ABSTRACT
Replication-dependent double-strand breaks (DSBs) are the main source of genomic instability as their inaccurate repair stimulates chromosomal rearrangements. In a recent work, we uncover a novel regulatory circuit that involves the Werner's syndrome helicase and CDK1, and that is essential for repair pathway choice at replication-dependent DSBs.
keywords: DNA repair, end-resection, WRN protein
Many endogenous or exogenous factors can affect the correct duplication of the genome, resulting in DNA damage and chromosome instability, both distinctive traits of cancer cells.1,2 Nicks or gaps in the template DNA strand are inevitably converted into a one-ended double-strand breaks (DSB) by an approaching replication fork, and such replication-dependent DSBs are thought to be the triggering lesion of most of the chromosomal rearrangements observed in cancer cells.3 Furthermore, replication-dependent DSBs accumulate because of the oncogene-induced replication stress and are commonly generated by Topoisomerase I and II inhibitors often employed in anticancer therapy, such as irinotecan, a camptothecin (CPT) derivative irinotecan.4
Hence, the correct repair of replication-dependent DSBs is crucial for cellular viability and for the maintenance of genome integrity. The precise view of all the factors implicated in the repair of a replication-dependent DSB is far to be complete; however, the pathways involved are basically those in charge of the repair of classical two-sided DSBs.5 In higher eukaryotes, the repair of DSBs is performed by homologous recombination (HR), single-strand annealing, and non-homologous end joining (NHEJ).5 The molecular mechanism underlying HR is pretty well defined, and it is initiated by processing of DNA ends by nucleases to produce single-strand DNA (ssDNA) tails that will be recognized by the major recombinase RAD51 to perform strand invasion and exchange.5 As a limiting step, resection of the DSB is tightly regulated and used as a switch to select for different DNA repair pathways. Indeed, the competition for DNA ends between HR and NHEJ factors implicates efficient formation of ssDNA as critical to promote loading of one class of factors or the other one.5
Resection is initiated by the MRE11 nuclease, part of a complex comprising also RAD50 and NBS1. Subsequently, the EXO1 or DNA2 exonucleases, in combination with the helicase activity of two RecQ-class proteins, the Bloom's syndrome (BLM), or the Werner's syndrome protein (WRN), take over in the process. Regulation of this process is performed by a coordinate phosphorylation of critical resection factors, including EXO1, DNA2 or CtIP, by the cell cycle kinase CDK1 and the DNA damage response factor ATM.5
In our paper, we wanted to decipher the regulation of one of the crucial end-resection helicases, the WRN protein, in response to replication-dependent DSBs.6
Indeed, although a key role of WRN during end-resection has not been firmly demonstrated, WRN-deficient cells are extremely sensitive to inhibitors of Topoisomerase I and II,7,8 showing enhanced replication stalling events and chromosome instability that might be related to defects in repairing replication-dependent DSBs.
Regulation of WRN involves many kinases, including the two checkpoint kinases ATM and ATR9; however, its primary sequence also contains putative consensus motif for CDKs, which are thought to be master regulators of DSB processing. Indeed, CDK1 phosphorylates WRN at Serine 1133 (Ser1133) in vitroand in vivo, and phosphorylation is increased upon treatment with CPT. Although phosphorylation of WRN by CDK1 is also seen under unperturbed cell growth, expression of a mutant containing Serine to Alanine substitution at residue 1133 (S1133A) does not overtly interfere with normal DNA replication and is sufficient to rescue almost completely the DNA replication defect associated with loss of WRN. This finding is potentially useful to understand the molecular pathology of Werner's syndrome (WS), the genetic disease linked to mutation of WRN.9 As the ATR-dependent regulation of WRN is essential to recover normal DNA replication in WS cells,9 our work might imply that only ATR-related functions of the protein may be relevant for the WS pathology. Further investigations will be needed to analyze senescence in cell models issued from cells expressing pathway-specific WRN mutations.
However, WRN phosphorylation by CDK1 at Ser1133 is highly relevant to faithful long-range resection taking place at the CPT-induced replication-dependent DSBs. Abrogation of Ser1133 phosphorylation reduces long-range resection similar to depletion of DNA2 or inhibition of the helicase activity of WRN, indicating that CDK1 regulates the reported cooperation between WRN and DNA2 in DSB processing. Furthermore, and worth noting, expression of the unphosphorylable WRN mutant S1133A interferes with the possible takeover by BLM or EXO1 as another long-resection duo, as elsewhere suggested.10 Indeed, while absence of WRN paves the way to BLM and loss of WRN or DNA2 stimulates the EXO1-dependent pathway of resection, the presence at DSBs of the unphosphorylable protein might shift the balance to end-joining possibly through additional and pathway-specific post-translational modifications of WRN, which also play roles in NHEJ repair pathway. This is an interesting starting point for further investigations on crosstalk between different layers of WRN regulation and their implications for DNA repair and genome integrity.
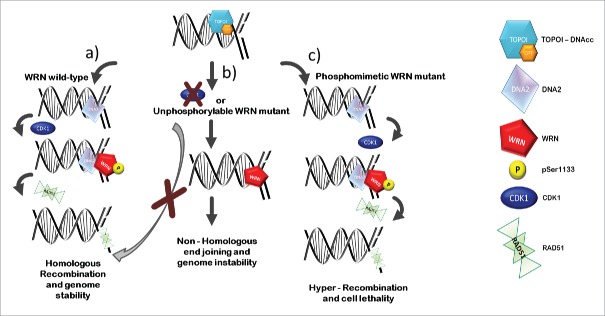
Of note, while the unphosphorylable S1133 WRN mutant affects resection of DSBs, the phosphomimicking mutant S1133D, in which Serine has been replaced by Aspartate, is able to stimulate end-resection and induce hyper-recruitment of RAD51 (Fig. 1). Stimulation of HR correlates with enhanced rate of resection as cells expressing S1133D WRN show faster disassembly of the MRE1 complex, which is implicated in the first step of resection.
Figure 1.
CDK1-mediated WRN phosphorylation promotes correct DNA repair at collapsed replication forks. Exposure to CPT entraps Topoisomerase I at DNA after production of the single-strand nick, resulting in accumulation of Topoisomerase I/DNA cleavable complexes (TOPOI-DNAcc). Formation of replication-dependent DSBs by CPT leads to fork collapse and engagement of homologous recombination. In wild-type condition, CDK1 phosphorylates WRN at residue Serine 1133 (pSer1133), promoting DNA2/WRN-dependent DNA end-resection (A). Fruitful resection supports homologous recombination-mediated DSBs repair by promoting recruitment of the RAD51 recombinase, thus ensuring viability and safeguarding against genome instability (A). Inhibition of CDK1 or abrogation of WRN phosphorylation through the expression of the WRNS1133A unphosphorylable mutant makes the end-resection pathway less efficient, affecting repair of DSBs by homologous recombination and switching to non-homologous end joining (B). On the other hand, expression of a phosphomimetic WRN mutant boosts end-resection, increasing RAD51 recruitment and inducing hyper-recombination, which eventually triggers cell lethality (C).
Interestingly, excessive recombination load undermines genome integrity and cell viability, as cells expressing S1133D WRN undergo cell death because of uncontrolled RAD51-dependent recombination (Fig. 1).
Altogether, our work uncovers a novel regulatory layer concerning the WRN function and repair of the replication-dependent DSBs, which may prove important in understanding how genomic instability accumulates under pathological conditions and in cancer cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank all members of the PP and AF laboratories for useful discussion and apologize to authors whose works cannot be cited because of space limitation.
Funding
This work was supported in part by grants from Associazione Italiana Ricerca sul Cancro (AIRC) to PP (IG17383) and AF (IG15410), and by a grant from Fondazione Telethon (GGP12144) to PP.
References
- 1.Hills SA, Diffley JFX. DNA Replication and Oncogene-Induced Replicative Stress. Curr Biol 2014; 24:R435-R444; PMID:24845676; http://dx.doi.org/ 10.1016/j.cub.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 2.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability-an evolving hallmark of cancer. Nat Rev Mol Cell Biol 2010; 11:220-8; PMID:20177397; http://dx.doi.org/ 10.1038/nrm2858 [DOI] [PubMed] [Google Scholar]
- 3.Burrell RA, McClelland SE, Endesfelder D, Groth P, Weller MC, Shaikh N, Domingo E, Kanu N, Dewhurst SM, Gronroos E, et al.. Replication stress links structural and numerical cancer chromosomal instability. Nature 2013; 494:492-6; PMID:23446422; http://dx.doi.org/ 10.1038/nature11935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pommier Y, Sun Y, Huang SN, Nitiss JL. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat Rev Mol Cell Biol 2016; 17:703-21; PMID:27649880; http://dx.doi.org/ 10.1038/nrm.2016.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceccaldi R, Rondinelli B, D'Andrea AD. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol 2015; 26:52-64; PMID:26437586; http://dx.doi.org/ 10.1016/j.tcb.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palermo V, Rinalducci S, Sanchez M, Grillini F, Sommers JA, Brosh RM Jr, Zolla L, Franchitto A, Pichierri P. CDK1 phosphorylates WRN at collapsed replication forks. Nat Commun 2016; 7:12880; PMID:27634057; http://dx.doi.org/ 10.1038/ncomms12880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pichierri P, Franchitto A, Mosesso P, Proietti de Santis L, Balajee A, Palitti F. Werner's syndrome lymphoblastoid cells are hypersensitive to topoisomerase II inhibitors in the G2 phase of the cell cycle. Mutat Res Repair 2000; 459:123-33; http://dx.doi.org/ 10.1016/S0921-8777(99)00065-8 [DOI] [PubMed] [Google Scholar]
- 8.Pichierri P, Franchitto A, Mosesso P, Palitti F. Werner's syndrome cell lines are hypersensitive to camptothecin-induced chromosomal damage. Mutat Res - Fundam Mol Mech Mutagen 2000; 456:45-57; PMID:11087895; http://dx.doi.org/21389352 10.1016/S0027-5107(00)00109-3 [DOI] [PubMed] [Google Scholar]
- 9.Pichierri P, Ammazzalorso F, Bignami M, Franchitto A. The Werner Syndrome protein: Linking the replication checkpoint response to genome stability. Aging (Albany NY) 2011; 3:311-8; PMID:21389352; http://dx.doi.org/ 10.18632/aging.100293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sturzenegger A, Burdova K, Kanagaraj R, Levikova M, Pinto C, Cejka P, Janscak P. DNA2 cooperates with the WRN and BLM RecQ helicases to mediate long-range DNA end resection in human cells. J Biol Chem 2014; 289:27314-26; PMID:25122754; http://dx.doi.org/ 10.1074/jbc.M114.578823 [DOI] [PMC free article] [PubMed] [Google Scholar]