Controlled trafficking of neutrophils is an important component of the host innate immunity.1 Neutrophils express a number of chemokine receptors, CXCR2 among them, which, following activation by its cognate chemokines CXCL1 and CXCL8, become mobilized and preferentially home to inflamed tissues.2,3 Dysregulated CXCR2 signaling that results in unresolving airway neutrophilia may contribute to the immunopathology of several chronic obstructive respiratory diseases, including bronchiectasis,4 cystic fibrosis,5 COPD6 and severe asthma.7
AZD5069 is a selective small-molecule antagonist of the human CXCR2 chemokine receptors, with greater than 100-fold selectivity over CXCR1 receptors.8 In preclinical models, airway neutrophil recruitment in response to inflammatory challenge is abrogated by selective targeting of CXCR2 using AZD5069.8 Clinically, oral administration of AZD5069 (80 mg, twice a day [b.i.d]) significantly reduced sputum neutrophilia by 69% in patients with bronchiectasis,4 whilst showing no impairment of optimal neutrophil immune responses.9 Given the potential for CXCR2 antagonists like AZD5069 as novel neutrophil-directed immunotherapies, further understanding the controlled regulation of neutrophil trafficking and their innate immune functions are of considerable clinical relevance. We have, therefore, examined the effects of chronic treatment with the chemokine receptor CXCR2 antagonist (AZD5069) on two key effector mechanisms of neutrophil-mediated host immunity in cynomolgus monkeys, namely phagocytosis and oxidative burst activities.
Healthy, adult cynomolgus monkeys (Macaca fascicularis) (age 27–36 months; weight 1.78–3.30 kg) were obtained from a purpose-breeding colony (Nafovanny, Vietnam). This study was conducted by Ricerca Biosciences SAS (France), and all procedures conformed to the National Decrees 2001-464 and 486 published in the Journal Officiel de la République Française regarding the use of laboratory animals in France. Protocols were approved by the testing facility Institutional Animal Care and Use Committee and studies conducted at AAALAC accredited laboratories in compliance with Good Laboratory Practice regulations. Four groups of eight cynomolgus monkeys (n= 4/sex) received placebo or AZD5069 administered orally (b.i.d) at doses of 30, 130 and 525 mg/kg/day for 39 weeks. This dosing regimen was chosen to explore multiples of the potential therapeutic dose range in humans. Circulating blood neutrophil counts were monitored longitudinally. Peripheral venous blood samples were taken at weeks 13, 26 and 39 post-treatment to assess the impact on neutrophil phagocytic and oxidative activities. Neutrophil phagocytic activity was determined using fluorescein isothiocyanate (FITC)-labeled opsonized Escherichia coli (E. coli) using a lysis-free whole blood imaging flow cytometry method, as reported previously.9 Superoxide anion production by neutrophils in whole blood was assessed by the intracellular oxidation of dihydrorhodamine (DHR) 123 and quantified by imaging flow cytometry.9 The efficiency of the phagocytic or oxidative burst responses were expressed as percentage of positive-stained cells; flow cytometry data acquisition of single-cell analysis were unobtainable. Plasma levels of CXCL8, IL-6, G-CSF, GM-CSF, IFN-γ and TNF-α aliquoted from venous blood obtained at weeks 26, 32 and 40 post-treatment were analyzed using a Luminex-based non-human primate multiplex cytokine panel (Merck Millipore). Neopterin levels were quantified by ELISA (IBL International) in accordance with the manufacturer’s instructions. Standard toxicology endpoints in terms of morbidity/mortality, clinical signs, ophthalmology, body weight, food consumption, cardiac examinations, hematology, coagulation, serum clinical chemistry, urine analysis, AZD5069 concentrations in plasma, organ weights, gross and microscopic pathology and bone marrow smears were monitored routinely throughout the study (data not shown). All data are expressed as mean ± S.D. and compared using analysis of variance (ANOVA) and Student’s t test (one-tailed, paired), where a P value of <0.05 was considered statistically significant.
Analysis of the drug exposure data from the cynomolgus monkeys revealed that the systemic concentrations of AZD5069 ranged from approximately 2- to 25-fold higher than those used clinically in humans (100 mg b.i.d).9 These high exposures of AZD5069 after continuous treatment suggested that they might exert adverse effects on host protective immune responses, so the potential impact on immunomodulatory actions on neutrophil effector function was investigated ex vivo. Chronic dosing of animals with AZD5069 (up to 39 weeks) at multiples of predicted human therapeutic exposure had no discernible effect on the neutrophil phagocytosis response, as evidenced by the percentage of cells engaging in the normal uptake of opsonized FITC-labeled E. coli particles relative to neutrophils from untreated monkeys (Table 1A). Correlating with this response, AZD5069 treatment did not interfere with the percentage of cells capable of normal superoxide anion production in E. coli-stimulated neutrophils compared with concurrent placebo-treated animals (Table 1B), in concordance with observations reported in humans.9 Furthermore, no significant difference was observed in the plasma inflammatory cytokine profile measured, i.e., IL-6, GM-CSF, IFN-γ, TNF-α and neopterin at any dose level (data not shown). However, we detected a trend towards an increasing abundance of the neutrophil-associated mediator, CXCL8, and to a lesser extent CXCL1, in plasma at 26 and 32 weeks, which was on the whole more apparent in female monkeys than in males, relative to the concurrent vehicle controls (Figure 1).
Table 1.
Pathogen-initiated phagocytosis and oxidative burst are preserved in circulating monkey neutrophils after long term treatment (39 weeks) with AZD5069.
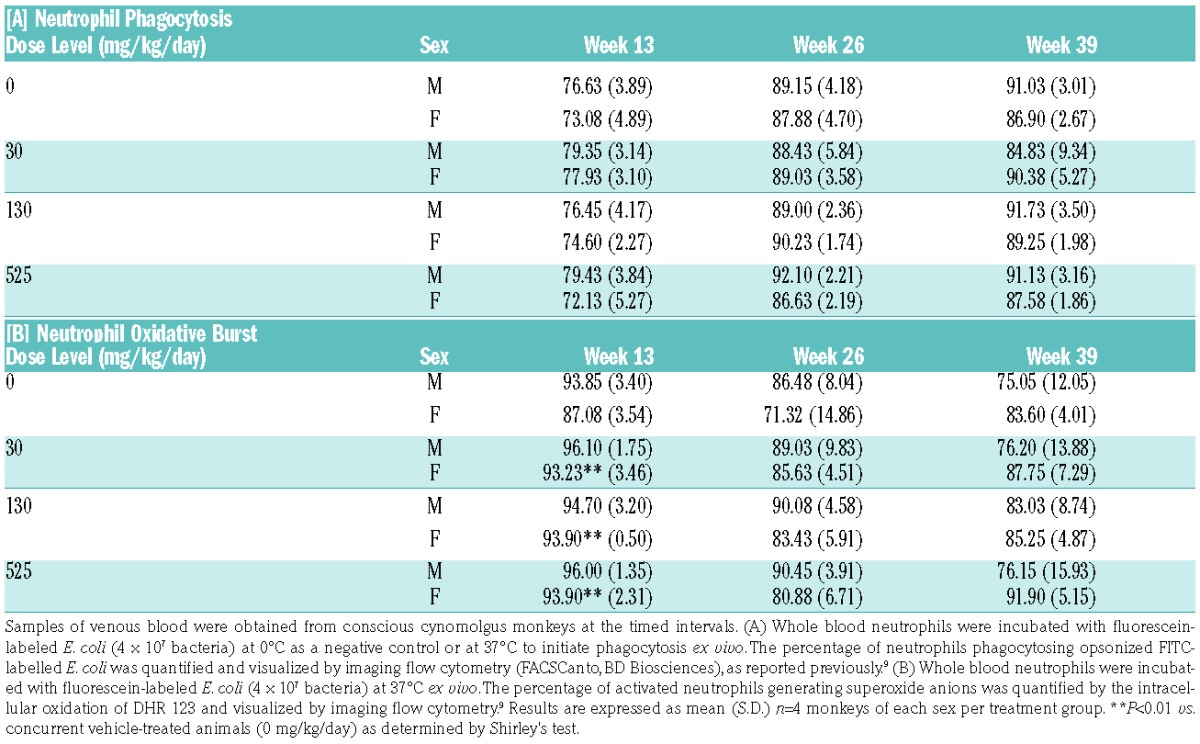
Figure 1.
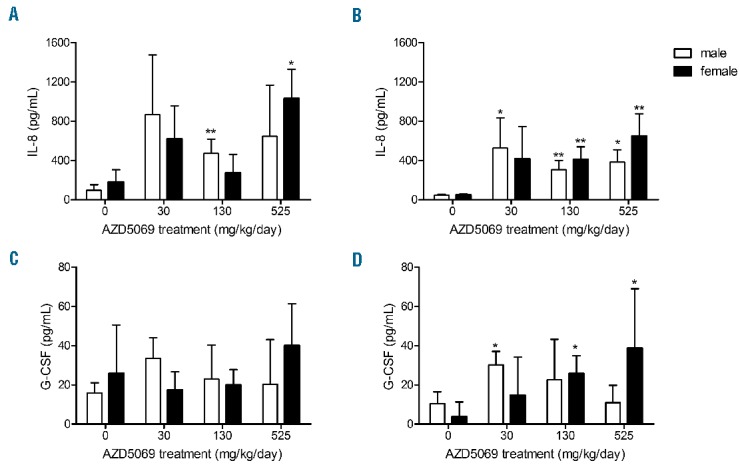
CXCL8 and G-CSF plasma concentrations in cynomolgus monkeys during the 39 week dosing interval. Samples of venous whole blood taken into K3-EDTA anticoagulant were obtained from conscious male (open bars) and female monkeys (closed bars) at week 26 (panels A & C) and week 32 (panels B & D). CXCL8 and G-CSF levels in samples collected at the end of compound treatment (week 40) were generally comparable to those obtained for week 32 (data not shown). Cytokine levels were determined by multiplex bead array (Millipore). Results are expressed as mean ± S.D. n=8 monkeys per treatment group. *P<0.05 and **P<0.01 vs. concurrent vehicle-treated animals (0 mg/kg/day) as determined by Shirley’s nonparametric test. IL-8: interleukin-8; G-CSF: granulocyte colony-stimulating factor.
Analysis of the general toxicological data from 39 weeks of treatment revealed that dosing regimens of up to 525 mg/kg/day were well tolerated in cynomolgus monkeys. AZD5069 had no significant impact on most of the measured parameters. However, an increase in circulating globulin and a decrease in albumin (with a consequent reduction in the albumin/globulin ratio) was seen in a reciprocal manner, that was not dose-related. Furthermore, AZD5069-related histopathological findings were limited to the bone marrow consisting of a dose-related increment in the myeloid/erythroid ratio, with an associated increase in segmented granulocytes at all dose levels. Notably, no compound-related changes in baseline circulating neutrophil numbers were evident (data not shown), despite an apparently similar expression pattern of CXCR2 receptors to humans,10 which indicated that AZD5069 did not affect the neutrophil mobilization from the bone marrow into the peripheral circulation under homeostatic conditions. The sparing effect of AZD5069 treatment on circulating neutrophils is particularly noteworthy in this context, since there was no increased risk of infection in animals chronically treated with AZD5069 over the 39 week interval in vivo, which is reflective of the preserved pathogen-initiated phagocytic and oxidative responses observed in the high percentage of neutrophils responding normally ex vivo.
Maintaining the immunostatic balance of neutrophils is vital to the host defense response.1 CXCR2 activation by chemokines coordinates neutrophil recruitment in homeostasis and during innate and inflammatory immune responses.2 Consistent with recent observations in humans,9 our findings in monkeys reveal that AZD5069 treatment did not impair the phagocytic or oxidative capacity of neutrophils, which are critical for anti-microbial functions. This underscores the fact that preserved neutrophil immunoprotection can be maintained during CXCR2 blockade in both these anthropoid primates.
The therapeutic rationale of targeting CXCR2 receptors in chronic obstructive respiratory diseases is emphasized by its clinical validation on airway neutrophils in patients with bronchiectasis,2 cystic fibrosis,5 COPD6 and severe asthma.7 However, larger studies are required in stratified patient populations to demonstrate that long-term neutrophil-directed therapies targeting airway inflammation result in a positive clinical outcome without impairing host protective immunity. Recently, there have been safety concerns about chronic neutrophil-directed therapies designed to constrain aberrant cell migration and activation, as this approach might also confer the risk of increasing infectious disease susceptibility in immunocompromised patients.11 In a recent phase 2b trial of the CXCR2 antagonist, MK-7123, in moderate to severe COPD, whilst the maximally effective dose, (50 mg/day, 6 months) was found to modestly improve lung function (by 67 ml FEV1 vs. placebo), this dose was also associated with significant neutropenia, resulting in the drug-related discontinuation and withdrawal of neutropenic subjects (18%).6 However, further subanalysis in current smokers on high dose MK-7123 showed a statistically and clinically significant increase in FEV1 (by up to 168 ml) and a reduction in sputum neutrophils, relative to placebo-treated patients.6 Although these data suggest that oral CXCR2 antagonists may offer a new treatment avenue for current smokers with COPD, further long-term follow-up studies in larger cohorts will be needed to determine the absolute therapeutic index of these neutrophil-directed agents.
While anti-neutrophil actions are commonly considered anti-inflammatory, recent translational evidence suggests the existence of distinct neutrophil subsets in humans that have bimodal immunomodulatory functions. The mature phenotype has a greater pro-inflammatory potential, whereas the hypersegmented phenotypes possess immunomodulatory activities that are essential for host protective immunity.12 Thus, it has been speculated that the rational design of chemokine receptor antagonists are selective for a specific neutrophil subset, in this case the disease-driving mature phenotype, whilst sparing the immunoprotective hypersegmented phenotypes will lead to the development of novel neutrophil-directed immunotherapies to advance precision respiratory medicine.13
One consequence of blocking the CXCR2 receptor pathway is the compensatory induction of CXCR2 ligands. Continuous treatment of animals with AZD5069 over a 39 week interval led to a marked increase in the high affinity CXCR2 ligand, CXCL8, compared with placebo, analogous to our prior study in humans.9 Dose-dependent increases in serum levels of CXCL8 have also been observed in patients with cystic fibrosis following treatment with a CXCR2 antagonist, SB-656933.5 Pharmacological and genetic ablation of the CXCR2 receptor in murine models of lung inflammation are related to reciprocal CXCR2 ligand expression in the circulation.14,15 However, the exact mechanisms underlying this intrinsic feed-forward regulation of systemic CXCR2 ligands is not known. We postulate that this compensatory adaptation could be a transient response to re-establish physiopharmacological homeostasis of the chemokine ligand-receptor disequilibrium elicited by CXCR2 receptor blockade in vivo.
In conclusion, our translational findings are believed to be unique in their demonstration of interspecies correlation between human and non-human primate neutrophil host immunity during CXCR2 antagonism. The net effect of chronic CXCR2 antagonism imparted by AZD5069 did not arrest neutrophil mobilization from the bone marrow to the blood, nor did it adversely impact on optimal neutrophil-mediated phagocytic and oxidative burst activities after bacterial challenge. A detailed understanding of the immunostatic mechanisms of CXCR2 signalling during host innate immunity should lead to a more cautious clinical approach, and, in all likelihood, therapeutic gains upon targeting phlogistic neutrophil trafficking pathways in the host, while potentially sparing immunoprotective pathways.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–182. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250(2):91–104. [DOI] [PubMed] [Google Scholar]
- 3.Proost P, Wuyts A, van Damme J. The role of chemokines in Inflammation. Int J Clin Lab Res. 1996;26(4):211–223. [DOI] [PubMed] [Google Scholar]
- 4.De Soyza A, Pavord I, Elborn JS, et al. A randomised, placebo-controlled study of the CXCR2 antagonist AZD5069 in bronchiectasis. Eur Respir J. 2015;46(4):1021–1032. [DOI] [PubMed] [Google Scholar]
- 5.Moss RB, Mistry SJ, Konstan MW, et al. Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis. J Cyst Fibros. 2013;12(3):241–248. [DOI] [PubMed] [Google Scholar]
- 6.Rennard SI, Dale DC, Donohue JF, et al. CXCR2 Antagonist MK-7123. CXCR2 antagonist MK-7123. A phase 2 proof-of-concept trial for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(9):1001–1011. [DOI] [PubMed] [Google Scholar]
- 7.Nair P, Gaga M, Zervas E, et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2012;42(7):1097–1103. [DOI] [PubMed] [Google Scholar]
- 8.Nicholls DJ, Wiley K, Dainty I, et al. Pharmacological characterization of AZD5069, a slowly reversible CXC chemokine receptor 2 antagonist. J Pharmacol Exp Ther. 2015;353(2):340–350. [DOI] [PubMed] [Google Scholar]
- 9.Jurcevic S, Humfrey C, Uddin M, Warrington S, Larsson B, Keen C. The effect of a selective CXCR2 antagonist (AZD5069) on human blood neutrophil count and innate immune functions. Br J Clin Pharmacol. 2015;80(6):1324–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hipkin RW, Deno G, Fine J, et al. Cloning and pharmacological characterization of CXCR1 and CXCR2 from Macaca fascicularis. J Pharmacol Exp Ther. 2004;310(1):291–300. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi SD, DeLeo FR. Role of neutrophils in innate immunity: a systems biology-level approach. Wiley Interdiscip Rev Syst Biol Med. 2009;1(3):309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pillay J, Kamp VM, van Hoffen E, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122(1):327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruijnzeel PL, Uddin M, Koenderman L. Targeting neutrophilic inflammation in severe neutrophilic asthma: can we target the disease-relevant neutrophil phenotype? J Leukoc Biol. 2015;98(4):549–556. [DOI] [PubMed] [Google Scholar]
- 14.Thatcher TH, McHugh NA, Egan RW, et al. Role of CXCR2 in cigarette smoke-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2005;289(2):L322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardona AE, Sasse ME, Liu L, et al. Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood. 2008; 112(2):256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


