Abstract
Composite endpoints that not only encompass mortality and relapse, but other critical post-transplant events such as graft-versus-host disease, are being increasingly utilized to quantify survival without significant morbidity after allogeneic blood or marrow transplantation. High-dose, post-transplantation cyclophosphamide reduces severe graft-versus-host disease with allogeneic marrow transplantation, making composite endpoints after this management particularly interesting. We retrospectively analyzed 684 adults with hematologic malignancies who received T-cell-replete bone marrow grafts and cyclophosphamide after myeloablative HLA-matched related (n=192) or unrelated (n=120), or non-myeloablative HLA-haploidentical (n=372) donor transplantation. The median follow up was 4 (range, 0.02–11.4) years. Graft-versus-host disease-free, relapse-free survival was defined as the time after transplantation without grade III–IV acute graft-versus-host disease, chronic graft-versus-host disease requiring systemic treatment, relapse, or death. Chronic graft-versus-host disease-free, relapse-free survival was defined as the time after transplantation without moderate or severe chronic graft-versus-host disease, relapse, or death. One-year graft-versus-host disease-free, relapse-free survival and chronic graft-versus-host disease-free, relapse-free survival estimates were, respectively, 47% (95% CI: 41–55%) and 53% (95% CI: 46–61%) after myeloablative HLA-matched related, 42% (95% CI: 34–52%) and 52% (95% CI: 44–62%) after myeloablative HLA-matched unrelated, and 45% (95% CI: 40–50%) and 50% (95% CI: 45–55%) after non-myeloablative HLA-haploidentical donor transplantation. In multivariable models, there were no differences in graft-versus-host disease-free, or chronic graft-versus-host disease-free, relapse-free survival after either myeloablative HLA-matched unrelated or non-myeloablative HLA-haploidentical, compared with myeloablative HLA-matched related donor transplantation. Although limited by inclusion of dissimilar cohorts, we found that post-transplantation cyclophosphamide-based platforms yield comparable composite endpoints across conditioning intensity, donor type, and HLA match.
Introduction
Graft-versus-host disease (GvHD) and infection (often related to immunosuppression used to prevent or treat GvHD) are leading causes of morbidity and non-relapse mortality after allogeneic blood or marrow transplantation (BMT).1–3 Chronic GvHD is the major cause of late non-relapse mortality and morbidity following allogeneic BMT.4 Successful control of GvHD without prolonged immunosuppression is fundamental for minimizing post-transplant complications and improving survival and quality of life.5–7 One strategy involves the use of high-dose, post-transplantation cyclophosphamide (PTCy), which is thought to target proliferating alloreactive cells stimulated early after transplant.8,9 When given on days +3 and +4, followed by mycophenolate mofetil and tacrolimus initiated on day +5, PTCy treatment results in low rates of severe acute GvHD, chronic GvHD, and non-relapse mortality after human leukocyte antigen (HLA)-haploidentical BMT, facilitating safe T-cell-replete allografting.10–14 The utility of PTCy in reducing GvHD in the haploidentical setting led to its expansion into HLA-matched BMT. After myeloablative (MA) conditioning and HLA-matched BMT utilizing T-cell-replete bone marrow grafts, PTCy can function as sole GvHD prophylaxis,15–17 and is associated with similar acute GvHD, survival, and reduced chronic GvHD rates when compared with outcomes achieved with calcineurin inhibitor-based immunosuppression.18
Selection of a single primary efficacy endpoint that is clinically relevant, consistently determined, readily interpretable, and sensitive to treatment changes is critical when evaluating the success of novel allogeneic BMT therapies, such as PTCy.19 Given the complexity of allogeneic BMT, in which decreases in non-relapse mortality or GvHD often come at the cost of increased relapse, no one factor is sufficient when examining outcomes. Composite endpoints may measure not only mortality, but critical post-transplant events, such as severe acute GvHD and chronic GvHD requiring treatment, which may allow determination of survival without significant morbidity. Two such endpoints, GvHD-free, relapse-free survival (GRFS) and chronic GvHD-free, relapse-free survival (CRFS) have been increasingly recognized as clinically meaningful in the evaluation of both standard and novel BMT platforms and are being used as primary outcomes in Blood and Marrow Transplant Clinical Trials Network (BMT CTN) studies. The BMT CTN defined GRFS as time from BMT without development of grade III–IV acute GvHD, chronic GvHD requiring systemic treatment, relapse, or death. CRFS was defined as time from BMT without development of moderate or severe chronic GvHD (according to National Institutes of Health consensus criteria),20 relapse, or death. Given their incorporation of multiple important outcomes, these composite endpoints may be more indicative of clinical success when comparing allogeneic BMT platforms, which carry differing risks of non-relapse mortality, GvHD, and relapse.
Herein, we retrospectively assessed GRFS and CRFS in 684 patients transplanted at Johns Hopkins over an 11-year period. Given the particular transplantation platforms employed at Johns Hopkins during that time, our analyses included three regimens that differed in terms of donor type, conditioning, and GvHD prophylaxis, although all uniformly used bone marrow as the graft source and PTCy. Thus, these analyses are restricted to comparing these transplantation platforms and are limited by the heterogeneity in the diseases and characteristics of the patients enrolled.
Methods
Outcome definitions
Non-relapse mortality was defined as death without disease recurrence or persistence. When estimating cumulative incidence, non-relapse mortality was a competing risk for relapse and vice versa. Disease-free survival events consisted of any detectable disease after transplantation or death from any cause. Disease-free survival was defined from the date of transplant to the date of the event. Overall survival was defined from the date of transplant to the date of death from any cause. Post-relapse survival within relapsed patients was defined from the date of relapse to death. Patients still alive at the time of last follow up were censored for disease-free survival, overall survival, and post-relapse survival. Acute GvHD and chronic GvHD were diagnosed and scored using the modified Keystone Criteria21 and the National Institutes of Health Consensus Criteria,20 respectively. Graft failure, donor lymphocyte infusion, relapse, and death were considered competing events when estimating the cumulative incidence of GvHD. GvHD scoring was performed by SM or CK with second independent assessment by the Johns Hopkins’ GvHD specialist (JBM). The definitions of composite endpoints were similar to those in ongoing BMT CTN randomized studies (#1203 and #1301). GRFS was defined from the date of transplant to the date of last follow up without grade III–IV acute GvHD, chronic GvHD requiring systemic treatment, relapse, progression, or death.22 CRFS was defined from the date of transplant to the date of last follow up without either moderate or severe chronic GvHD, relapse, progression, or death.
Statistical analysis
The primary objective of this study was to evaluate GvHD composite endpoints after PTCy-based transplantation platforms. The data were locked on August 3rd, 2015. The patients’ characteristics and clinical variables are described and summarized. Disease-free survival, overall survival, GRFS, and CRFS curves were estimated using the Kaplan-Meier method and survival distributions between groups were compared with stratified log-rank tests.23 All group comparisons were tested stratifying by year of transplant (2009–2012 versus 2002–2008) based on the median year of transplantation. There was a higher proportion of patients undergoing haploidentical BMT than MA HLA-matched BMT from 2009–2012 and thus we stratified by BMT year to account for the impact of experience with PTCy-based transplantation platforms. The cumulative incidences of GvHD and relapse were estimated by competing risks and the distributions between groups were compared using the Gray k-sample test.24 Cox proportional hazard models were fitted to evaluate associations between risk factors and survival outcomes. Regression models for outcomes accounting for competing risks were evaluated using the stratified approach of Fine and Gray.25 Additional descriptions of multivariable and post-relapse survival analyses are provided in the Online Supplementary Material.
Patients and treatment
After Institutional Review Board approval, we retrospectively evaluated 684 consecutive patients with hematologic malignancies aged ≥18 years who received MA matched related donor (MRD), MA matched unrelated donor (MUD), or non-myeloablative (NMA) haploidentical BMT with PTCy at Johns Hopkins between 2002 and 2012. Recipients of NMA MRD and NMA MUD transplants were not included given the small numbers of patients treated after establishment of a uniform treatment regimen (n=21). Patients were treated either on Institutional Review Board-approved clinical trials or off-study using identical transplantation platforms. The majority of patients in this analysis were included in previous reports.10,11,16,17,26,27 Additional details on donor selection and treatment plan are provided in the Online Supplementary Material.
Results
Patients’ characteristics
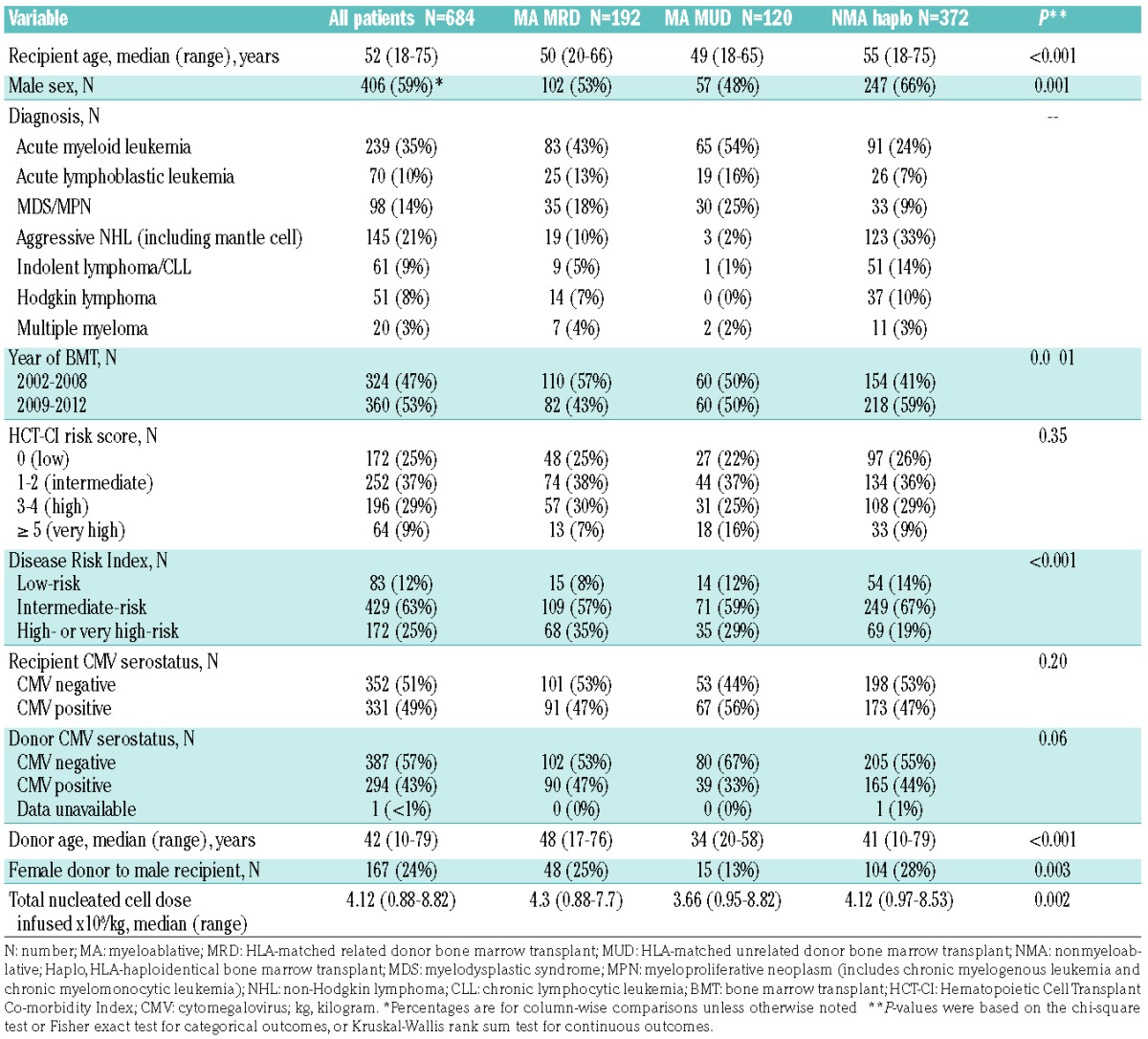
The characteristics of the patients and their transplants, overall and divided by BMT platform, are shown in Table 1. The median follow up was 4 (range, 0.02–11.4) years. Median recipient age was 52 (range, 18–75) years for the group overall, 50 (range, 20–66) years for the MA MRD cohort, 49 (range, 18–65) years for the MA MUD cohort, and 55 (range, 18–75) for the NMA haploidentical cohort. Median donor age was 42 (range, 10–79) years, 48 (range, 17–76) years, 34 (range, 20–58) years, and 41 (range, 10–79) years for the MA MRD, MA MUD, and NMA haploidentical cohorts, respectively. The most common diagnosis was acute myeloid leukemia (35%), followed by aggressive non-Hodgkin lymphoma including mantle cell lymphoma (21%), then myelodysplastic syndromes or myeloproliferative neoplasms (14%). There was a lower proportion of patients with aggressive non-Hodgkin lymphoma and a higher proportion of patients with acute myeloid leukemia in the MA MRD and MA MUD cohorts compared with the NMA haploidentical cohort. Forty-three percent of patients overall had active disease (46% for MA MRD, 28% for MA MUD, and 46% for NMA haploidentical). Thirty-eight percent of patients had Hematopoietic Cell Transplant-Comorbidity Index (HCT-CI)28 scores of ≥3 (high-risk), and the distribution of HCT-CI scores was similar between the groups. Twelve percent of patients overall were low-risk, 63% intermediate-risk, and 25% high or very high-risk according to the Disease Risk Index (DRI).29 The percentage of patients with low-risk, intermediate-risk, and high or very high-risk disease differed between the groups (P<0.001) with a higher proportion of low-risk patients (14% versus 8%) and a lower proportion of high or very high-risk patients (19% versus 35%) in the NMA haploidentical versus MA MRD BMT cohort.
Table 1.
Patients’ characteristics: overall and according to post-transplantation cyclophosphamide bone marrow transplant platform.
Kaplan-Meier estimates of composite endpoints
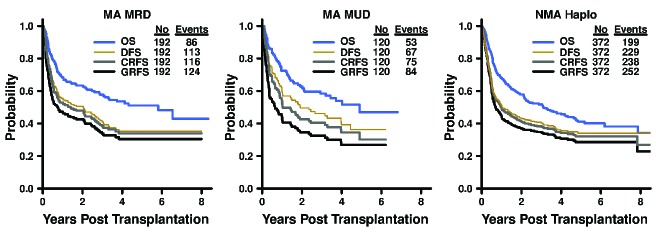
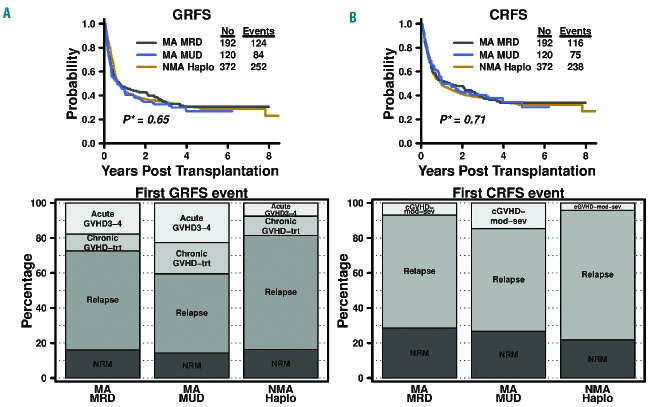
Estimates for 1-year overall survival, disease-free survival, GRFS, and CRFS rates were 68%, 56%, 47%, and 53% after MA MRD; 72%, 60%, 42%, and 52% after MA MUD; and 68%, 51%, 45%, and 50% after NMA haploidentical BMT (Figure 1 and Table 2 for 95% CI). Three-year overall survival, disease-free survival, GRFS, and CRFS estimates were 58%, 41%, 34%, and 38% after MA MRD; 58%, 46%, 32%, and 40% after MA MUD; and 52%, 41%, 35%, and 38% after NMA haploidentical BMT. There were no significant differences in GRFS (P=0.65) and CRFS (P=0.75) among the three transplantation platforms based on stratified log-rank tests (Figure 2).
Figure 1.
Kaplan-Meier curves* for overall survival, disease-free survival, GRFS, and CRFS after post-transplantation cyclophosphamide platforms for myeloablative HLA-matched related, myeloablative HLA-matched unrelated, and non-myeloablative HLA-haploidentical bone marrow transplantation. GRFS: graft-versus-host disease-free, relapse-free survival; CRFS: chronic graft-versus-host disease-free, relapse-free survival; MA: myeloablative; MRD: HLA-matched related donor; MUD: HLA-matched unrelated donor; NMA: non-myeloablative; Haplo: HLA-haploidentical; OS: overall survival; DFS: disease-free survival. *P-values shown in the plots were based on stratified log-rank tests and the curves were truncated at 8 years.
Table 2.
Survival and composite endpoints according to post-transplantation cyclophosphamide bone marrow transplant platform.
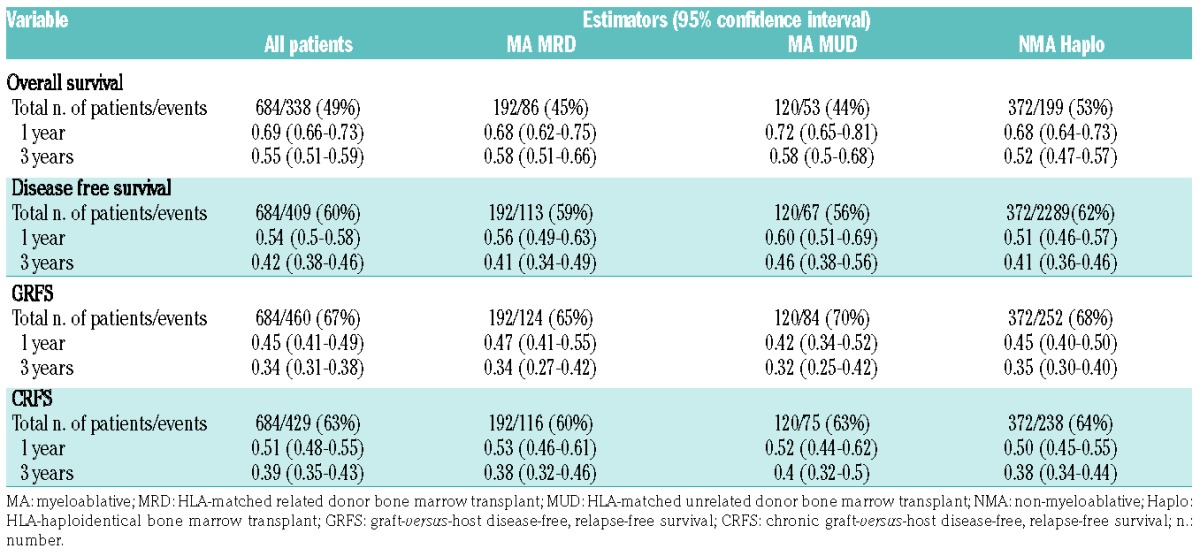
Figure 2.
(A) Kaplan-Meier curves* of GRFS and CRFS and (B) distribution of first event components of GRFS and CRFS following different transplant platforms with post-transplantation cyclophosphamide. GRFS: graft-versus-host disease-free, relapse-free survival; CRFS: chronic graft-versus-host disease-free, relapse-free survival; MA: myeloablative; MRD: HLA-matched related donor; MUD: HLA-matched unrelated donor; NMA: non-myeloablative; Haplo: HLA-haploidentical; aGVHD 3–4: grade 3–4 acute graft-versus-host disease; cGVHD trt: chronic graft-versus-host disease requiring systemic treatment; NRM: non-relapse mortality; cGVHD-modsev: moderate or severe chronic graft-versus-host disease. *P-values shown in the plots were based on stratified log-rank tests and the curves were truncated at 8 years.
Multivariable analysis of composite endpoints
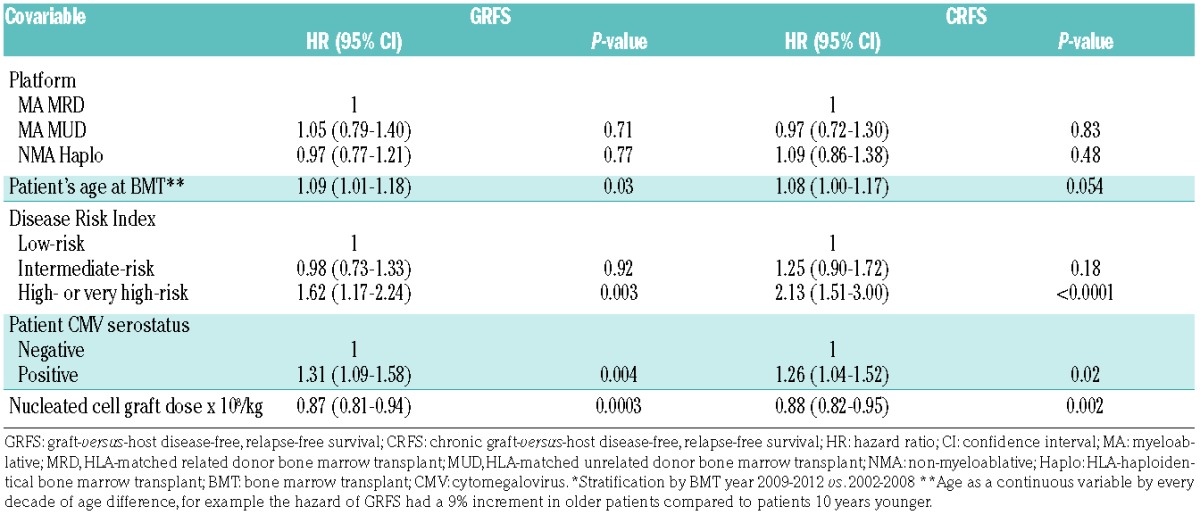
HCT-CI score, donor cytomegalovirus serostatus, donor age, and female into male allografting were not significantly independently associated with GRFS or CRFS. Of the assessed variables, DRI, patient’s age, patient’s cytomegalovirus serostatus, and nucleated cell graft dose were found to significantly influence GRFS and CRFS in multivariable analysis. Older age of the patients (analyzed as a continuous variable in increments of 10 years) was associated with inferior GRFS (HR=1.09; 95% CI: 1.01–1.18, P=0.03) and CRFS (HR=1.08; 95% CI: 1.00–1.17, P=0.05). High or very high-risk disease by DRI was also associated with inferior GRFS (HR=1.62; 95% CI: 1.17–2.24, P=0.003) and CRFS (HR=2.13; 95% CI: 1.51–3.0, P<0.0001) when compared with low-risk disease. GRFS (HR=1.31; 95% CI: 1.09–1.58, P=0.004) and CRFS (HR=1.26; 95% CI: 1.04–1.52, P=0.02) were also inferior in patients who were cytomegalovirus-seropositive compared to those who were cytomegalovirus-seronegative. In contrast, higher nucleated cell graft dose was associated with superior GRFS (HR 0.87; 95% CI: 0.81–0.94, P=0.0003) and CRFS (HR 0.88; 95% CI: 0.82–0.95, P=0.002). Finally, MA MUD was not significantly different from MA MRD for GRFS (HR=1.05; 95% CI: 0.79–1.40, P=0.71) or CRFS (HR= 0.97; 95% CI: 0.72–1.30, P=0.83) when including the above variables in the model and stratifying by BMT year. Similarly, GRFS (HR=0.97; 95% CI: 0.77–1.21, P=0.77) and CRFS (HR=1.09; 95% CI: 0.86–1.38, P=0.48) were not significantly different after NMA haploidentical when compared with MA MRD BMT in the multivariable model (Table 3).
Table 3.
Multivariable model for graft-versus-host disease-free, relapse-free survival and chronic graft-versus-host disease-free, relapse-free survival adjusting for potential cofounders.*
Distribution of first events in the composite outcomes
Despite the similarity of GRFS and CRFS outcomes, the distribution of overall first events differed between the transplant platforms (Figure 2). While non-relapse mortality and chronic GvHD requiring systemic treatment made up a similar proportion of first GRFS events, relapse was different between the groups, occurring as a first event (among those having an event) in 56% of patients after MA MRD, 45% after MA MUD, and 65% of patients after NMA haploidentical BMT (P=0.005). Grade III–IV acute GVHD as a first GRFS event was also significantly different, occurring in 18% of patients having an event after MA MRD, 23% of patients after MA MUD, and 8% of patients after NMA haploidentical BMT (P=0.0003). For CRFS, non-relapse mortality as a first event was similar after each transplant platform at 28% after MA MRD, 27% after MA MUD, and 22% after NMA haploidentical BMT (P=0.35). However, relapse comprised a different proportion of first CRFS events after MA MRD, MA MUD, and NMA haploidentical BMT with 65%, 59%, and 74%, respectively (P=0.02). Moderate or severe chronic GvHD as first CRFS events also differed between the groups, occurring in 7%, 15%, and 4% after MA MRD, MA MUD, and NMA haploidentical BMT, respectively (P=0.007).
Relapse and post-relapse survival
Relapse was the most common event within the GRFS and CRFS endpoints and was statistically different between the cohorts. We, therefore, examined the cumulative incidence of relapse by transplant platform stratified by BMT year and found that it was statistically significantly different (P=0.03) (Figure 3). The cumulative incidence of relapse at 3 years was 41% (95% CI: 33–48%) after MA MRD, 36% (95% CI: 38–45%) after MA MUD, and 46% (95% CI: 41–51%) after NMA haploidentical BMT. The median time to relapse was also different between the groups, being 164 days, 302 days, and 171 days after MA MRD, MA MUD, and NMA haploidentical BMT, respectively.
Figure 3.
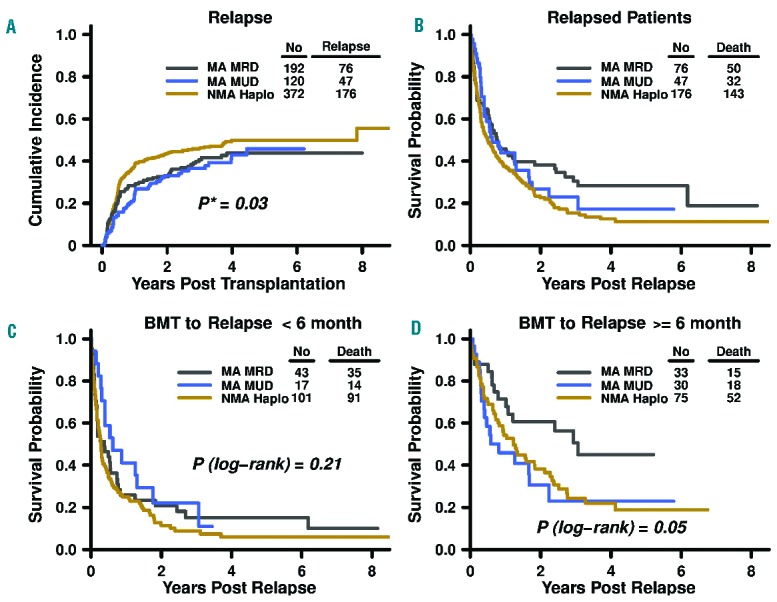
Relapse according to post-transplantation cyclophosphamide bone marrow transplant platform. (A) Cumulative incidence of relapse (P-value in the model is a result of testing the differences of cumulative incidence of relapse among transplant platforms stratified by bone marrow transplant year). (B–D) Kaplan-Meier curves of post-relapse survival by (B) post-transplantation cyclophosphamide bone marrow transplant platform and (C–D) time from transplantation to relapse. MA: myeloablative; MRD, HLA-matched related donor bone marrow transplant; MUD: HLA-matched unrelated donor bone marrow transplant; NMA: non-myeloablative; Haplo: HLA-haploidentical bone marrow transplant; BMT: bone marrow transplant.
Subsequent analysis revealed 1-year survival after relapse estimates of 46% (95% CI: 35–59), 44% (95% CI: 31–62%), and 37% (95% CI: 31–45) for patients after MA MRD, MA MUD, and NMA haploidentical BMT, respectively (Figure 3B). Given the correlation of transplant platform and time to relapse, we stratified post-relapse survival by time to relapse less than or greater than/equal to 6 months (Figure 3C,D). There was no difference in survival (P=0.21) by BMT with PTCy platform in the patients who relapsed early. Point estimates for 1-year post-relapse survival in patients who relapsed before 6 months were 26% (95% CI: 15–44%), 41% (95% CI: 23–73%), and 25% (95% CI: 18–35%) for MA MRD, MA MUD, and NMA haploidentical BMT, respectively. Among patients who relapsed at or after 6 months, those with prior MA MRD had relatively better survival than either MA MUD or NMA haploidentical patients (P=0.05). The 1-year post-relapse survival rate was 71% (95% CI: 57–89%) for MA MRD, 46% (95% CI: 30–70%) for MA MUD, and 54% (95% CI: 44–67%) for NMA haploidentical BMT.
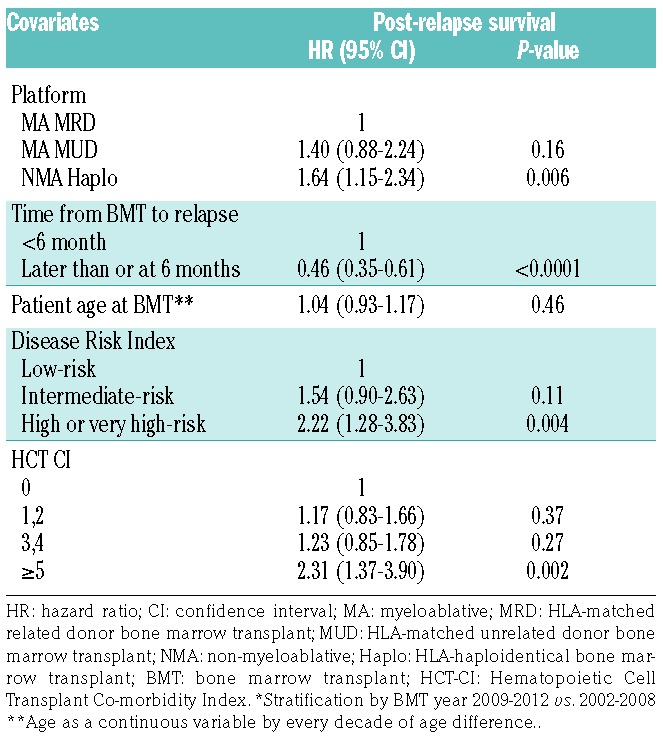
Multivariable analysis for post-relapse survival indicated that recipient’s age, donor’s age, female into male allografting, total nucleated cell dose, and recipient and donor cytomegalovirus serostatus were not independently associated with post-relapse survival (Table 4). Compared with patients who underwent MA MRD BMT, those who underwent MA MUD BMT (HR 1.40; 95% CI: 0.88–2.24, P=0.16) or NMA haploidentical BMT (HR 1.64; 95% CI: 1.15–2.34, P=0.006) had an inferior post-relapse survival, although the difference did not reach significance for MA MUD compared to MA MRD BMT. Patients who relapsed at or beyond 6 months had a longer post-relapse survival (HR 0.46; 95% CI: 0.35–0.61, P<0.0001) compared to patients who relapsed within 6 months of their transplant. Patients with high or very high-risk DRI scores had inferior post-relapse survival (HR 2.22; 95% CI: 1.28–3.83, P=0.004). Finally, patients with very high HCT-CI scores ≥ 5 also had an inferior post-relapse survival (HR 2.31; 95% CI: 1.37–3.90, P=0.002) relative to patients with HCT-CI scores of 0.
Table 4.
Multivariable analysis for post-relapse survival.*
Discussion
GRFS and CRFS incorporate GvHD, relapse, and survival endpoints to allow measurement of the success of BMT defined as relapse-free survival without ongoing morbidity. These composite outcomes were initially developed from data reported to the Center for International Blood and Marrow Transplant Research regarding HLA-matched allogeneic BMT using a variety of different GvHD prophylaxis regimens, with a calcineurin inhibitor combined with methotrexate being the most commonly utilized. Estimates of 1-year GRFS and CRFS were 23% and 28%, respectively (BMT CTN #1203). In a mixture of MRD, MUD, and umbilical cord blood transplant recipients who predominately received peripheral blood stem cell transplants and calcineurin inhibitor-based GvHD prophylaxis, Holtan et al. and Arora et al. demonstrated 1-year GRFS rates of 31%30 and 38%,31 respectively. In addition, work by Mehta et al. that included predominantly calcineurin inhibitor-based GvHD prophylaxis found improved GRFS in patients who received MRD BMT compared with patients grafted with MRD peripheral blood stem cells, MUD peripheral blood stem cells, umbilical cord blood, or mismatched unrelated allogeneic bone marrow.32 The 1-year GRFS rate of 45% in our PTCy cohort as a whole and 47%, 42%, and 45% after MA MRD, MA MUD, and NMA haploidentical BMT, respectively, compare favorably with GRFS rates previously reported for calcineurin inhibitor-based regimens.
GvHD composite endpoints have also been described following in vivo or ex vivo T-cell-depleted allogeneic BMT regimens. A recent analysis of HLA-matched peripheral blood stem cell transplants by Kroger et al.33 demonstrated a 2-year cumulative incidence of chronic GvHD of 32% (95% CI: 22–47%) in patients receiving antithymocyte globulin and 69% (95% CI: 58–81%) in patients not given antithymocyte globulin. Our 2-year incidences of chronic GvHD using the same competing risk factors as those utilized in the analysis by Kroger et al. were 9% (95% CI: 5–13%), 16% (95% CI: 9–23%), and 11% (95% CI: 8–14%) after MA MRD, MA MUD, and NMA haploidentical BMT, respectively. Furthermore, in their HLA-matched peripheral blood stem cell recipients, 2-year CRFS was 37% (95% CI: 24–48%) with antithymocyte globulin and 17% (95% CI: 9–26%) without antithymocyte globulin. Two-year CRFS rates for our PTCy platforms are similar to those in the antithymocyte globulin arm in the analysis by Kroger et al., with 48% (95% CI: 41–56%) after MA MRD, 43% (95% CI: 35–53%) after MA MUD, and 41% (95% CI: 37–47%) after NMA haploidentical BMT. Finally, considering CD34+ selection, Pasquini et al. reported a 2-year GRFS of 41% for CD34+ selected allogeneic BMT compared with 19% after standard calcineurin inhibitor-based GvHD prophylaxis, with corresponding 2-year chronic GVHD cumulative incidences of 19% and 50%.34 In all, GvHD composite endpoint outcomes after PTCy-based platforms were comparable to those reported using T-cell depletion with either antithymocyte globulin or CD34+ selection.
Furthermore, within our study, we found that GRFS and CRFS after MA MRD, MA MUD, or NMA haploidentical were not significantly different. While GRFS estimates were not dissimilar between treatment groups, the distributions of first events were significantly different, with a higher proportion of relapse and a lower proportion of GvHD in the NMA haploidentical group, compared to the other treatment groups. The lower incidence of grade III–IV acute GvHD and chronic GvHD requiring systemic treatment as a first event in the NMA haploidentical BMT cohort is likely explained by the additional immunosuppression (i.e. tacrolimus and mycophenolate mofetil) utilized and the decreased conditioning intensity.
Although relapse remains a significant problem after all allogeneic BMT platforms, higher relapse as a first GRFS or CRFS event and in cumulative incidence curves was seen after NMA haploidentical BMT. These findings might be due, in part, to the reduction in conditioning intensity. Furthermore, GvHD has been shown to correlate with graft-versus-leukemia effects, and thus a reduction in GvHD likely contributed to the increase in relapse. Time to relapse was comparable after MA MRD and NMA haploidentical BMT, but longer after MA MUD BMT. Given this difference in time to relapse, we examined survival after relapse in patients who relapsed before 6 months and those who relapsed at 6 months or later. Early relapse after BMT was associated with similarly poor outcomes regardless of the PTCy platforms. In keeping with studies by Bashey et al.13 and Solh et al.,35 survival after relapse for patients who underwent haploidentical BMT was worse than that for patients treated with MA MRD BMT. However, in our NMA haploidentical cohort we observed a higher 1-year post-relapse survival than that recorded in these prior analyses (37% compared with 17% reported in previous studies). Furthermore, Solh et al. demonstrated similar 1-year post-relapse survival for MA MRD and MA MUD BMT recipients of 46% and 40%, compared with 46% and 44% in our analysis, respectively. In their study post-relapse survival was inversely related to the use of donor lymphocyte infusion and increasing DRI score.35 We postulate that worse survival after relapse in our study may have been due to decreased use of donor lymphocyte infusion for MA MUD and NMA haploidentical36 BMT patients and/or resistance to donor lymphocyte infusion through “HLA loss” in the haploidentical BMT cohort.37 However, Bashey et al. found that survival after relapse was inferior even when patients with prior donor lymphocyte infusion were excluded and they attributed this result to a higher proportion of patients with a history of prior autologous transplantation in the haploidentical group, indicating worse disease. Our haploidentical cohort contained a higher proportion of patients with a history of lymphoma and autologous transplantation, which may indicate more resistant disease in this group, but may also indicate that fewer patients underwent a second BMT after relapse. The haploidentical cohort also included older patients who tend to have worse disease and poorer tolerance to salvage therapy (i.e. higher DRI and HCT-CI scores). Regardless, poor survival after relapses, particularly after early relapses, highlights the importance of relapse prevention. The very low rates of grade III–IV acute GvHD and moderate/severe chronic GvHD in the NMA haploidentical group provides an ideal platform to study post-transplant relapse prevention strategies, such as decreasing the duration of immunosuppression and/or early initiation of post-transplant maintenance therapies.
In our multivariable analysis of all transplant platforms, high or very high-risk disease according to the DRI, older patient’s age as a continuous variable, patient’s cytomegalovirus seropositivity, and lower total nucleated cell graft dose were associated with a higher risk of a GRFS or CRFS event. The relationship of DRI with inferior survival is consistent with past studies.11,29 The effect of age on GRFS and CRFS is due in part to higher-risk disease as defined by the DRI and an increased risk of non-relapse mortality in older patients. The 3-year non-relapse mortality rate was 17% (95% CI: 11–22%) after MA MRD, 18% (95% CI: 11–25%) after MA MRD, and 12% (95% CI: 9–16%) after NMA haploidentical BMT. The risk of non-relapse mortality was greater for older patients (examined as a continuous variable by 10-year intervals) after MA MRD [subdistribution hazard ratio (SDHR) 1.76; 95% CI: 1.12–2.76, P=0.01] and MA MUD (SDHR 1.69; 95% CI: 1.16–2.46, P=0.006), but not statistically higher after NMA haploidentical BMT (SDHR 1.22; 95% CI: 0.96–1.54, P=0.10). Furthermore, in yet unpublished data (McCurdy et al. Johns Hopkins, Improved Outcomes with Grade II Acute Graft-Versus-Host Disease after HLA-Haploidentical Transplantation using Posttransplantation Cyclophosphamide, 2017), older age was found to be associated with an increased risk of acute GvHD. After adjustment for DRI score, year of BMT, patient’s cytomegalovirus seropositivity, total nucleated cell graft dose, and transplant platform, older patients continued to have an inferior GRFS, but not CRFS, which suggests that acute GvHD (which was not included in the CRFS end-point) contributed to their worse outcomes. The relationship of patient’s cytomegalovirus seropositivity is also consistent with prior analyses.38 Finally, our finding of improved GRFS and CRFS with higher total nucleated cell dose is consistent with the association of graft dose and improved overall survival in past studies.39–41
By incorporating GvHD endpoints, GRFS and CRFS may prove useful in comparing allogeneic BMT platforms especially when reduction in GvHD is the desired clinical outcome. Moreover, GvHD composite endpoints may be better measures of successful allogeneic BMT, affording a more comprehensive picture of patients’ outcomes than overall survival or disease-free survival alone. The utility of GRFS was highlighted by Holtan et al. who found that after HLA-matched and umbilical cord transplantation only 31% of patients survive to 1 year without experiencing a GRFS event, which indicates a highly different transplant outcome than that suggested by the 1-year overall survival of 63% seen in the same cohort.30 While overall survival may be an appropriate endpoint for therapies that are not associated with high morbidity, outcomes after allogeneic BMT may be more accurately represented when GvHD events, which carry ongoing morbidity, are included. Although further studies are necessary to evaluate these endpoints, we believe that GRFS and CRFS are useful in determining relapse-free survival without ongoing morbidity, generating a more complete assessment of successful allogeneic BMT than standard endpoints. Furthermore, we posit that CRFS may be more clinically meaningful given that chronic GvHD is associated with protracted morbidity and a long-term requirement for immunosuppression,42 whereas non-fatal grade III–IV acute GvHD that is included in the GRFS outcome usually has a limited course.
This study is inherently limited by its retrospective nature and its incorporation of patients with heterogeneous risks of relapse and complications. To adjust for differences between the cohorts we formulated a multivariable model that included factors that were different between the cohorts as well as those which are known to affect outcomes after BMT such as age, DRI score, HCT-CI score, cytomegalovirus serostatus, and total nucleated cell graft dose. Moreover, because these platforms differ in the conditioning, HLA-matching, and post-grafting immunosuppression employed, we cannot make definitive conclusions about HLA-matching or conditioning intensity independently and only about these platforms as a whole. Furthermore, the sample size limits our statistical power to detect small differences in GRFS and CRFS between the PTCy platforms.
While the specific transplant sequelae may differ slightly, composite endpoints that incorporate several important post-transplant outcomes are similar across conditioning intensities and HLA-matching with PTCy. Our study supports earlier data indicating that HLA-matched and haploidentical allografting yeild similar survival,11,13,14,43–45 but demonstrates this comparability for the first time in MRD and MUD platforms that also utilize PTCy. This analysis emphasizes the safety and tolerability of PTCy-based transplant platforms, which may facilitate the early initiation of novel targeted post-transplant therapies to prevent relapse in the future. Given the growing data on the similarity of outcomes after HLA-matched and haploidentical BMT, further studies are required to determine whether others factors, such as donor’s age, may be more important for donor selection than HLA-matching.
Supplementary Material
Acknowledgments
The authors would like to thank the Cell Therapy Laboratory at Johns Hopkins for graft data.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/2/391
Funding
This study was supported by National Institutes of Health, National Cancer Institute grants P01 CA015396 (RJJ) and P30 CA006973.
References
- 1.Thomas E, Storb R, Clift RA, et al. Bone-marrow transplantation (first of two parts). N Engl J Med. 1975;292(16):832–843. [DOI] [PubMed] [Google Scholar]
- 2.Storb R, Prentice RL, Buckner CD, et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med. 1983;308(6):302–307. [DOI] [PubMed] [Google Scholar]
- 3.Clift RA, Buckner CD, Thomas ED, et al. The treatment of acute non-lymphoblastic leukemia by allogeneic marrow transplantation. Bone Marrow Transplant. 1987;2(3): 243–258. [PubMed] [Google Scholar]
- 4.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacigalupo A, Lamparelli T, Barisione G, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12(5):560–565. [DOI] [PubMed] [Google Scholar]
- 6.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–233. [DOI] [PubMed] [Google Scholar]
- 7.Pidala J, Kurland B, Chai X, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117(17):4651–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98(12):3456–3464. [DOI] [PubMed] [Google Scholar]
- 9.Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39(6):683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nature Rev Clin Oncol. 2016;13(1):10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31(10):1310–1316. [DOI] [PubMed] [Google Scholar]
- 14.Raiola AM, Dominietto A, di Grazia C, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014;20(10):1573–1579. [DOI] [PubMed] [Google Scholar]
- 15.Luznik L, Bolanos-Meade J, Zahurak M, et al. High-dose cyclophosphamide as singleagent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanakry CG, O’Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32(31):3497–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanakry CG, Tsai HL, Bolanos-Meade J, et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. 2014;124(25): 3817–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303–2314. [PubMed] [Google Scholar]
- 19.Sankoh AJ, Li H, D’Agostino RB., Sr Use of composite endpoints in clinical trials. Stat Med. 2014;33(27):4709–4714. [DOI] [PubMed] [Google Scholar]
- 20.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. [DOI] [PubMed] [Google Scholar]
- 21.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 22.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125(8):1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric-estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 24.Gray RJ. A Class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 25.Zhou B, Latouche A, Rocha V, Fine J. Competing risks regression for stratified data. Biometrics. 2011;67(2):661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasamon YL, Bolanos-Meade J, Prince GT, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33(28): 3152–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16(4):482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120(4):905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125(8):1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arora M, Cutler CS, Jagasia MH, et al. Late acute and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(3):449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta RS, Peffault de Latour R, DeFor TE, et al. Improved graft-versus-host disease-free, relapse-free survival associated with bone marrow as the stem cell source in adults. Haematologica. 2016;101(6):764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroger N, Solano C, Wolschke C, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med. 2016;374(1):43–53. [DOI] [PubMed] [Google Scholar]
- 34.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30(26):3194–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solh M, Zhang X, Connor K, et al. Post-relapse survival after haploidentical transplantation vs matched-related or matched-unrelated hematopoietic cell transplantation. Bone Marrow Transplant. 2016;51(7): 949–954. [DOI] [PubMed] [Google Scholar]
- 36.Zeidan AM, Forde PM, Symons H, et al. HLA-haploidentical donor lymphocyte infusions for patients with relapsed hematologic malignancies after related HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2014;20(3):314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCurdy SR, Iglehart BS, Batista DA, et al. Loss of the mismatched human leukocyte antigen haplotype in two acute myelogenous leukemia relapses after haploidentical bone marrow transplantation with post-transplantation cyclophosphamide. Leukemia. 2016;30(10):2102–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broers AE, van Der Holt R, van Esser JW, et al. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood. 2000;95(7):2240–2245. [PubMed] [Google Scholar]
- 39.Martin PS, Li S, Nikiforow S, et al. Infused total nucleated cell dose is a better predictor of transplant outcomes than CD34+ cell number in reduced-intensity mobilized peripheral blood allogeneic hematopoietic cell transplantation. Haematologica. 2016; 101(4):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rocha V, Labopin M, Gluckman E, et al. Relevance of bone marrow cell dose on allogeneic transplantation outcomes for patients with acute myeloid leukemia in first complete remission: results of a European survey. J Clin Oncol. 2002;20(21):4324–4330. [DOI] [PubMed] [Google Scholar]
- 41.Mehta J, Powles R, Treleaven J, Kulkarni S, Horton C, Singhal S. Number of nucleated cells infused during allogeneic and autologous bone marrow transplantation: an important modifiable factor influencing outcome. Blood. 1997;90(9):3808–3810. [PubMed] [Google Scholar]
- 42.Stewart BL, Storer B, Storek J, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104(12):3501–3506. [DOI] [PubMed] [Google Scholar]
- 43.Bashey A, Zhang X, Jackson K, et al. Comparison of outcomes of hematopoietic cell transplants from T-replete haploidentical donors using post-transplantation cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allele-matched unrelated donors and HLA-identical sibling donors: a multivariable analysis including disease risk index. Biol Blood Marrow Transplant. 2016;22(1):125–133. [DOI] [PubMed] [Google Scholar]
- 44.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127(7):938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.