinase 70 expression correlated with central nervous system disease in B-cell precursor acute lymphoblastic leukemia, and CCR7/CXCR4 correlated with central nervous system involvement in T-cell acute lymphoblastic leukemia patients. In multivariate analysis, zeta-chain-associated protein kinase 70 expression levels in the upper third and fourth quartiles were associated with central nervous system involvement in B-cell precursor acute lymphoblastic leukemia (odds ratio=7.48, 95% confidence interval, 2.06–27.17; odds ratio=6.86, 95% confidence interval, 1.86–25.26, respectively). CCR7 expression in the upper fourth quartile correlated with central nervous system positivity in T-cell acute lymphoblastic leukemia (odds ratio=11.00, 95% confidence interval, 2.00–60.62). We propose zeta-chain-associated protein kinase 70, CCR7 and CXCR4 as markers of central nervous system infiltration in acute lymphoblastic leukemia warranting prospective investigation.
Introduction
Diagnosis and treatment of central nervous system (CNS) infiltration and/or CNS relapse in childhood acute lymphoblastic leukemia (ALL) remain challenging.1,2 CNS leukemia is mainly a leptomeningeal disease, but leukemic cells may be detectable in the cerebrospinal fluid.3 Survival and homing mechanisms of leukemic cells into the CNS remain largely unclear. Factors associated with CNS involvement are peripheral hyperleukocytosis, a pro-B- or T-cell immunophenotype,4,5 and certain cytogenetics (e. g., BCR-ABL in B-cell precursor ALL (BCP-ALL)).4,6 Additionally, the E2A-PBX1 fusion (t(1;19)(q23;p13)) has been associated with an increased risk of CNS relapses in BCP-ALL,7 and we recently identified the MER tyrosine kinase as a marker for CNS involvement in that entity.8 Most chemotherapeutic drugs applied for ALL treatment poorly penetrate the blood-brain barrier. Accordingly, current pediatric treatment protocols advocate extensive intrathecal and systemic chemotherapy,9–11 which has been associated with long-term neurologic sequelae.11,12 Thus, more precise diagnostic and prognostic markers for CNS involvement in ALL are needed, not only to control CNS infiltration but also to avoid systemic overtreatment.
Zeta-chain-associated protein kinase 70 (ZAP70), a tyrosine kinase mainly expressed in T cells and natural killer (NK) cells, is also found to be expressed at low levels in B cells.13 The role of ZAP70 in BCP-ALL is poorly described. Few reports have shown that ZAP70 is expressed in some ALL cell lines and in a report of 5 patients with the E2A-PBX1 fusion.14–16 ZAP70 has been shown to be overexpressed in B-cell chronic lymphocytic leukemia (B-CLL), indicating an aggressive course of the disease.17 ZAP70 expression and phosphorylation have been associated with B-cell receptor (BCR) signaling in B-CLL.18,19 Further, ZAP70 may function as an adaptor protein and enhance BCR signaling independently of its kinase activity.20 ZAP70 has been shown to enhance the migration of malignant B cells to the bone marrow (BM) by upregulating adhesion molecules and chemokine receptors (CCRs),21,22 and is also required for C-X-C motif chemokine ligand 12 (CXCL12)-mediated T-cell transendothelial migration.23,24 In T-cell acute lymphoblastic leukemia (T-ALL), the chemokine receptors CCR7 and CXCR4 have been associated with an increased capability of T-ALL cells to enter the CNS, mainly in cell line models.25,26
We hypothesized that ZAP70 mediates the infiltration and the survival of ALL cells in the CNS. Downregulating ZAP70 in ALL cell lines resulted in a reduced CCR7/CXCR4 expression and an impaired CNS infiltration in NSG mice via the regulation of ERK. In contrast, up-regulating ZAP70 caused an enhanced CCR7/CXCR4 expression and an improved migratory capacity towards chemokine ligand 19 (CCL19) and CXCL12 gradients. Blocking CCR7 with a monoclonal antibody resulted in a decreased homing capacity of ALL cells in vivo, including the CNS. High ZAP70 expression in patient samples correlated with CNS infiltration in xenografts. Furthermore, patient CNS-positivity correlated with a high ZAP70 expression in BCP-ALL, and with a high CCR7/CXCR4 expression in T-ALL patients. Multivariate analysis confirmed that a high expression of ZAP70 and CCR7 conferred an increased risk for CNS involvement in these patients. Our data suggest an important role of these mechanisms for homing and survival of ALL cells in the CNS niche.
Methods
Cell lines, antibodies
REH and 697 (EU-3) cell lines were purchased from DSMZ. JURKAT cells were provided by Dr. Renate Burger (University of Kiel, Germany). Cell viability was measured using trypan blue. CD19, CD3ε, hCD45, mCD45, CCR7, CXCR4 and ZAP70 antibodies were purchased from eBioscience or Santa Cruz Biotechnology, p-ERK/ERK and β-tubulin antibodies from Cell Signaling Technology, anti-Immunoglobulin M (IgM) F(ab)2 from SouthernBiotech, and U0126 from LC Laboratories.
Patients
130 BCP-ALL and 117 T-ALL patients were treated according to the Berlin-Frankfurt-Münster (BFM) ALL 2000 or 2009 protocols. Informed consent was obtained according to institutional regulations, in accordance with the Declaration of Helsinki.
Xenografts
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) and NOD/SCID mice were purchased from Charles River Laboratories and bred. The mice were maintained as approved by the governmental animal care and use committees. Eight to twelve week old female mice were injected intrafemorally with 1 × 106 ALL cells from patient BM (>90% blasts), or intravenously with 1 × 104 – 1 × 105 ALL cells from cell lines.8,27 Animals were sacrificed upon detection of >75% leukemic blasts or clinical leukemia (weight or activity loss, organomegaly, hind limb paralysis). CNS-leukemic cells were recovered from meninges and separated on 30% percoll.28
Expression assays
Western blotting was performed as described.27 Mouse heads were decalcified, paraffin-embedded and cut. CNS infiltration was scored negative (−), intermediate (+) and high (++) as described.8 Quantitative real-time polymerase chain reaction (qRT-PCR) analyses were performed on an ABI7900HT using QuantiTect primer assays (Qiagen) and real-time quantitative PCR value (RQ) (2^-DCCT) was calculated.
Knockdown and upregulation of ZAP70
A short hairpin ribonucleic acid (shRNA) against ZAP70 (TRCN0000000438) was cloned into pSicoR-Ef1a-mCh (Addgene vector 31847). pSicoR-Ef1a-mCh expressing an shRNA against green fluorescent protein (GFP) was used as control (Addgene vector 31849). 697, REH and JURKAT cells were transduced with viruses and sorted for mCherry-positivity.8,27 pMIG or pMIG-ZAP70 vectors, provided by Prof. Michael Reth (University of Freiburg, Germany), were used to transduce leukemic cells.29
Chemotaxis
Chemotaxis was measured as described.21 5 × 105 cells were added to the top chamber of a transwell culture insert (Corning) and allowed to migrate toward media containing 2 μg/ml CCL19 or 0.1 μg/ml CXCL12 (ImmunoTools) for 16 h. The cell number in the lower chamber was determined.
Antibody treatment in vivo
NSG mice were injected with 10,000 697 or JURKAT cells/animal (day 0). 1 mg/kg of anti-CCR7 antibody (clone 150503, R&D Systems) was applied on day +1, +3 and +7. All mice were sacrificed when control animals showed signs of leukemia. Leukemic infiltration was assayed by FACS or histology.
Statistical analysis
Statistical tests are indicated in the Figure legends. A P-value of <0.05 was considered significant. For the in vivo histology (Figures 1 and 2) one-sided testing was chosen, since death rates at a time point in the control groups were known and we aimed to detect a difference in one direction. In vitro panels are representative of at least 2 independent experiments. Densitometry was performed using ImageJ.30 The association between gene expression and CNS status was examined by unconditional logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs).
Figure 1.
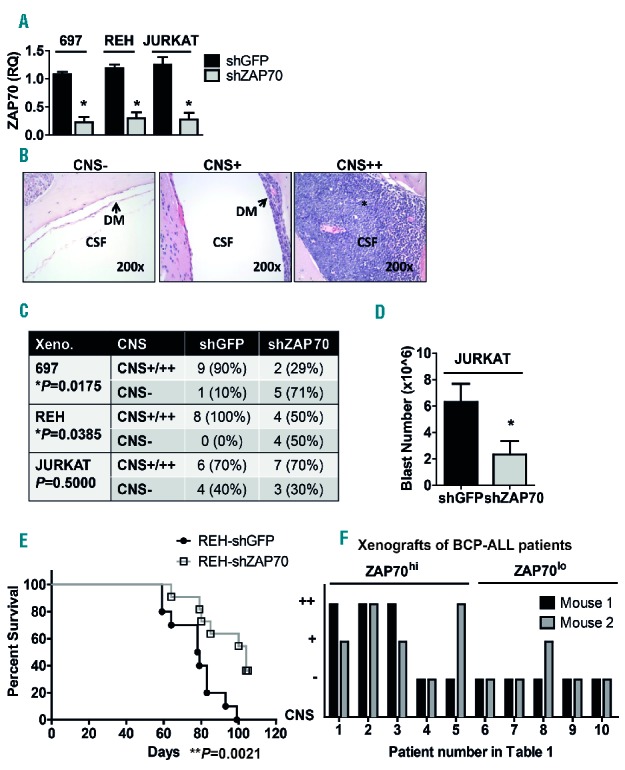
ZAP70 expression is associated with CNS infiltration in vivo. A: Efficiency of ZAP70 knockdown by shRNA, as determined by qRT-PCR. RQ=2^-DDCT. B: Semi-quantitative measurement of CNS infiltration as described in Methods. Hematoxylin and Eosin staining of a negative (−, left), an intermediate (+, middle), and a high (++, right) sample as examples for the scoring method, 200× magnification. * signifies leukemic infiltration. C: Mice were xenografted with 697 (n=10 per group), REH (n=8 per group) and JURKAT (n=10 per group) cells bearing an shRNA against ZAP70 (shZAP70) or a control (shGFP). Mice were sacrificed upon appearance of hind limb paralysis, and semi-quantitative scoring of the xenograft CNS is shown in the table (Fisher´s exact test, one-sided P-values. *P is significant). In the 697-shZAP70 group, 1 cage containing 3 mice was accidentally eliminated from the experiment on day +2. These animals were not included in the statistical analysis. D: Number of leukemic blasts recovered from the CNS of mice xenografted with either JURKAT-shGFP (n=4) or JURKAT-shZAP70 (n=3) (unpaired t-test, one-sided *P-value 0.041). E: 1×105 REH-shGFP or REH-shZAP70 cells/mouse were xenografted into 10 NOD/SCID mice/group by tail vein injection, xenograft survival (Kaplan-Meier log-rank test, **P=0.0021). F: 10 primary samples of pediatric BCP-ALL patients chosen according to ZAP70 expression (5 ZAP70lo and 5 ZAP70hi) were xenografted into duplicate NSG-mice. Mice were sacrificed when leukemic symptoms were visible and semi-quantitative scoring of the CNS was performed. Patient and xenograft characteristics are depicted in the Online Supplementary Table S1. *P<0.05; **P<0.01. CSF: cerebrospinal fluid; DM: dura mater; CNS: central nervous system; BCP-ALL: B-cell precursor acute lymphoblastic leukemia; Xeno: xenograft; shGFP: shRNA against GFP(green fluorescent protein); shZAP70: shRNA targeting ZAP70 (zeta-chain-associated protein kinase 70).
Figure 2.
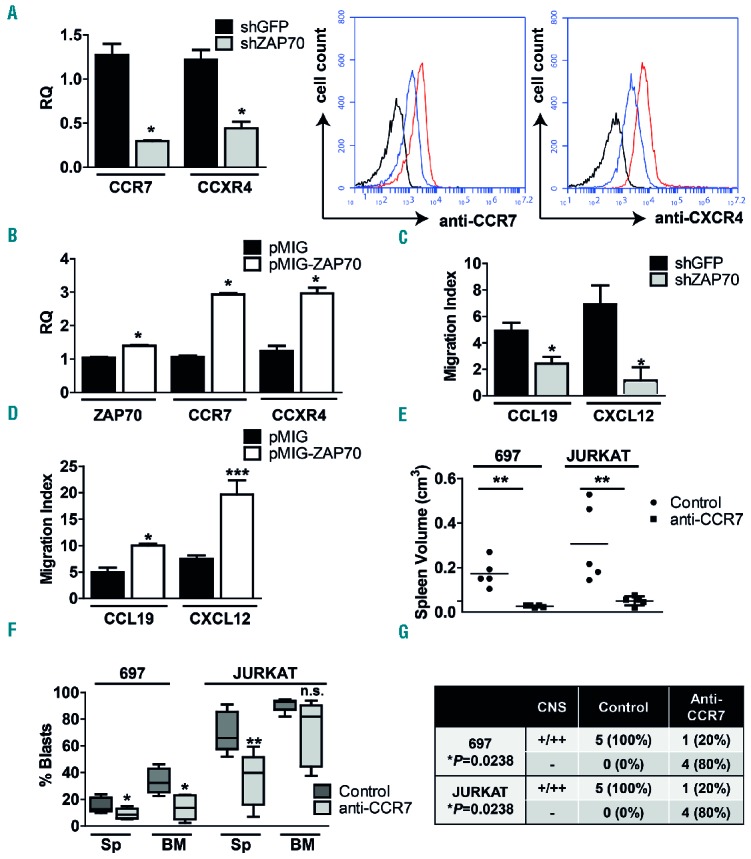
ZAP70 regulates CCR7 and CXCR4 expression, and CCR7 inhibition is relevant for homing processes. A: CCR7 and CXCR4 expression in 697-shZAP70 in comparison to 697-shGFP cells as measured by qRT-PCR (left) and by extracellular FACS staining (right), black: isotype, red: 697-shGFP and blue: 697-shZAP70. Unpaired t-test, two-sided P-value. B: ZAP70, CCR7 and CXCR4 expression in 697 cells transduced with either pMIG or pMIG-ZAP70, unpaired t-test, two-sided P-value. C: 697-shGFP or -shZAP70 cells were subjected to a migration assay toward CCL19 or CXCL12. Cells in the lower chamber were counted by flow cytometry or trypan blue. The migration index is defined as the number of transmigrating cells in the presence of the chemokine divided by the number of transmigrating cells to control medium. Results are shown as the mean ± SEM of 4 independent experiments (unpaired t-test, two-sided P-value). D: Migration assay of 697-pMIG and 697-pMIG-ZAP70 toward CCL19 or CXCL12 (unpaired t-test, two-sided P-value). E: 697 or JURKAT cells were injected into NSG mice, 5 mice were treated with 1 mg/kg anti-CCR7 antibody on day +1, +3 and +7, 5 mice were treated with vehicle alone. Spleen volume as assessed by the formula longest length × highest height × broadest width (unpaired t-test, two-sided P-value). F: Spleen and BM infiltration by human leukemic blasts in control and treated animals (unpaired t-test, two-sided P-value). G: Semi-quantitative CNS scoring of control and treated animals (Fisher´s exact test, one-sided P-value. *P is significant). *P<0.05; **P<0.01, ***P<0.001, n.s. = not significant. BM: bone marrow; CNS: central nervous system; Sp: spleen; shGFP: shRNA against GFP(green fluorescent protein); shZAP70: shRNA targeting ZAP70 (zeta-chain-associated protein kinase 70).
Results
ZAP70 expression influences CNS infiltration in vivo
First, we studied the effects of ZAP70 downregulation in ALL cell lines known to infiltrate the CNS of NSG and NOD/SCID mice. 697 and REH BCP-ALL and JURKAT T-ALL cells were transduced with a non-targeting shRNA (shGFP) or with an shRNA targeting ZAP70 (shZAP70). ZAP70 messenger RNA (mRNA) expression was reduced by >70% in all shZAP700 cells (Figure 1A). 697- and JURKAT-shGFP and -shZAP70 cells were then injected into NSG and REH-shGFP and -shZAP70 cells into NOD/SCID mice, a model for an almost isolated CNS leukemia with this cell line. Mice were sacrificed when hind limb paralysis as a symptom of CNS leukemia was visible. Median BM infiltration in shZAP70 xenografts was not different from shGFP controls in any cell line (Online Supplementary Figure S1A). For 697 and JURKAT, both groups had a similar median survival (Online Supplementary Figures S1B and S1C). For 697, the survival difference between 697-shGFP and 697-shZAP70 was statistically significant but small (median 28 vs. 26 days, Online Supplementary Figure S1B). Standardized histological semi-quantitative scoring of CNS infiltration8 (Figure 1B) revealed that 9/10 animals (90%) in the 697-shGFP group were CNS++ or CNS+, and only 2/7 animals (29%) in the 697-shZAP70 group showed a CNS-positive status in the xenografts (Figure 1C). In the REH-shGFP controls, 8/8 animals (100%) were CNS-positive as compared to 4/8 (50%) in the REH-shZAP70 test group (Figure 1C). For JURKAT, scoring was not significantly different in JURKAT-shGFP vs. JURKAT-shZAP70 cells (Figure 1C), however, the total number of blasts recovered from the CNS of xenografts in controls was significantly higher than those from JURKAT-shZAP70 (Figure 1D). For REH cells, ZAP70 knockdown significantly prolonged the median xenograft survival by 25 days in the REH-shZAP70 group as compared to REH-shGFP (104 vs. 79 days, Figure 1E). These data suggest that despite a similar leukemic burden in the periphery, knockdown of ZAP70 hampers the infiltration of 697 and REH BCP-ALL cells into the CNS. In the REH NOD/SCID model, CNS leukemia outweighs peripheral leukemia and mice show low levels of blood, BM and splenic engraftment, but massive CNS infiltration. Our data show that knockdown of ZAP70 in REH cells delays CNS infiltration as a cause of xenograft death in vivo. The data also suggest that knockdown of ZAP70 is not sufficient to prevent CNS infiltration in xenografts bearing JURKAT cells.
We next investigated if ZAP70 influences the ability of primary patient cells to infiltrate the CNS of NSG mice. ZAP70 mRNA levels were measured in pediatric BCP-ALL patients. Patients with ZAP70 expression levels higher than the median were considered as ZAP70hi, and the remaining patients as ZAP70lo. 1 × 106 primary cells (>90% blasts) of 5 ZAP70hi and 5 ZAP70lo patients were injected into replicate NSG mice. The clinical characteristics of these patients are shown in the Online Supplementary Table S1. Interestingly, 7/10 (70%) mice injected with ZAP70hi primary cells showed CNS++ or CNS+ phenotypes by histology, whereas only 1/10 (10%) mice bearing ZAP70lo patient cells were CNS positive (Figure 1F, Online Supplementary Table S1). These data suggest that ZAP70 expression levels play a role for the homing and/or the survival of primary patient BCP-ALL cells in the CNS, and corroborate our cell line data.
ZAP70 regulates CCR7 and CXCR4
ZAP70 was shown to upregulate the expression of adhesion molecules and chemokine receptors in B-CLL.21,24 Chemokine receptors CCR7 and CXCR4 in turn have been associated with an increased capability of T-ALL cells to enter the CNS.25,26 We hypothesized that ZAP70 mediated upregulation of CCR7 and CXCR4 may enhance the homing and the survival of CNS-prone BCP-ALL cells in the CNS niche. Downregulation of ZAP70 in 697 cells resulted in a reduced CCR7 and CXCR4 mRNA expression and a reduction of the proteins on the cell surface (Figure 2A). On the other hand, ectopic overexpression of ZAP70 in 697 cells resulted in a 3-fold upregulation of CCR7 and CXCR4 mRNA levels (Figure 2B). In order to test if ZAP70 mediates the migration of ALL cells via CCR7, we performed transwell assays with or without CCL19 or CXCL12, which are known ligands of CCR7 and CXCR4. The migratory capacity of 697-shZAP70 cells was reduced by ≈50% as compared to controls with CCL19, and by ≈80% with CXCL12 (Figure 2C). Similarly, 697 cells expressing pMIG-ZAP70 showed a ≈2-fold higher migration index toward these cytokines (Figure 2D). These data show that CCR7 and CXCR4 expression, in this cell line, are ZAP70 dependent, which correlates with migratory properties. In order to exemplify that CCR7 is required for CNS homing, we injected 697 and JURKAT cells into NSG mice (day 0). Treatment with 1 mg/kg of anti-CCR7 antibody on day +1, +3 and +7 was then initiated in the test group. On day +23 3/5 (60%) of the 697 mice had developed signs of CNS-leukemia, and on day +35 2/5 (40%) of the JURKAT control mice had developed signs of CNS-leukemia; all mice in both groups were sacrificed. Interestingly, spleens from control mice were enlarged, whereas spleen volumes in the treatment group were normal (Figure 2E). Mice from both groups showed leukemic engraftment, however median blast percentages in the spleen and BM were lower in the anti-CCR7 groups (8.6% vs. 12.7% and 13.6% vs. 32.2% for 697 and 39.8% vs. 65.8% and 81.8% vs. 92.9% for JURKAT, respectively, Figure 2F). The difference in BM engraftment for JURKAT was not statistically significant, suggesting that leukemic burden at the end of the experiment was equal in both groups. Most importantly, CNS histology showed that 5/5 (100%) control mice (both 697 and JURKAT) were CNS++/+ as compared to 1/5 (20%) in both treatment groups (Figure 2G). These data suggest that CCR7 inhibition in 697 and JURKAT cells reduces the homing to hematopoietic organs, including CNS infiltration.
ZAP70 regulates CCR7 and CXCR4 via ERK
CNS infiltration in BCP-ALL has been associated with Ras-mutations activating mitogen-activated protein kinase (MAPK) pathways.31 ZAP70 was shown to enhance the migration of B-CLL cells via ERK1/2 activation.21 Furthermore, ERK1/2 is required for the migration of naïve T lymphocytes toward CCL21, another ligand of CCR7.32 We hypothesized that ZAP70 activates MAPK pathways to regulate CCR7 and CXCR4 and to mediate CCL19- and CXCL12-induced migration. In order to examine ZAP70 expression and ERK activation, we analyzed 9 patient xenografts by western blotting (Figure 3A, left). ZAP70 expression correlated with ERK1/2 phosphorylation (Figure 3A, right). To examine the role of ERK1/2 in response to CCL19, ALL cells were stimulated with CCL19 (Figure 3B). In order to mimic pre-BCR and pre-T-cell receptor (TCR) activation as controls, 697 cells were additionally stimulated with anti-IgM F(ab)2 and JURKAT cells with anti-CD3 (Figure 3B). Upon stimulation with CCL19, 697 cells displayed an increase in p-ERK1/2 as compared to unstimulated cells, but to a lesser extent than in response to anti-IgM F(ab)2 (Figure 3B, left). Similarly, JURKAT cells showed an increase in p-ERK after 5 min of CCL19 stimulation (Figure 3B, right), which declined after 30 min (data not shown). In BCP-ALL primary xenograft cells, p-ERK1/2 was also enhanced by 30 min of CCL19 stimulation in most samples (Figure 3C, Online Supplementary Figure S2A). p-ERK was also induced by CXCL12 in 697 and JURKAT cells (Online Supplementary Figure S2B) as well as in BCP-ALL primary xenograft cells (Online Supplementary Figure S2C). These data suggest that CCL19/CCR7 and CXCL12/CXCR4 activate ERK in 697, JURKAT and BCP-ALL primary cells. In order to examine p-ERK in the context of ZAP70 downregulation, we analyzed p-ERK in 697-shZAP70 and JURKAT-shZAP70 cells. Both cell lines bearing shZAP70 showed a reduction in basal p-ERK (Figure 3D). Furthermore, the induction of p-ERK by CCL19 and CXCL12 was entirely abrogated (Figure 3E). The treatment of 697 and JURKAT cells with the MAPK kinase (MEK) inhibitor U0126, thereby abrogating ERK activation, resulted in a ≈2-fold downregulation of CCR7 mRNA in 697 and JURKAT cells, and a >2-fold downregulation of CXCR4 mRNA (Figure 3F). Additionally, 697 and JURKAT cells treated with U0126 showed significantly reduced migration indices towards both cytokines, as compared to cells treated with vehicle alone (Figure 3G). Our data show that high expression of ZAP70 correlates with ERK activation. Furthermore, CCL19 and CXCL12 induce p-ERK. Finally, the inhibition of ZAP70 reduces p-ERK, and MEK inhibition results in a downregulation of CCR7 and CXCR4 and impaired migration towards CCL19 and CXCL12. The data suggest that ERK may be a missing link between ZAP70 and CCR7/CXCR4.
Figure 3.
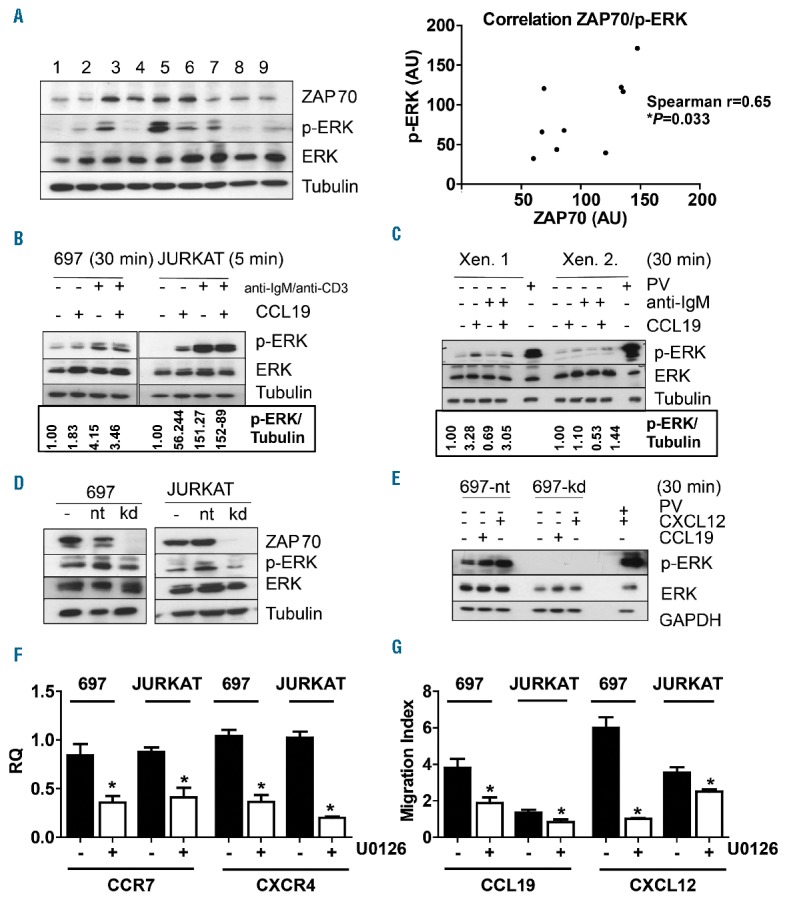
ZAP70 regulates CCR7 and CXCR4 via ERK. A: Bone marrow cells from initial diagnosis from 9 BCP-ALL patients were xenografted into NSG mice, and leukemic blasts from xenograft spleens were analyzed by western blotting for ZAP70, p-ERK, ERK and β-Tubulin (left). The ZAP70/p-ERK ratios were calculated using Image J software for each individual lane (Spearman’s rank correlation). B: 697 (left) and JURKAT cells (right) were serum starved for 30 min, then left without treatment or stimulated with one or more of the following: CCL19 1 μg/ml, anti-IgM F(ab)2 10μg/ml, and anti-CD3 10μg/ml for 5 or 30 min. Samples were analyzed by western blotting for p-ERK, ERK and β-Tubulin. Densitometry was performed using ImageJ software. C: Primary leukemic blasts from xenograft spleens were treated and analyzed as the cell lines in Panel B. Densitometry was performed using ImageJ software. D: 697-shGFP and 697-shZAP70 cells (left) and JURKA-shGFP and JURKAT-ZAP70 cells (right) were lysed and studied by western blotting for the indicated proteins. E: 697-shGFP and 697-shZAP70 cells were stimulated with CCL19 or CXCL12 as indicated. Cells were then lysed and studied by western blotting for the indicated proteins. F: CCR7 and CXCR4 expression was quantified via qRT-PCR of 697 and JURKAT cells in the presence or absence of U1026 (20μM) for 48 h. G: Cells were treated with U1026 (20μM) for 1 h, then subjected to a migration assay for 16 h toward CCL19 or CXCL12 and cells in the lower chamber were counted by FACS or trypan blue. *P<0.05. Xen: xenograft; RQ: real-time quantitative PCR value; AU: arbitrary unit; IgM: immunoglobulin M; PV: pervanadate, positive control; nt: shGFP (shRNA against GFP(green fluorescent protein)); kd: shZAP70 (shRNA targeting ZAP70 (zeta-chain-associated protein kinase 70)).
Association of ZAP70 and CCR7 with CNS involvement in ALL patients
To test whether ZAP70/CCR7/CXCR4 is associated with CNS involvement in patients, we analyzed the mRNA levels of these markers in diagnostic BM samples of patients with BCP-ALL and T-ALL. ZAP70 mRNA expression correlated with protein levels in 9 patient xenografts tested as examples (Spearman r=0.5667, Online Supplementary Figure S3), however the P-value did not reach statistical significance (P=0.0603). The cohort of 130 BCP-ALL patients (Online Supplementary Table S2, top) was selected to contain 46 patients that were initially CNS-positive and developed no CNS relapse (CNS positive/no relapse), 18 patients that were CNS-negative at diagnosis but developed CNS relapse (CNS negative/relapse) as well as 2 patients that were CNS-positive at diagnosis and developed CNS relapse (CNS positive/relapse), matched to 64 CNS-negative patients that developed no CNS relapse at all (CNS negative/no relapse). The cohort of 117 T-ALL patients (Online Supplementary Table S2, bottom) included 24 CNS positive/no relapse, 6 CNS negative/relapse, 3 CNS positive/relapse and 84 CNS negative/no relapse patients. There were no statistical differences in sex, age, prednisone response or cytogenetics between the CNS negative/no relapse, CNS positive/no relapse and CNS negative/relapse groups. CNS positive/relapse patients were not included in the analysis due to low numbers. However, in the T-ALL CNS positive/no relapse group, patients had a significantly higher initial white blood cell (WBC) count as compared to CNS negative/no relapse control patients. In BCP-ALL patients, there were a significantly higher number of patients in the CNS negative/relapse group stratified into the minimal residual disease (MRD) intermediate-risk (IR) group than in CNS negative/no relapse control patients. These data confirm the importance of hyperleukocytosis as a risk factor for CNS involvement in T-ALL, and show that outcomes in the MRD-IR group are difficult to predict.
ZAP70 expression levels in patient samples were correlated with CCR7 expression levels in the BCP-ALL cohort as well as in the T-ALL cohort, but to a lesser extent in the latter cohort (Figure 4A). ZAP70 also correlated with CXCR4 in both cohorts, however, more markedly in T-ALL (Figure 4B). Importantly, ZAP70 expression was found to be significantly elevated in CNS positive/no relapse as compared to CNS negative/no relapse BCP-ALL, but not T-ALL patients (Figure 4C, Online Supplementary Figure S4A). CNS positive/no relapse T-ALL patients showed a significantly higher CCR7 expression as compared to CNS negative/no relapse patients, which was not detectable in BCP-ALL patients (Figure 4D, Online Supplementary Figure S4B). Similarly, CXCR4 expression was higher in CNS positive/no relapse T-ALL in comparison to CNS negative/no relapse patients (Figure 4E). Again, this difference was not detectable in BCP-ALL (Online Supplementary Figure S4C). ZAP70 may thus be important for CNS infiltration in BCP-ALL, and the upregulation of chemokine receptors may be more relevant in T-ALL patients, which matches the discrepancies seen in our in vivo experiments between BCP-ALL and T-ALL cells. CNS negative/relapse patients showed no elevations in ZAP70 or CCR7 mRNA levels as compared to CNS negative/no relapse patients, neither in BCP-ALL nor in T-ALL (Figure 4C,D, Online Supplementary Figure S4A,S4B). In fact, CNS negative/relapse BCP-ALL patients had significantly lower ZAP70 mRNA expression levels than CNS negative/no relapse patients (Figure 4C). This suggests that ZAP70/CCR7 expression in ALL blasts may be relevant as a homing/survival mechanism initially, and not as a predictor of CNS relapse in the course of the disease. For CXCR4, CNS negative/relapse T-ALL patients showed significantly higher levels than CNS negative/no relapse patients (Figure 4E). Interestingly, the 2 CNS positive/relapse BCP-ALL patients showed the highest expression levels of both ZAP70 and CCR7 (Figure 4C, Online Supplementary Figure S4B), however, evaluation of more patients is needed before conclusions can be drawn.
Figure 4.
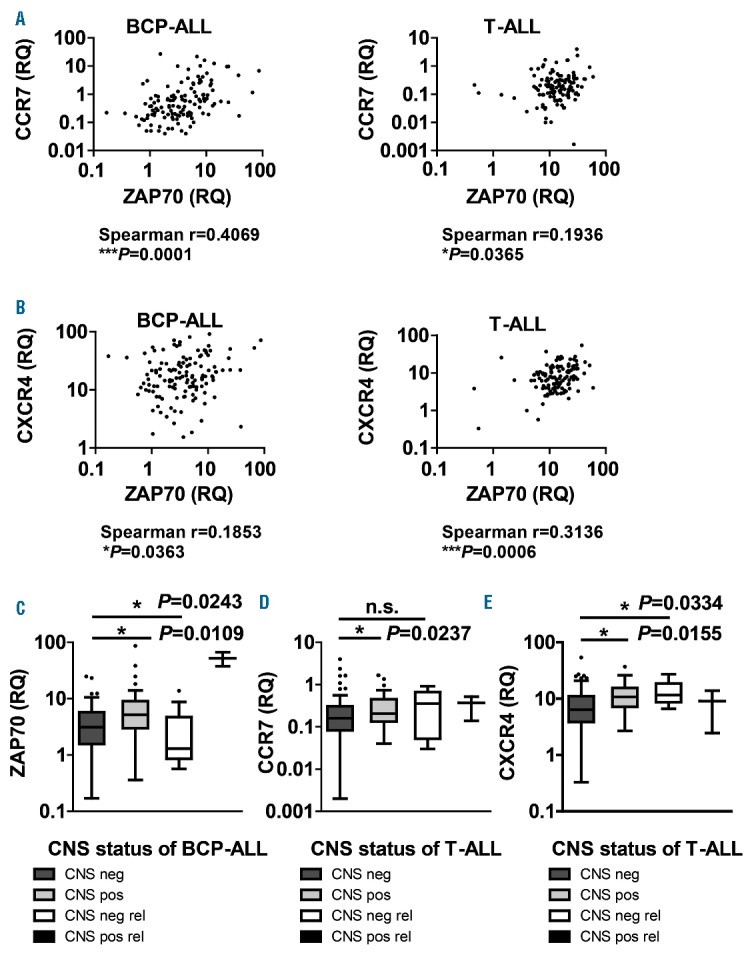
Correlation of ZAP70, CCR7 and CXCR4 with CNS status in ALL patients. Correlation of ZAP70, CCR7 and CXCR4 with CNS status in ALL patients. ZAP70, CCR7 and CXCR4 expression levels were determined with qRT-PCR in 130 pediatric BCP-ALL and 117 pediatric T-ALL patients. Correlation analysis between ZAP70 and CCR7 (A) and ZAP70 and CXCR4 (B) for BCP-ALL (left) and T-ALL (right) patients. Correlation between ZAP70 in BCP-ALL (C) and CCR7 (D)/CXCR4 (E) in T-ALL patients and CNS group (CNS negative/no relapse, CNS positive/no relapse, CNS negative/relapse, CNS positive/relapse). Further definitions are provided in the Online Supplementary Table S2. BCP-ALL: B-cell precursor acute lymphoblastic leukemia; T-ALL: T-cell acute lymphoblastic leukemia; CNS: central nervous system; n.s: not significant; neg: negative; pos: positive; rel: relapse; RQ: real-time quantitative PCR value; ZAP70: zeta-chain-associated protein kinase 70.
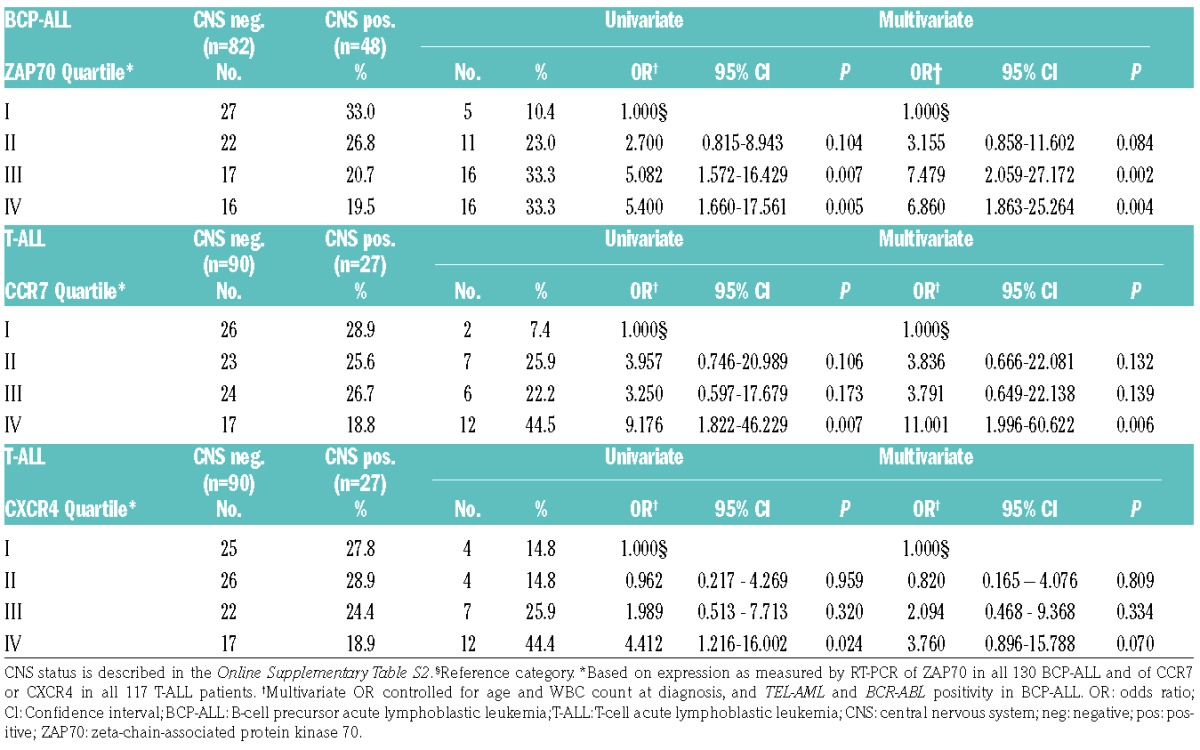
In order to evaluate the independent impact of high ZAP70 and CCR7/CXCR4 mRNA levels on CNS status, we performed a binary logistic regression analysis controlling for age, WBC and the presence of certain cytogenetics (BCR-ABL or TEL-AML1) in BCP-ALL with CNS infiltration at the initial diagnosis (yes/no) as the dependent variable. In BCP-ALL patients, ZAP70 expression levels in the upper two quartiles conferred a ≈7-fold increased risk of CNS-positivity compared with the lowest expression quartile (OR=7.48, 95% CI 2.06–27.17, P=0.002 and OR=6.86, 95% CI 1.86–25.26, P=0.004, respectively) (Table 1). Similarly, in T-ALL patients, CCR7 expression levels in the highest quartile conferred an 11-fold increased risk of CNS3 status compared with the lowest quartile (OR=11.00, 95% CI 2.00–60.62, P=0.006) (Table 1). In T-ALL patients, CXCR4 expression levels in the highest quartile conferred a ≈4-fold increased risk of CNS3 status compared with the lowest quartile in univariate analysis (OR=4.41, 95% CI 1.22–16.00, P=0.024), however, this effect did not hold up in multivariate testing (P=0.070, Table 1). Taken together, these data suggest that the expression of ZAP70 is higher in leukemic cells from diagnostic BM samples of BCP-ALL with overt CNS involvement, and that the same is true for CCR7 and CXCR4 in T-ALL. Also, ZAP70 has an independent predictive impact on the CNS status in BCP-ALL and CCR7 in T-ALL.
Table 1.
Univariate and multivariate associations for ZAP70 expression quartiles and CNS status in 130 childhood BCP-ALL patients, and for CCR7 or CXCR4 expression quartiles and CNS status in 117 childhood T-ALL patients, as indicated.
Discussion
CNS involvement in ALL is linked to leukemic relapses in the CNS. The ability of some ALL blasts to penetrate the blood brain barrier exposes these cells to suboptimal levels of chemotherapeutic drugs, contributing to their survival in a protected niche.33,34 We hypothesized that ZAP70 regulates CNS-leukemia. ZAP70 and SYK, the two members of the Syk kinase family, exert overlapping and distinct functions in lymphocytes. The ZAP70 tyrosine kinase, constitutively expressed in T cells and NK cells, mediates signals downstream of the pre-TCR and mature TCR.35 ZAP70 is involved in early B-lymphocyte development and activation,13 and is also important for B-cell signaling. A recent report identified a subset of ALLs that depend on tonic pre-BCR signaling and is selectively sensitive to the inhibition of SYK and SRC downstream of the pre-BCR.36 In the same study, ZAP70 was shown to be a target of the E2A-PBX1 fusion.36 An upregulation of ZAP70 in E2A-PBX1 positive BCP-ALL has been observed.16,37 Downregulating the expression of ZAP70 in the E2A-PBX1-positive 697 cell line reduced CNS-positivity in xenografts (Figure 1C). This also applied to REH BCP-ALL cells bearing the TEL-AML1 translocation. Similarly, t(1;19) xenografts bearing ZAP70hi patients exhibited a pronounced CNS-positive phenotype as compared to ZAP70lo patients (Figure 1F). The reduction in ZAP70 expression did not alter the migration of ALL cells to the BM, but rather to the CNS. This observation differs from the situation in B-CLL, where cells with lower ZAP70 expression showed a reduced BM homing.21,22 In JURKAT T-ALL cells, downregulation of ZAP70 did not prevent CNS infiltration; however, it was associated with a reduction in CNS blasts recovered from xenografts (Figure 1D). Downregulating ZAP70 in JURKAT cells did not prolong xenograft survival. On the other hand, animals bearing 697-shZAP70 lived slightly longer and REH-shZAP70 xenografts lived considerably longer than controls (Figure 1E, Online Supplementary Figure S1B,S1C). This may be explained by the REH-model, which causes an almost isolated CNS leukemia in NOD/SCID mice.
To investigate how ZAP70 facilitates the migration of ALL cells to the CNS, we hypothesized that ZAP70 regulates chemokine receptors. Chemokines control leukocyte homing into organs, including the CNS.38,39 ALL cells upregulate chemokine receptors, such as CXCR4, CCR3, CCR4 and CCR7,25,40,41 and ZAP70 was shown to enhance the migration of lymphocytes towards chemokines, e.g., CXCL12, CCL21 or CCL19.21,23,24 Herein we show that CCR7/CXCR4 expression was reduced in 697-shZAP70 cells, which impaired their migration toward CCL19/CXCL12. Similarly, enforced ZAP70 expression in 697 cells increased the expression of CCR7/CXCR4 and subsequent migration toward their ligand (Figure 2). Importantly, CCR7 blockade resulted in a reduction in homing processes in vivo analogous to B-CLL42 and, importantly, a reduction in CNS infiltration (Figure 2E–G), which is an extremely rare event in B-CLL.43 Stimulating cells with CCL19/CXCL12 increased p-ERK in 697, JURKAT and BCP-ALL primary cells. Also, basal and CXCL19/CXCL12-inducible p-ERK was reduced in cells bearing a ZAP70 knockdown. CCR7/CXCR4 expression and in vitro migration toward CCL19/CXCL12 were reduced when cells were pre-treated with a MEK inhibitor (Figure 3F,G). The RAS/RAF/MEK/ERK cascade is essential for many cancer cells,44 and hyper-activation of this pathway is common in malignancies due to genetic alterations in the RAS pathway45 and oncogenic tyrosine kinases.46 The use of MEK inhibitors reduced RAS-mutated CNS leukemia in xenografts.31 On the other hand, ERK negative feedback is also a mechanism of leukemia progression in ALL.47 We propose that ZAP70 functions as a regulator of CNS leukemia in ALL via CCR7/CXCR4 and ERK in preclinical in vitro and in vivo models. In addition to providing a chemotactic stimulus, this may confer a niche-specific survival advantage.
Most notably, we demonstrated an importance of these markers in large exploratory patient cohorts (Online Supplementary Table S2). We found a correlation between ZAP70 and CCR7/CXCR4 expression (Figure 4A,B) and showed that CNS positive/no relapse BCP-ALL patients expressed higher levels of ZAP70 (Figure 4C). Similarly, CNS positive/no relapse T-ALL patients expressed higher levels of CCR7/CXCR4 (Figure 4D,E). However, there were no significant correlations between CCR7/CXCR4 expression and CNS-positivity in BCP-ALL, and ZAP70 expression and CNS-positivity in T-ALL patients (Online Supplementary Figure S4). Even though we demonstrated a ZAP70-mediated regulation of CCR7/CXCR4 in pre-clinical models, the situation in patients differs. In T-ALL, CCR7/CXCR4 may be regulated by additional mechanisms. In BCP-ALL, the activation of pre-BCR signaling by ZAP70 may be more important than a regulation of chemokine receptors. It is critical to emphasize that a correlation between ZAP70 and CCR7/CXCR4 was observed for all patients, regardless of the immunophenotype. Furthermore, ZAP70 in BCP-ALL and CCR7 in T-ALL hold up in multivariate analyses, suggesting that high marker expression predicts initial CNS infiltration (Table 1). In contrast to recent experimental findings,48 our data suggest that chemokine receptors and their regulation are relevant in CNS leukemia. As our analysis is limited by patient selection in order to analyze sufficient numbers of CNS-positive cases, prospective validation will be important.
We conclude that ZAP70 plays a role for the homing to and/or the survival of ALL cells in the CNS and that ZAP70 may represent a therapeutic target. Furthermore, targeting CCR7/CXCR4 may be particularly promising in treating T-ALL.49,50
Supplementary Material
Acknowledgments
D. M. S. is supported by the Max-Eder group leader program by the Deutsche Krebshilfe. We thank Michael Reth and David Medgyesi for providing the pMIG plasmids. We thank Katrin Timm-Richert, Katrin Neumann, Juliane Schmäh and Birthe Fedders for the excellent technical assistance.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/2/346
References
- 1.Forestier E, Heyman M, Andersen MK, et al. Outcome of ETV6/RUNX1-positive childhood acute lymphoblastic leukaemia in the NOPHO-ALL-1992 protocol: frequent late relapses but good overall survival. Br J Haematol. 2008;140(6):665–672. [DOI] [PubMed] [Google Scholar]
- 2.Gandemer V, Chevret S, Petit A, et al. Excellent prognosis of late relapses of ETV6/RUNX1-positive childhood acute lymphoblastic leukemia: lessons from the FRALLE 93 protocol. Haematologica. 2012;97(11):1743–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price RA, Johnson WW. The central nervous system in childhood leukemia. I. The arachnoid. Cancer. 1973;31(3):520–533. [DOI] [PubMed] [Google Scholar]
- 4.Burger B, Zimmermann M, Mann G, et al. Diagnostic cerebrospinal fluid examination in children with acute lymphoblastic leukemia: significance of low leukocyte counts with blasts or traumatic lumbar puncture. J Clin Oncol. 2003;21(2):184–188. [DOI] [PubMed] [Google Scholar]
- 5.Howard SC, Gajjar AJ, Cheng C, et al. Risk factors for traumatic and bloody lumbar puncture in children with acute lymphoblastic leukemia. Jama. 2002;288(16): 2001–2007. [DOI] [PubMed] [Google Scholar]
- 6.Gajjar A, Harrison PL, Sandlund JT, et al. Traumatic lumbar puncture at diagnosis adversely affects outcome in childhood acute lymphoblastic leukemia. Blood. 2000;96(10):3381–3384. [PubMed] [Google Scholar]
- 7.Jeha S, Pei D, Raimondi SC, et al. Increased risk for CNS relapse in pre-B cell leukemia with the t(1;19)/TCF3-PBX1. Leukemia. 2009;23(8):1406–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krause S, Pfeiffer C, Strube S, et al. Mer tyrosine kinase promotes the survival of t(1;19)-positive acute lymphoblastic leukemia (ALL) in the central nervous system (CNS). Blood. 2015;125(5):820–830. [DOI] [PubMed] [Google Scholar]
- 9.Pinkel D. Five-year follow-up of “total therapy” of childhood lymphocytic leukemia. Jama. 1971;216(4):648–652. [PubMed] [Google Scholar]
- 10.Silverman LB, Declerck L, Gelber RD, et al. Results of Dana-Farber Cancer Institute Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1981–1995). Leukemia. 2000;14(12):2247–2256. [DOI] [PubMed] [Google Scholar]
- 11.Clarke M, Gaynon P, Hann I, et al. CNS-directed therapy for childhood acute lymphoblastic leukemia: Childhood ALL Collaborative Group overview of 43 randomized trials. J Clin Oncol. 2003;21(9):1798–1809. [DOI] [PubMed] [Google Scholar]
- 12.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–178. [DOI] [PubMed] [Google Scholar]
- 13.Au-Yeung BB, Deindl S, Hsu LY, et al. The structure, regulation, and function of ZAP-70. Immunol Rev. 2009;228(1):41–57. [DOI] [PubMed] [Google Scholar]
- 14.Chakupurakal G, Bell A, Griffiths M, et al. Analysis of ZAP70 expression in adult acute lymphoblastic leukaemia by real time quantitative PCR. Mol Cytogenet. 2012;5(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wandroo F, Bell A, Darbyshire P, et al. ZAP-70 is highly expressed in most cases of childhood pre-B cell acute lymphoblastic leukemia. Int J Lab Hematol. 2008;30(2):149–157. [DOI] [PubMed] [Google Scholar]
- 16.Chiaretti S, Guarini A, De Propris MS, et al. ZAP-70 expression in acute lymphoblastic leukemia: association with the E2A/PBX1 rearrangement and the pre-B stage of differentiation and prognostic implications. Blood. 2006;107(1):197–204. [DOI] [PubMed] [Google Scholar]
- 17.Rassenti LZ, Huynh L, Toy TL, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351(9):893–901. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Apgar J, Huynh L, et al. ZAP-70 directly enhances IgM signaling in chronic lymphocytic leukemia. Blood. 2005;105(5): 2036–2041. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Widhopf G, Huynh L, et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2002;100(13): 4609–4614. [DOI] [PubMed] [Google Scholar]
- 20.Gobessi S, Laurenti L, Longo PG, et al. ZAP-70 enhances B-cell-receptor signaling despite absent or inefficient tyrosine kinase activation in chronic lymphocytic leukemia and lymphoma B cells. Blood. 2007; 109(5):2032–2039. [DOI] [PubMed] [Google Scholar]
- 21.Calpe E, Codony C, Baptista MJ, et al. ZAP-70 enhances migration of malignant B lymphocytes toward CCL21 by inducing CCR7 expression via IgM-ERK1/2 activation. Blood. 2011;118(16):4401–4410. [DOI] [PubMed] [Google Scholar]
- 22.Calpe E, Purroy N, Carpio C, et al. ZAP-70 promotes the infiltration of malignant B-lymphocytes into the bone marrow by enhancing signaling and migration after CXCR4 stimulation. PLoS One. 2013;8(12):e81221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ticchioni M, Charvet C, Noraz N, et al. Signaling through ZAP-70 is required for CXCL12-mediated T-cell transendothelial migration. Blood. 2002;99(9):3111–3118. [DOI] [PubMed] [Google Scholar]
- 24.Ottoson NC, Pribila JT, Chan AS, et al. Cutting edge: T cell migration regulated by CXCR4 chemokine receptor signaling to ZAP-70 tyrosine kinase. J Immunol. 2001; 167(4):1857–1861. [DOI] [PubMed] [Google Scholar]
- 25.Buonamici S, Trimarchi T, Ruocco MG, et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature. 2009;459(7249):1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jost TR, Borga C, Radaelli E, et al. Role of CXCR4-mediated bone marrow colonization in CNS infiltration by T-cell acute lymphoblastic leukemia. J Leukoc Biol. 2016;99(6):1077–1087. [DOI] [PubMed] [Google Scholar]
- 27.Alsadeq A, Strube S, Krause S, et al. Effects of p38alpha/beta inhibition on acute lymphoblastic leukemia proliferation and survival in vivo. Leukemia. 2015;29(12):2307–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammad MG, Tsai VWW, Ruitenberg MJ, et al. Immune cell trafficking from the brain maintains CNS immune tolerance. J Clin Invest. 2014;124(3):1228–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulathu Y, Hobeika E, Turchinovich G, et al. The kinase Syk as an adaptor controlling sustained calcium signalling and B-cell development. Embo J. 2008;27(9):1333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irving J, Matheson E, Minto L, et al. Ras pathway mutations are prevalent in relapsed childhood acute lymphoblastic leukemia and confer sensitivity to MEK inhibition. Blood. 2014;124(23): 3420–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shannon LA, Calloway PA, Welch TP, et al. CCR7/CCL21 migration on fibronectin is mediated by phospholipase Cgamma1 and ERK1/2 in primary T lymphocytes. J Biol Chem. 2010;285(50):38781–28787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinkel D. CNS relapse in childhood leukaemia. Lancet. 1981;2(8255):1115. [DOI] [PubMed] [Google Scholar]
- 34.Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9(3):257–268. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Kadlecek TA, Au-Yeung BB, et al. ZAP-70: an essential kinase in T-cell signaling. Cold Spring Harb Perspect Biol. 2010;2(5):a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geng H, Hurtz C, Lenz KB, et al. Self-enforcing feedback activation between BCL6 and pre-B cell receptor signaling defines a distinct subtype of acute lymphoblastic leukemia. Cancer Cell. 2015;27(3):409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duque-Afonso J, Wei MC, Lin C-H, et al. Oncogenic role for the Lck/ZAP70/PLCG2 signaling pathway in pre-B-ALL pathogenesis. Blood. 2015;126(23):810. [Google Scholar]
- 38.Colmone A, Amorim M, Pontier AL, et al. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322(5909):1861–1865. [DOI] [PubMed] [Google Scholar]
- 39.Gomez AM, Martinez C, Gonzalez M, et al. Chemokines and relapses in childhood acute lymphoblastic leukemia: A role in migration and in resistance to antileukemic drugs. Blood Cells Mol Dis. 2015;55(3):220–227. [DOI] [PubMed] [Google Scholar]
- 40.Wong S, Fulcher D. Chemokine receptor expression in B-cell lymphoproliferative disorders. Leukemia Lymphoma. 2004; 45(12):2491–2496. [DOI] [PubMed] [Google Scholar]
- 41.Ishida T, Utsunomiya A, Iida S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003;9(10 Pt 1):3625–3634. [PubMed] [Google Scholar]
- 42.Cuesta-Mateos C, Loscertales J, Kreutzman A, et al. Preclinical activity of anti-CCR7 immunotherapy in patients with high-risk chronic lymphocytic leukemia. Cancer Immunol Immunother. 2015;64(6):665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akintola-Ogunremi O, Whitney C, Mathur SC, et al. Chronic lymphocytic leukemia presenting with symptomatic central nervous system involvement. Ann Hematol. 2002;81(7):402–404. [DOI] [PubMed] [Google Scholar]
- 44.Steelman LS, Abrams SL, Shelton JG, et al. Dominant roles of the Raf/MEK/ERK pathway in cell cycle progression, prevention of apoptosis and sensitivity to chemotherapeutic drugs. Cell Cycle. 2010;9(8):1629–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Mullighan CG, Harvey RC, et al. Key pathways are frequently mutated in high-risk childhood acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2011; 118(11): 3080–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fielding AK. How I treat Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2010;116(18):3409–3417. [DOI] [PubMed] [Google Scholar]
- 47.Shojaee S, Caeser R, Buchner M, et al. Erk negative feedback control enables pre-B cell transformation and represents a therapeutic target in acute lymphoblastic leukemia. Cancer Cell. 2015;28(1):114–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams MT, Yousafzai YM, Elder A, et al. The ability to cross the blood-cerebrospinal fluid barrier is a generic property of acute lymphoblastic leukaemia blasts. Blood. 2016;127(16):1998–2006. [DOI] [PubMed] [Google Scholar]
- 49.Alfonso-Pérez M, López-Giral S, Quintana NE, et al. Anti-CCR7 monoclonal antibodies as a novel tool for the treatment of chronic lymphocyte leukemia. J Leukoc Biol. 2006;79(6):1157–1165. [DOI] [PubMed] [Google Scholar]
- 50.Kashyap MK, Kumar D, Jones H, et al. Ulocuplumab (BMS-936564 / MDX1338): a fully human anti-CXCR4 antibody induces cell death in chronic lymphocytic leukemia mediated through a reactive oxygen species-dependent pathway. Oncotarget. 2016;7(3): 2809–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


