Abstract
In the treatment of diffuse large B-cell lymphoma, a persistently positive [18F]fluorodeoxyglucose positron emission tomography (PET) scan typically carries a poor prognosis. In this prospective multi-center phase II study, we sought to establish whether treatment intensification with R-ICE (rituximab, ifosfamide, carboplatin, and etoposide) chemotherapy followed by 90Y-ibritumomab tiuxetan–BEAM (BCNU, etoposide, cytarabine, and melphalan) for high-risk diffuse large B-cell lymphoma patients who are positive on interim PET scan after 4 cycles of R-CHOP-14 (rituximab, cyclophosphamide, doxorubicin, and prednisone) can improve 2-year progression-free survival from a historically unfavorable rate of 40% to a rate of 65%. Patients received 4 cycles of R-CHOP-14, followed by a centrally-reviewed PET performed at day 17–20 of cycle 4 and assessed according to International Harmonisation Project criteria. Median age of the 151 evaluable patients was 57 years, with 79% stages 3–4, 54% bulk, and 54% International Prognostic Index 3–5. Among the 143 patients undergoing interim PET, 101 (71%) were PET-negative (96 of whom completed R-CHOP), 42 (29%) were PET-positive (32 of whom completed R-ICE and 90Y-ibritumomab tiuxetan-BEAM). At a median follow up of 35 months, the 2-year progression-free survival for PET-positive patients was 67%, a rate similar to that for PET-negative patients treated with R-CHOP-14 (74%, P=0.11); overall survival was 78% and 88% (P=0.11), respectively. In an exploratory analysis, progression-free and overall survival were markedly superior for PET-positive Deauville score 4 versus score 5 (P=0.0002 and P=0.001, respectively). Therefore, diffuse large B-cell lymphoma patients who are PET-positive after 4 cycles of R-CHOP-14 and who switched to R-ICE and 90Y-ibritumomab tiuxetan-BEAM achieved favorable survival outcomes similar to those for PET-negative R-CHOP-14-treated patients. Further studies are warranted to confirm these promising results. (Registered at: ACTRN12609001077257).
Introduction
In the treatment of diffuse large B-cell lymphoma (DLBCL), combination immuno-chemotherapy with R-CHOP given for 6–8 cycles at intervals of 14–21 days is considered standard of care in most parts of the world.1–3 Despite the clear benefit from the addition of rituximab to CHOP chemotherapy, still nearly 40% of patients have disease which will either fail to respond or manifest early relapse.1–3 Furthermore, patients with high-risk International Prognostic Index (IPI) have a 5-year progression-free survival (PFS) of only 40% following R-CHOP.4 Therefore, more intensive treatment protocols, such as high-dose therapy (HDT) and autologous stem cell transplantation (ASCT), have been advocated.5,6 These, however, are associated with considerable increased cost and toxicity.5,6 One of the largest randomized trials incorporating ASCT conducted without positron emission tomography (PET) scans failed to show an obvious benefit of early dose-escalated sequential HDT.7 Similar trials used a variety of study designs, and the value of treatment intensification remains unresolved; suffice to say that such treatment is feasible, but at the price of greater toxicity than that associated with the current standard treatment. A more individualized approach, such as interim PET-based risk stratification, would allow the selection of high-risk patients for treatment intensification, while at the same time avoiding unnecessary toxicity in patients destined to do well with R-CHOP alone.
Early studies indicated that FDG-PET performed after 2–4 cycles of R-chemotherapy is predictive of outcome in patients with DLBCL.8–12 More recent reports have called this into question, perhaps due to the diversity of imaging and interpretative methodologies that has led to wide variations in reported negative and positive predictive values for interim PET (iPET) scans.13–17 One confounding factor is that iPET is typically performed after 2 cycles of R-chemotherapy when FDG-avidity likely reflects a mix of residual cancer cells and inflammation.18–20 In contrast, iPET assessment after cycle 4 R-chemotherapy may more specifically identify resistant lymphoma15,21 and thereby enable more accurate identification of high-risk patients on the basis of therapeutic response who may benefit most from early treatment intensification. Therefore, in this study we scheduled the iPET in the week following completion of cycle 4 R-CHOP (iPET-4), delaying cycle 5 by seven days to reduce any confounding inflammatory effects within the tumor bed of immuno-chemotherapy. Few studies in DLBCL have prospectively explored a change in treatment strategy guided by iPET responses.22–25 We chose to evaluate a change to HDT since it is the most widely accepted curative strategy for patients with DLBCL failing R-CHOP. We hypothesized that improved clinical outcomes for high-risk DLBCL patients with poor prognosis as identified by iPET after 4 chemotherapy cycles would be achieved with early HDT and ASCT delivered when there is a lower burden of chemo-resistant disease than if instituted at the time of radiological progression. Given the favorable reports of the use of radio-immunotherapy combined with ASCT for patients with relapsed/refractory DLBCL, the study combined 90Y-ibritumomab tiuxetan (Zevalin®) with high-dose BEAM (Z-BEAM) chemotherapy.26–28
Methods
Study design
This prospective multi center phase II study enrolled patients aged 18–70 years with ECOG performance status (PS) 0–3, with high-risk DLBCL (either IPI 2–5 or 0–1 with bulk >7.5 cm), who were previously untreated (except for pre-phase prednisone), were considered fit for ASCT, and who had a positive baseline PET scan with more than one FDG-avid lesion.
All patients underwent a baseline PET prior to receiving 4 cycles of dose-dense R-CHOP-14 (see Online Supplementary Appendix).2 Cycle 5 R-CHOP-14 was delayed seven days and an iPET was performed at day (d)17-d20 of cycle 4. Central nervous system (CNS) prophylaxis was administered according to institutional practice and could include systemic methotrexate. Following the completion of R-CHOP-14, consolidative radiation therapy (RT) was permitted but not mandated as part of the treatment regimen. After HDT, iPET-positive patients could receive RT to those sites of residual metabolic activity. The study was registered at ANZCTR (ACTRN12609001077257) (www.anzctr.org.au/Trial/Registration) and approved by the Ethics Committees at each of the 20 participating sites.
PET imaging and analysis
Patients were injected with 370 MBq of [18F]-labeled FDG for a 70 kg subject and dosed at 5.28 MBq/kg ±5%, with a minimum of 250 MBq and maximum of 550 MBq. Anonymized DICOM file images of baseline and interim PET scans were transmitted to the central reporting site at the Peter MacCallum Cancer Centre for consensus assessment by 2 experienced nuclear imaging specialists. Interim PET scans were subject to visual interpretation according to International Harmonisation Project (IHP) criteria.19 In the case of discordance between imaging specialists, a consensus result was recorded after face-to-face discussion. Following RICE chemotherapy, PET scans were performed and assessed locally, since only in the event of interval progressive disease (PD) did patients not proceed to Z-BEAM.
Post-iPET treatment
Interim PET-negative patients completed 2 further cycles of R-CHOP-14 followed by 2 doses of 2-weekly rituximab. Interim PET-positive patients received R-ICE chemotherapy (see Online Supplementary Appendix) every 21 days for 3 cycles. Peripheral blood stem cells were mobilized with filgrastim at 10 μg/kg daily. Subsequently, patients without PD after 3 cycles of R-ICE proceeded to ASCT using Z-BEAM conditioning 2–6 weeks (typically 3) from d1 of cycle 3 R-ICE; patients with PD were taken off study. At a central site, ibritumomab tiuxetan was labeled to 90Yttrium (see Online Supplementary Appendix) as part of the Z-BEAM regimen as previously published.26
Statistical analysis
The primary end point was 2-year PFS for patients remaining iPET-positive and treated with R-ICE and Z-BEAM. PFS was defined as the time from the iPET scan to disease progression or death from any cause. The expected 2-year PFS rate for iPET-4-positive patients treated with R-CHOP based on historical data was considered to be 40% (range 36%–47%).11,12,15,17,21 By switching these patients to early treatment intensification, the aim was to increase the 2-year PFS to 65%. A one-stage design with 33 iPET-positive patients provided a probability of at least 90% to detect a difference in 2-year PFS of 40% versus 65%, with a two-sided alpha of 0.05. Since it was expected that at least 20% would be iPET-positive, the planned accrual was 165. The major secondary end point was overall survival (OS). A subsequent exploratory analysis involved iPET assessment using the Deuville 5-point score (5-PS) by 2 blinded readers. Statistical analyses were performed using Stata MP v.13.1 software (StataCorp, USA).
Results
Patients’ characteristics
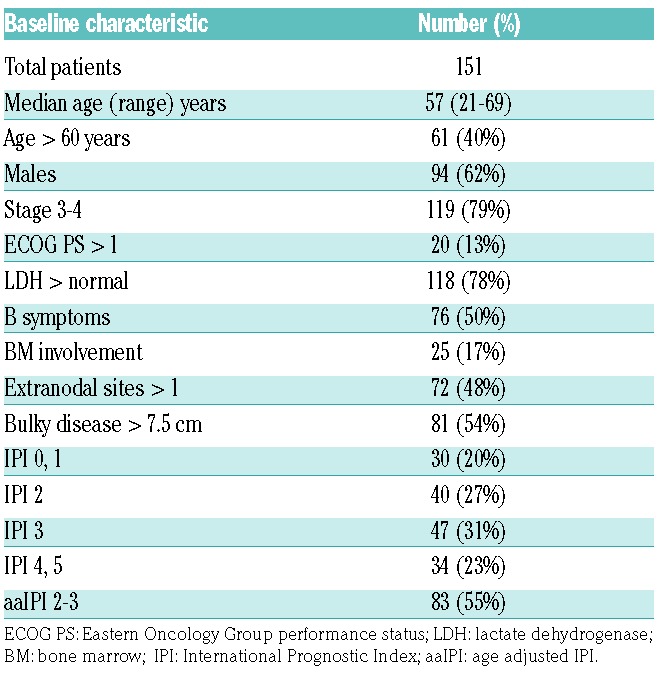
Between July 2009 and December 2012, 162 patients were enrolled from 20 Australian centers. Subsequently, 11 patients were excluded for failure to meet the eligibility criteria. Baseline characteristics of the 151 evaluable patients include: median age 57 years (range 21–69), 79% stage 3–4, 54% bulky disease, and 54% IPI 3–5 (Table 1).
Table 1.
Characteristics of the 151 patients at baseline.
Interim PET results
No iPET scan was performed in 8 patients due to PD (n=1), bowel perforation (n=2), organ toxicity (n=3), and dose delays of two weeks or more (n=2) (Figure 1). In the remaining 143 patients, iPET was undertaken between d17-20 in 89 and d14-16 in 34. By central review, 101 (71%) were iPET-negative and 42 (29%) iPET-positive. Of the 101 iPET-negative patients, 96 completed protocol therapy while the remaining 5 did not due to PD (n=3, each with CNS progression), toxicity (n=1), and omission of the last rituximab dose (n=1). Five of the 42 iPET-positive patients scheduled to receive R-ICE salvage did not due to PD before R-ICE (n=1) and withdrawal of consent (n=4). Of the remaining 37 patients completing R-ICE, repeat PET (assessed locally) prior to Z-BEAM demonstrated responses as follows: PET-negative in 11 (30%), PET-positive in 26 [including partial response (PR) in 20 (54%)], stable disease (SD) in 1 (3%), and PD in 5 (14%). Thirty-two patients completed Z-BEAM, of whom on restaging 20 (63%) were PET-negative and 10 (31%) remained PET-positive, including PR in 8 (25%) and SD in 2 (6%), while 2 (6%) were not evaluated.
Figure 1.
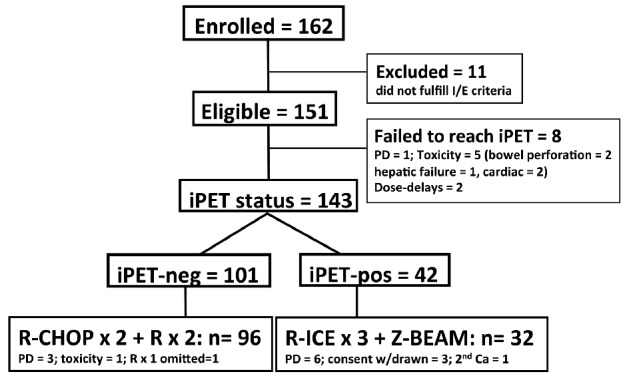
Consort diagram. Flow of patients from enrollment to completion of therapy.
Treatment outcomes
The principle aim of the study was achieved. On an intention-to-treat basis, at a median follow-up time of 35 months, the iPET-positive patients showed a 2-year PFS of 67% [95% confidence interval (CI): 50%–79%], a rate similar to that seen among iPET-negative patients (74%) (95%CI: 64%–81%) (P=0.32) (Figure 2A). The 2-year OS was similar for iPET-positive and iPET-negative cohorts at 78% (95%CI: 60%–88%) and 88% (95%CI: 79%–93%) (P=0.11), respectively (Figure 2B). Among patients with high IPI 3–5, 2-year PFS and OS rates were comparable (Online Supplementary Figure S1A and B).
Figures 2.
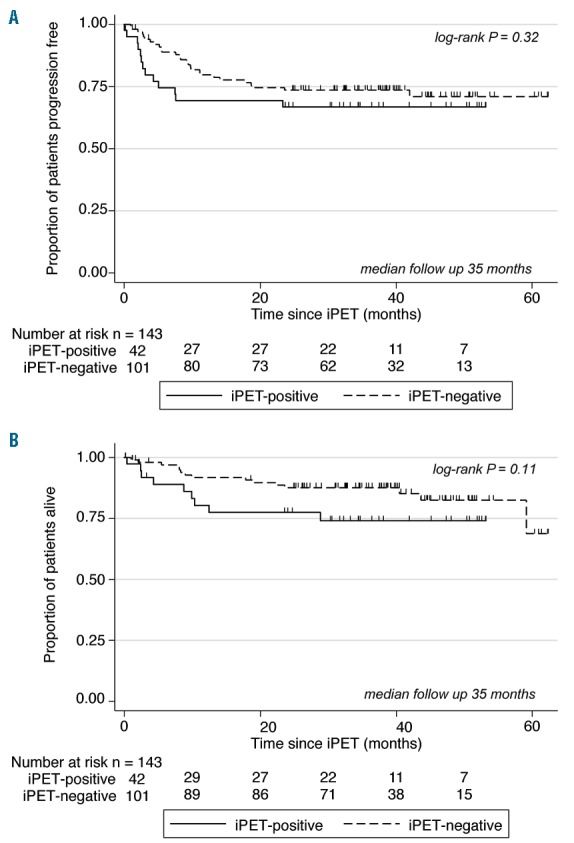
Interim positron emission tomography (iPET)-negative (n=101) versus iPET-positive (n=42). (A) Progression-free survival (PFS): 2-year PFS for iPET-negative versus iPET-positive patients. (B) Overall survival: 2-year OS for iPET-negative versus iPET-positive patients.
At least one grade 3–4 adverse event was reported in 58% of iPET-negative patients receiving R-CHOP-14 and 76% (P=0.04) of iPET-positive patients receiving R-ICE. There were more grade 3–4 neutropenia (44% vs. 28%; P=0.06), febrile neutropenia (46% vs. 10%; P<0.0001), and thrombocytopenia (49% vs. 7%; P<0.0001) events among those receiving R-ICE compared to those continuing R-CHOP. Among the 32 patients who completed Z-BEAM, the median time to engraftment of neutrophils over 1.0×109/L and platelets over 50×109/L was as expected at 11 days (range 9–104), and 16 days (range 12–105), respectively; 4 patients had delayed platelet engraftment beyond 30 days. There was one treatment-related death; this was due to viral pneumonitis on d33 post transplant. There have been 3 subsequent cancers: 2 in the iPET-negative (glioblastoma, myelodysplastic syndrome) and one in the iPET-positive group (lung). Of the 143 patients who underwent iPET, 15 (10%) received RT; 5 (4 to site of initial bulk) of 101 (5%) iPET-negative patients and 8 (to site of initial bulk) of 42 (19%) iPET-positive patients. CNS prophylaxis was given to 7 patients, including 6 as intrathecal and one as high-dose intravenous methotrexate.
iPET assessment using the Deauville 5-point score
In a post-hoc exploratory analysis iPET-positive scans were scored by 2 blinded readers with a visual interpretation using the Deauville 5-PS,15,29–32 and survival outcomes were evaluated accordingly. Of the entire 42 iPET-positive patients, 27 were Deauville score 4 and 15 were score 5. There was a notable difference in 2-year PFS for Deauville score 4 versus score 5, at 88% (95%CI: 66%–96%) versus 33% (95%CI: 12%–57%) (P=0.0002), respectively (Figure 3A), and similarly for 2-year OS at 96% (95%CI: 74%–99%) versus 42% (95%CI: 15%–67%) (P=0.001) (Figure 3B).
Figure 3.
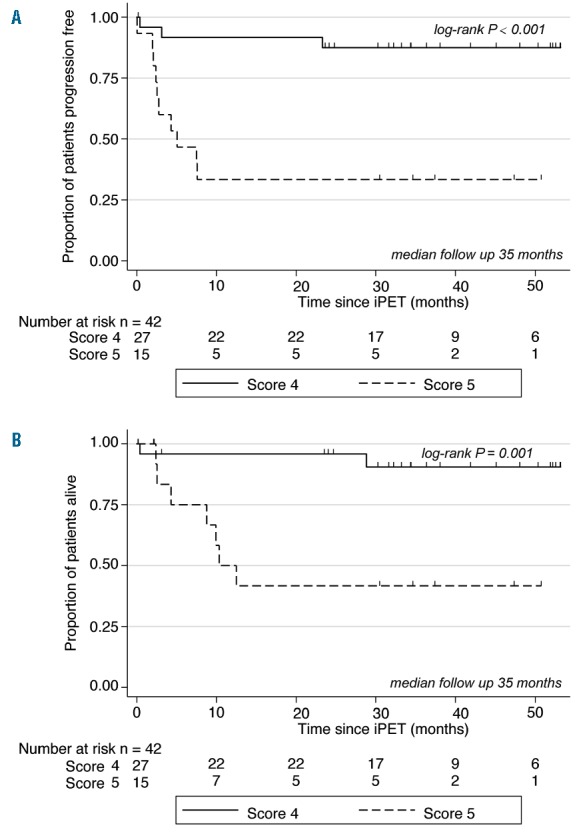
Interim positron emission tomography (iPET)-positive Deauville score 4 (n=27) versus score 5 (n=15). (A) Progression-free survival (PFS): 2-year PFS for Deauville score 4 versus score 5. (B) Overall survival (OS): 2-year OS for Deauville Score 4 versus score 5.
Univariate and multivariate analyses
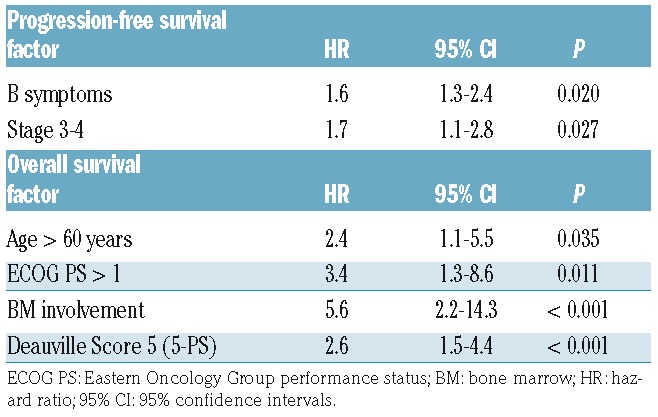
In univariate Cox proportional hazards model, status on iPET was not significant for either PFS (P=0.327) or OS (P=0.122), respectively. Multivariate analyses (MVA) factors significantly associated with PFS included B symptoms, stage 3–4 disease, and, with OS included age over 60 years, ECOG PS over 1, BM involvement, and iPET positive Deauville score 5 (Table 2).
Table 2.
Multivariate analyses for 2-year progression-free survival and overall survival.
Discussion
We present the largest study published to date examining iPET-directed treatment intensification in DLBCL. Historically, the expected 2-year PFS rate for iPET-4-positive patients treated with R-CHOP is approximately 40%.11,12,15,17,21 The aim of this study was to determine if patients with poor prognosis DLBCL who are iPET-positive after 4 cycles of R-CHOP-14 and receive treatment intensification with R-ICE followed by Z-BEAM ASCT could obtain 2-year PFS of more than 65%. Not only was this achieved, but we show that this high-risk group of patients had survival outcomes similar to those for iPET-negative patients who receive standard R-CHOP. Furthermore, in an exploratory analysis of the Deauville 5-PS to quantify the iPET scan, both PFS and OS were markedly superior in patients who are iPET-positive scoring 4 on the 5-PS compared to those scoring 5.
In combining Zevalin with BEAM, we confirm the feasibility and efficacy of the intensified combination in this adverse risk patient group, particularly for those with Deauville score 4. As expected, however, this approach is associated with greater toxicity, supporting restriction of its use to those patients with a high risk of treatment failure. Four patients displayed somewhat delayed platelet engraftment beyond day 30, as has been described previously.27,28 This study pre-dated the results of the randomized study in chemo-sensitive relapsed DLBCL comparing 131iodine-tosituzumab-BEAM and rituximab-BEAM (R-BEAM) regimens and which produced similar 2-year PFS and OS rates.33 Accordingly, we cannot exclude the possibility that the results would have been the same had we used R-BEAM instead of Z-BEAM.
At the time of study design, the issue of the optimal timing for iPET still had to be established.32,34,35 Consideration has to be made of the need to deliver potentially curative therapy in a timely manner while avoiding treatment toxicity in patients with a favorable prognosis, as against the desire to delay iPET and improve specificity by reducing the extent of rebound inflammation after the most recent immuno-chemotherapy cycle. Given the need to robustly identify poor responders, and given the reports indicating 2-year PFS rates for iPET4-positive patients of approximately 40%,11,12,15,17,21 we chose to perform the iPET after 4 cycles of R-CHOP-14. Secondly, 2- versus 3-weekly R-CHOP has been shown in randomized controlled studies to have equivalent efficacy.3 This suggests that our approach of delaying cycle 5 by seven days to permit a central review of an iPET scan performed at d17-d20 post 4th R-CHOP did not impact outcome, while, at the same time, it should be noted that a 7-day delay alone may not have necessarily improved the PPV.
Despite using IHP criteria in this study, none of the iPET scans initially rated as positive were subsequently assessed on the 5-PS as Deauville score 3 (uptake ≤ liver but > mediastinum). There is often heterogeneity of liver FDG activity as well as significant inter-subject variability in patients with DLBCL, likely due to the reversible changes in the liver metabolism during immuno-chemotherapy, and often leading to variable agreement among PET readers.36 In the present study, some iPET scans potentially considered Deauville score 3 may have been interpreted as score 2 for the purposes of IHP criteria; however, the 29% rate of iPET-positivity is highly consistent with that reported among high-risk patients in other studies using the 5-PS.16,29,30
Some investigators have evaluated a quantitative PET approach, with reports suggesting that iPET analysis using ΔSUVmax provides a higher PPV than qualitative analysis and therefore lower rates of PET-positivity.21,37–40 However, these studies have predominantly assessed the ΔSUVmax at iPET-2, as well as using somewhat variable cut-off values, with a lack of congruence between PET centers and limited validation in multi-center trials.21,37–40 At the iPET-4 time-point, ΔSUVmax has been less commonly utilized,37 while some studies have suggested that visual assessments using the liver as background may be equivalent to quantitative models for predicting PFS.21,32 Secondly, some recent studies have shown end-of-treatment PET to carry the highest PPV compared to iPET.16,41,42 Consequently, early biopsy of FDG-avid sites has been recommended, since, in one large prospective study, iPET-positive patients who were biopsy-negative displayed outcomes equivalent to those for iPET-negative patients, with the caveat that both patient groups were switched to R-ICE treatment intensification.43 As in the vast majority of studies, however, we did not assess biopsies taken from iPET-positive residual masses. Carrying out biopsies during treatment can be problematic since the affected sites are often very difficult to access surgically or radiologically, with the associated risks of bleeding, infection, sampling error, inadequate tissue, and resultant treatment delays that may adversely affect outcomes.
The feasibility and efficacy of the use of salvage therapy and ASCT among iPET-positive DLBCL patients has been assessed in 3 smaller prospective studies.22–24 Notably, these trials showed higher rates of iPET-positivity of 39%, 51%, and 71%, compared to 29% in the present study, meaning that more patients were subject to HDT yet still displayed rates of 2-year PFS (66%, 57%, and 65%) that were no better than those seen in the current study (67%). Other notable differences with these 3 studies were that iPET scans were performed earlier in the treatment course (after 2 or 3 induction cycles), and that the 5-PS was not utilized. In contrast, 3 prospective trials have evaluated treatment intensification with chemotherapy alone for iPET-positive patients and showed relatively inferior outcomes compared to the trials incorporating ASCT.25,44,45 Importantly, and in contrast to our study, iPET-positive patients either failed to complete the salvage therapy (due primarily to toxicity)26,44 and/or derived minimal benefit from the treatment intensification,45 and therefore displayed relatively unfavorable survival outcomes.
We cannot exclude the possibility that some iPET-positive patients may have converted to PET-negativity with additional cycles of standard R-CHOP treatment, and may have gone on to be cured. As against this, there was a substantial difference in 2-year PFS observed between iPET-positive Deauville score 4 (88%) and score 5 (33%) patients, as well as favorable comparisons for PFS in iPET-positive patients compared to the iPET-negative cohort and to historical controls. Only a randomized study comparing treatment intensification to continued R-CHOP in iPET-positive patients can definitively address this issue.
In summary, this study provides support for the further investigation of early selection of poor prognosis DLBCL patients, as identified by iPET scanning, who might benefit from alternative therapeutic approaches to improve outcomes. It lends weight to the role of treatment intensification for those patients who are iPET-positive Deauville score 4, and suggests that novel strategies need to be explored for those who are score 5.
Supplementary Material
Acknowledgments
Jason Callahan of the Peter MacCallum Cancer Centre provided expert technical support of the PET imaging component of this study.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/2/356
References
- 1.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23(18):4117–4126. [DOI] [PubMed] [Google Scholar]
- 2.Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2): 105–116. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013; 381(9880):1817–1826. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Z, Sehn LH, Rademaker A, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the Rituximab era. Blood. 2014;123(6):837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass B, Ziepert M, Reiser M, et al. High-dose therapy followed by autologous stem-cell transplantation with and without rituximab for primary treatment of high-risk diffuse large B-cell lymphoma. Ann Oncol. 2010;21(11):2255–2261. [DOI] [PubMed] [Google Scholar]
- 6.Stiff PJ, Unger JM, Cook JR, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013;369(18):1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitz N, Nickelsen M, Ziepert M, et al. Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol. 2012;13(12):1250–1259. [DOI] [PubMed] [Google Scholar]
- 8.Jerusalem G, Beguin Y, Fassotte M-F, et al. 18F-FDG uptake after a few cycles of poly-chemotherapy is predictive of treatment failure in non-Hodgkin lymphoma. Haematologica. 2000;85(6):613–618. [PubMed] [Google Scholar]
- 9.Spaepen K, Stroobants S, Dupont P, et al. Early restaging positron emission tomography with (18)F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2002;13(9):1356–1363. [DOI] [PubMed] [Google Scholar]
- 10.Mikhaeel NG, Hutchings M, Fields PA, O’Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16(9):1514–1523. [DOI] [PubMed] [Google Scholar]
- 11.Haioun C, Itti E, Rahmouni A, et al. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood. 2005; 106(4):1376–1381. [DOI] [PubMed] [Google Scholar]
- 12.Dupuis J, Itti E, Rahmouni A, et al. Response assessment after an inductive CHOP or CHOP-like regimen with or without rituximab in 103 patients with diffuse large B-cell lymphoma: integrating 18fluorodeoxyglucose positron emission tomography to the International Workshop Criteria. Ann Oncol. 2009;20(3):503–507. [DOI] [PubMed] [Google Scholar]
- 13.Han HS, Escalon MP, Hsiao B, Serafini A, Lossos IS. High incidence of false-positive PET scans in patients with aggressive non-Hodgkin’s lymphoma treated with rituximab-containing regimens. Ann Oncol. 2009;20(2):309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cashen AF, Dehdashti F, Luo J, Homb A, Siegel BA, Bartlett NL. 18F-FDG PET/CT for early response assessment in diffuse large B-cell lymphoma: poor predictive value of international harmonization project interpretation. J Nucl Med. 2011; 52(3):386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang DH, Min JJ, Song HC, et al. Prognostic significance of interim (1)(8)F-FDG PET/CT after three or four cycles of R-CHOP chemotherapy in the treatment of diffuse large B-cell lymphoma. Eur J Cancer. 2011; 47(9):1312–1318. [DOI] [PubMed] [Google Scholar]
- 16.Pregno P, Chiappella A, Bello M, et al. Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood. 2012; 119(9):2066–2073. [DOI] [PubMed] [Google Scholar]
- 17.Safar V, Dupuis J, Itti E, et al. Interim [18F]fluorodeoxyglucose positron emission tomography scan in diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy plus rituximab. J Clin Oncol. 2012;30(2):184–190. [DOI] [PubMed] [Google Scholar]
- 18.Spaepen K, Stroobants S, Dupont P, et al. [18F]FDG PET monitoring of tumour response to chemotherapy: does [18F]FDG uptake correlate with the viable tumour cell fraction? Eur J Nucl Med Mol Imaging. 2003;30(5):682–688. [DOI] [PubMed] [Google Scholar]
- 19.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25(5):571–576. [DOI] [PubMed] [Google Scholar]
- 20.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itti E, Lin C, Dupuis J, et al. Prognostic value of interim 18F-FDG PET in patients with diffuse large B-Cell lymphoma: SUV-based assessment at 4 cycles of chemotherapy. J Nucl Med. 2009;50(4):527–533. [DOI] [PubMed] [Google Scholar]
- 22.Kasamon YL, Wahl RL, Ziessman HA, et al. Phase II study of risk-adapted therapy of newly diagnosed, aggressive non-Hodgkin lymphoma based on midtreatment FDG-PET scanning. Biol Blood Marrow Transplant. 2009;15(2):242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart DA, Kloiber R, Owen C, et al. Results of a prospective phase II trial evaluating interim positron emission tomography-guided high dose therapy for poor prognosis diffuse large B-cell lymphoma. Leuk Lymphoma. 2014;55(9):2064–2070. [DOI] [PubMed] [Google Scholar]
- 24.Pardal E, Coronado M, Martin A, et al. Intensification treatment based on early FDG-PET in patients with high-risk diffuse large B-cell lymphoma: a phase II GELTA-MO trial. Br J Haematol. 2014;167(3):327–336. [DOI] [PubMed] [Google Scholar]
- 25.Swinnen LJ, Li H, Quon A, et al. Response-adapted therapy for aggressive non-Hodgkin’s lymphomas based on early [18F] FDG-PET scanning: ECOG-ACRIN Cancer Research Group study (E3404). Br J Haematol. 2015;170(1):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nademanee A, Forman S, Molina A, et al. A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood. 2005;106(8):2896–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan A, Nademanee A, Fung HC, et al. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(1):90–95. [DOI] [PubMed] [Google Scholar]
- 28.Shimoni A, Avivi I, Rowe JM, et al. A randomized study comparing yttrium-90 ibritumomab tiuxetan (Zevalin) and high-dose BEAM chemotherapy versus BEAM alone as the conditioning regimen before autologous stem cell transplantation in patients with aggressive lymphoma. Cancer. 2012; 118(19):4706–4714. [DOI] [PubMed] [Google Scholar]
- 29.Nols N, Mounier N, Bouazza S, et al. Quantitative and qualitative analysis of metabolic response at interim positron tomography scan combined with International Prognostic Index is highly predictive of outcomes in diffuse large B-cell lymphoma. Leuk Lymphoma. 2014;55(4):773–780. [DOI] [PubMed] [Google Scholar]
- 30.Fuertes S, Setoain X, Lopez-Guillermo A, et al. Interim FDG PET/CT as a prognostic factor in diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2013; 40(4):496–504. [DOI] [PubMed] [Google Scholar]
- 31.Itti E, Meignan M, Berriolo-Riedinger A, et al. An international confirmatory study of the prognostic value of early PET/CTR in diffuse large B-cell lymphoma: comparison between Deauville criteria and SUVmax. Eur J Nucl Med Mol Imaging. 2013; 40(9):1312–1320. [DOI] [PubMed] [Google Scholar]
- 32.Meignan M, Gallamini A, Haioun C, Polliack A. Report on the Second International Workshop on interim positron emission tomography in lymphoma held in Menton, France, 8–9 April 2010. Leuk Lymphoma. 2010;51(12):2171–2180. [DOI] [PubMed] [Google Scholar]
- 33.Vose J, Carter S, Burns LJ, et al. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: results from the BMT CTN 0401 trial. J Clin Oncol. 2013;31(13):1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terasawa T, Lau J, Bardet S, et al. Fluorine-18-fluorodeoxyglucose positron emission tomography for interim response assessment of advanced-stage Hodgkin’s lymphoma and diffuse large B-cell lymphoma: a systematic review. J Clin Oncol. 2009; 27(11):1906–1914. [DOI] [PubMed] [Google Scholar]
- 35.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceriani L, Suriano S, Ruberto T, Zucca E, Giovenalla L. 18F-FDG uptake changes in liver and mediastinum during chemotherapy in patients with diffuse large B-cell lymphoma. Clin Nucl Med. 2012;37(10): 949–952. [DOI] [PubMed] [Google Scholar]
- 37.Casasnovas RO, Meignan M, Berriolo-Riedinger A, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood. 2011; 118(1):37–43. [DOI] [PubMed] [Google Scholar]
- 38.Lin C, Itti E, Haioun C, et al. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med. 2007;48(10):1626–1632. [DOI] [PubMed] [Google Scholar]
- 39.Ishii Y, Tomita N, Tateisha U, et al. The rate of reduction in the maximum standardized uptake value from the initial to the post-R-CHOP therapy in positron emission tomography scan predicts disease progression in diffuse large B cell lymphoma patients. Med Oncol. 2014;31(3):880–887. [DOI] [PubMed] [Google Scholar]
- 40.Itti E, Meignan M, Berriolo-Riedinger A, et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and SUVmax. Eur J Nuc Med Mol Imaging. 2013;40(9):1312–1320. [DOI] [PubMed] [Google Scholar]
- 41.Micallef IN, Maurer MJ, Wiseman J, et al. Epratuzumab with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with previously untreated diffuse large B-cell lymphoma. Blood. 2013;118(15):4053–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mamot C, Klingbiel D, Hitz F, et al. Final Results of a Prospective Evaluation of the Predictive Value of Interim Positron Emission Tomography in Patients With Diffuse Large B-Cell Lymphoma Treated With R-CHOP-14 (SAKK 38/07). J Clin Oncol. 2015;33(23):2523–2529. [DOI] [PubMed] [Google Scholar]
- 43.Moskowitz CH, Schoder H, Teruya-Feldstein J, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in Advanced-stage diffuse large B-Cell lymphoma. J Clin Oncol. 2010;28(11):1896–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sehn LH, Gill KK, Al-Tourah AJ, et al. Phase 2 Trial of Interim PET Scan-Tailored Therapy in Patients with Advanced Stage Diffuse Large B-Cell Lymphoma (DLBCL) in British Columbia (BC). Blood. 2014;124:394a. [Google Scholar]
- 45.Dührsen U, Hüttmann A, Müller S, et al. Positron Emission Tomography (PET) Guided Therapy of Aggressive Lymphomas: a Randomized Controlled Trial Comparing Different Treatment Approaches Based on Interim PET Results (PETAL Trial). Blood. 2014;124(21):391a. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


