Abstract
Cytogenetic data using multiple cell culture conditions to induce different classes of fragile sites from males with mental retardation are limited. Thus, the frequency and distribution of chromosome fragile sites were assessed from peripheral blood lymphocytes of 165 institutionalized males (age range 18–86 years) with mental retardation screened for the fragile X syndrome but with no recognizable cause of their retardation. The cells were grown in folate-replete RPMI 1640 culture medium with bromodeoxyuridine (BrdU) or fluorodeoxyuridine (FUdR) and in folate-deficient Medium 199. A significantly higher number of fragile sites or lesions (1,118 vs. 301) was observed in cells grown in RPMI 1640 with FUdR than in Medium 199. Specifically, 6q26 and Xp22 sites were observed much more frequently in RPMI 1640 with FUdR than in Medium 199. Similarly, a significantly higher number of sites (612 vs. 332) was observed in cells from RPMI 1640 with BrdU than in Medium 199. Specifically, 3q27, 9q13 and 12q24 sites were observed more frequently in RMPI 1640 with BrdU. However the 3p14 site was observed more frequently in Medium 199. The fragile sites were non-random and unevenly distributed with the highest number of sites located on chromosome 3 (band 3p14) for both Medium 199 and RPMI 1640 with FUdR and for chromosome 16 (band 16q22) for RPMI 1640 with BrdU. Significantly more fragile sites were located on chromosomes 3 and 16 from cells grown in Medium 199; for chromosomes 3, 6, 7, and 16 for cells grown in RPMI 1640 with FUdR; and for chromosomes 4, 9, 10, and 16 for cells grown in RPMI 1640 with BrdU. In addition, the 301 fragile sites or lesions in cells grown in Medium 199 were found on 103 of a total of 306 chromosome bands: 1,118 sites on 203 bands from RPMI 1640 with FUdR and 612 sites on 145 bands from RPMI 1640 with BrdU. Eight sites each representing at least 2% of the total number of sites were found in cells grown in Medium 199; seven sites in cells from RPMI 1640 with FUdR and eight sites in cells from RPMI 1640 with BrdU. Only one site (3p14) was classified as a common site in >50% of the population while the other sites were polymorphic (in 1–50%) or rare (in <1%). The BrdU site at 9q13 was seen in 10% of males with mental retardation, accounted for 3.8% of all BrdU sites, and appears to be a newly reported or underreported site enhanced by BrdU.
Fragile sites are chromosome lesions which appear as gaps or breaks on chromosomes at specific locations prone to breakage and are inducible by certain culture conditions. Chromosome fragile sites have been recognized for over 20 years and are classified as either rare or common. Hecht (1986) suggested that fragile sites may be classified into three categories, based on their occurrence in the general population: rare (<1%), polymorphic (1–50%), and common (>50%). There are at least 107 chromosome fragile sites (24 rare and 83 common) (Hecht et al., 1990). Rare fragile sites are grouped into four categories (folate-sensitive, distamycin A, bromodeoxyuridine (BrdU), and unclassified) and there are four groups of common sites (aphidicolin, 5-azacytidine, BrdU, and unclassified) (Hecht, et al., 1990). An example of a rare site is Xq27.3, which is observed in cells grown in folate-deficient culture medium and associated with one form of X-linked mental retardation (fragile X syndrome).
Folate-deficient culture conditions increase chromosome breakage relative to folate-replete culture conditions (Zhou et al., 1982; Steinbach et al., 1982; Butler 1990), although increased chromosome instability has not been observed in patients with fragile X syndrome compared with cells from control subjects (Vekemens et al., 1983; Branda et al., 1984; Butler et al., 1988). Cells grown in fluorodeoxyuridine (FUdR), an inhibitor of thymidylate synthetase which limits the dTMP available for DNA synthesis (Glover, 1981), have shown increased chromosome breakage (Sutherland and Hecht, 1985; Butler, 1990) compared with cells grown in folate-deficient culture medium. No significant relationship was found between age and frequency of chromosome lesions induced by folate-deficient culture conditions in individuals with mental retardation with or without the fragile X syndrome (Butler, 1990). However, others have suggested there is an association between sites induced by BrdU, a thymidine analog that induces a separate class of fragile sites, and mental retardation (Sanfilippo et al., 1983).
The frequency of folate-sensitive autosomal or non sex chromosome fragile sites in mentally retarded and/or mentally normal populations has been reported in a few studies (Mavrou et al., 1991; Kahkonen et al., 1986). However, studies comparing the location and frequency of fragile sites in individuals with mental retardation using different culture conditions are lacking.
This report is a detailed descriptive controlled study of the frequency, distribution, and comparison of fragile sites or lesions in BrdU or FUdR folate-replete culture medium and folate-deficient medium in institutionalized males with mental retardation.
Materials and Methods
The patients included 165 institutionalized mentally retarded males ranging in age from 18 to 86 years with no recognizable cause of their mental retardation, (i.e., no chromosomal abnormalities recognized with routine (350–400 band level) G-banded analysis, environmental insults or genetic syndromes identified after clinical genetics evaluation). Clinical information including family and medical histories and anthropometric data were obtained on each individual and reported elsewhere (Butler et al., 1991a; Butler and Singh, 1993). Butler and others (1991a, 1991b, 1993) and Hagerman et al. (1991) reported that certain physical characteristics (e.g., ear width, testicular volume, bizygomatic diameter, head breadth, plantar crease, and hyperfiexibility) could be used to correctly classify the fragile X syndrome in over 90% of mentally retarded males. In addition, discriminant analysis of anthropometric variables and other physical and historical variables could be used to distinguish between mentally retarded males with or without the fragile X syndrome at an overall correct classification rate greater than 95% (Butler et al., 1991a; Butler et al., 1991b). Molecular genetic screening was undertaken on 20 of 165 cytogenetically normal mentally retarded males but with manifestations of the fragile X syndrome using two DNA probes for detection of the fragile X syndrome, but were found to be normal (Butler et al., 1994). In order to analyze a similar number of cells grown in different culture conditions, a subset of the 165 mentally retarded males was used to compare fragile sites induced by either BrdU or FUdR with folate-deficient culture conditions (Medium 199) following established protocols (Butler et al., 1990).
Aliquots (0.5 ml) of a peripheral blood specimen collected at room temperature in sodium heparin vacutainer tubes and three separate cultures were established on the same day of collection using Medium 199, or RPMI 1640 medium supplemented with either FUdR, or BrdU. Medium 199 is a folate-deficient medium which allows expression of folate-sensitive fragile sites. FUdR, a thymidylate synthetase inhibitor, promotes expression of these sites, and BrdU, a thymidine analog, induces a separate class of fragile sites (Sutherland and Hecht, 1985).
Peripheral blood cultures were prepared using Medium 199 (Gibco BRL, Grand Island, New York) supplemented with 5% fetal bovine serum (Gibco BRL, Grand Island, New York) inactivated at 65°C, for 30 min, phytohemagglutinin (M form–Gibco BRL, Grand Island, New York), and antibiotics (penicillin/streptomycin–Gibco BRL, Grand Island, New York) with the pH (7.8) adjusted at the time of culture preparation. The cultures were incubated at 37°C for 96 h and colcemid (0.15μg/ml) (Gibco BRL, Grand Island, New York) added 45 min prior to harvest.
Peripheral blood samples also were cultured in RPMI 1640 medium (Gibco BRL, Grand Island, New York) supplemented with 15% fetal bovine serum, phytohemagglutinin (M-form), and antibiotics (penicillin/streptomycin). The cultures were incubated for 96 h at 37°C with FUdR (10−7M) (Sigma, St Louis, Missouri) added 24 h prior to harvest and colcemid (0.15μg/ml) added 45 min before harvest.
Peripheral blood samples were cultured by using RPMI 1640 medium supplemented with 5% fetal bovine serum, phytohemagglutinin (M-form) and antibiotics (penicillin/streptomycin). Incubation was for 72 h at 37°C and BrdU (30mg/L) (Sigma, St. Louis, Missouri) was added 6 h before harvest. Colcemid (0.15μg/ml) was added 45 min prior to harvest.
Cells were harvested following treatment with hypotonic (0.56%) KCl solution (8 min at 37°C). Cells were fixed with 3: 1 methanol/acetic acid and washed four times. Chromosome slides were air dried using conventional methods and stained with Giemsa. A minimum of 125 cells (e.g., 50 from Medium 199, 25 from RPMI 1640 with FUdR, and 50 from RPMI 1640 with BrdU) were analyzed by the same observer from each subject for chromosome fragile sites. The location of the chromosome lesions or fragile sites was identified after destaining and GTG banding by comparing photographic images of the uniformly stained chromosomes with the same chromosomes after banding at the microscopic level (Butler et al., 1990). Chromosome lesions were scored as gaps (width of a chromatid) or breaks (greater than the width of a chromatid or out of the plane) involving one or both chromatids.
To compare the frequency, distribution and significance of fragile sites or lesions induced by BrdU and FUdR in folate-replete culture medium with folate-deficient medium in mentally retarded males, a similar number of cells was analyzed for each culture condition (e.g., 5,846 cells grown in RPMI 1640 with BrdU and compared with a similar number of cells in Medium 199). The location and frequency of fragile sites or lesions were recorded on chromosome idiograms and the comparison of sites was undertaken for Medium 199 and RPMI 1640 with BrdU as well as Medium 199 and RPMI 1640 with FUdR. Chi-square test with Yates’ correction was used throughout for statistical analysis.
Results
The number of patients studied and the cytogenetic data are shown in Table 1.
Table 1.
Summary of chromosome fragile site data from the mentally retarded males.
| Medium 199 | RPMI 1640 with FUdR | χ2 | Medium 199 | RPMI 1640 with BrdU | χ2 | |
|---|---|---|---|---|---|---|
| Subjects | 100 | 165 | — | 117 | 121 | — |
| Cells Analyzed | 5,096 | 4,738 | — | 6,009 | 5,846 | — |
| Fragile Sites | 301 | 1,118 | — | 332 | 612 | — |
| Cells with Fragile Sites | 294 | 837 | 340.23* | 321 | 578 | 86.70* |
| Cells with Fragile Sites (%) | 5.8 | 17.7 | — | 5.3 | 9.9 | — |
FUdR is fluorodeoxyuridine; BrdU is bromodeoxyuridine, χ2 is chi-square.
P < 0.001, two-tailed test.
For comparison studies, 5,846 cells grown in RPMI 1640 medium with BrdU were analyzed from 121 individuals and 6,009 cells grown in Medium 199 were recorded from 117 patients. No fragile sites or lesions were observed on chromosomes 19, 21, or Y in cells grown in Medium 199, while sites were seen in at least one cell on all chromosomes in cells grown in RPMI 1640 with FUdR. Fragile sites or lesions also were seen (in at least one cell) for all chromosomes except for chromosomes 20 and 21 in cells grown in RPMI 1640 with BrdU.
In 5,096 cells grown in Medium 199 and 4,738 cells in RPMI with FUdR from our mentally retarded males, a significantly higher number (P < 0.05) of fragile sites or lesions (1,118 vs. 301) were observed in cells grown in RPMI 1640 with FUdR than in Medium 199. Similarly, in 6,009 cells grown in Medium 199 and 5,846 cells in RPMI 1640 with BrdU, a significantly higher number (P < 0.05) of fragile sites or lesions (612 vs. 332) were observed in the cells grown in RPMI 1640 with BrdU than in Medium 199 (Table 1).
The average percentage of fragile sites per cell in cells grown in Medium 199 compared with those grown in RPMI 1640 with FUdR was 5.9% and 23.6%, respectively. A range of 0% was observed for chromosomes 19, 21, and Y and 26.9% for chromosome 3 in cells grown in Medium 199 and a range of 0.1% for chromosome Y and 20.8% for chromosome 3 in cells grown in RPMI 1640 with FUdR (Table 2). The average percentage of fragile sites per cell in cells grown in Medium 199 compared with those grown in RPMI 1640 with BrdU was 5.5% and 10.5%, respectively. A range of 0% was observed for chromosomes 19, 21, and Y and 27.1% for chromosome 3 in cells grown in Medium 199 and a range of 0% for chromosomes 20 and 21 and 10.5% for chromosome 16 in cells grown in RPMI 1640 with BrdU (Table 3). The range of fragile site expression in cells grown in Medium 199 for the 117 mentally retarded males was 0 to 22% while the range of expression in cells grown in RPMI 1640 with BrdU for the 121 males was 0 to 38% and 0 to 48% for cells grown in RPMI 1640 with FUdR for the 165 males.
Table 2.
Chromosome fragile site data from cells grown in Medium 199 or RPMI 1640 with fluorodeoxyuridine from mentally retarded males.
| Chromosome Number | Medium 1991
|
RPMI 1640 with FUdR1
|
|||
|---|---|---|---|---|---|
| Fragile Sites2 | Expression of Fragile Sites (%) | Fragile Sites3 | Expression of Fragile Sites (%) | χ2 | |
| 1 | 24 | 8.0 | 87 | 7.8 | 0.00 |
| 2 | 18 | 6.0 | 104 | 9.3 | 2.92 |
| 3 | 81 | 26.9 | 232 | 20.8 | 4.88a |
| 4 | 9 | 3.0 | 47 | 4.2 | 0.63 |
| 5 | 15 | 5.0 | 70 | 6.3 | 0.48 |
| 6 | 12 | 4.0 | 98 | 8.8 | 6.92b |
| 7 | 18 | 6.0 | 85 | 7.6 | 0.70 |
| 8 | 8 | 2.7 | 31 | 2.8 | 0.01 |
| 9 | 11 | 3.6 | 35 | 3.1 | 0.07 |
| 10 | 13 | 4.3 | 30 | 2.7 | 1.64 |
| 11 | 10 | 3.3 | 31 | 2.8 | 0.10 |
| 12 | 8 | 2.7 | 28 | 2.5 | 0.00 |
| 13 | 6 | 2.0 | 18 | 1.6 | 0.04 |
| 14 | 6 | 2.0 | 31 | 2.8 | 0.30 |
| 15 | 3 | 1.0 | 16 | 1.4 | 0.09 |
| 16 | 42 | 14.0 | 86 | 7.7 | 10.58b |
| 17 | 2 | 0.7 | 14 | 1.2 | 0.30 |
| 18 | 5 | 1.7 | 22 | 2.0 | 0.01 |
| 19 | 0 | 0.0 | 9 | 0.8 | 1.33 |
| 20 | 1 | 0.3 | 7 | 0.6 | 0.03 |
| 21 | 0 | 0.0 | 5 | 0.4 | 0.38 |
| 22 | 1 | 0.3 | 5 | 0.4 | 0.05 |
| X | 8 | 2.7 | 26 | 2.3 | 0.01 |
| Y | 0 | 0.0 | 1 | 0.1 | 0.50 |
5,096 cells grown in Medium 199 were analyzed from 100 patients; 4,738 cells grown in RPMI with fluorodeoxyuridine (FUdR) were analyzed from 165 patients, χ2 is Chi-square.
Total fragile sites = 301.
Total fragile sites = 1,118.
P < 0.05.
P < 0.01, two-tailed test.
Table 3.
Chromosome fragile site data from cells grown in Medium 199 or RPMI 1640 with bromodeoxyuridine from mentally retarded males.
| Chromosome Number | Medium 1991
|
RPMI 1640 with BrdU1
|
|||
|---|---|---|---|---|---|
| Fragile Sites2 | Expression of Fragile Sites (%) | Fragile Sites3 | Expression of Fragile Sites (%) | χ2 | |
| 1 | 27 | 8.1 | 25 | 4.1 | 6.02a |
| 2 | 21 | 6.3 | 40 | 6.6 | 0.00 |
| 3 | 90 | 27.1 | 41 | 6.7 | 73.32c |
| 4 | 10 | 3.0 | 56 | 9.2 | 11.54c |
| 5 | 15 | 4.5 | 48 | 7.9 | 3.31 |
| 6 | 13 | 3.9 | 29 | 4.8 | 0.18 |
| 7 | 22 | 6.6 | 32 | 5.2 | 0.54 |
| 8 | 9 | 2.7 | 17 | 2.8 | 0.02 |
| 9 | 11 | 3.3 | 55 | 9.0 | 9.80b |
| 10 | 14 | 4.2 | 55 | 9.0 | 6.54a |
| 11 | 10 | 3.0 | 20 | 3.3 | 0.00 |
| 12 | 8 | 2.4 | 41 | 6.7 | 7.20b |
| 13 | 6 | 1.8 | 17 | 2.8 | 0.49 |
| 14 | 7 | 2.1 | 27 | 4.4 | 2.66 |
| 15 | 3 | 0.9 | 4 | 0.7 | 0.00 |
| 16 | 48 | 14.5 | 64 | 10.5 | 2.92 |
| 17 | 2 | 0.6 | 8 | 1.3 | 0.46 |
| 18 | 6 | 1.8 | 7 | 1.2 | 0.30 |
| 19 | 0 | 0.0 | 3 | 0.5 | 0.45 |
| 20 | 1 | 0.3 | 0 | 0.0 | 0.10 |
| 21 | 0 | 0.0 | 0 | 0.0 | — |
| 22 | 1 | 0.3 | 1 | 0.2 | 0.09 |
| X | 8 | 2.4 | 15 | 2.4 | 0.03 |
| Y | 0 | 0.0 | 7 | 1.2 | 2.43 |
6,009 cells grown in Medium 199 were analyzed from 117 patients; 5,846 cells grown in RPMI with bromodeoxyuridine (BrdU) were analyzed from 121 patients, χ2 is Chi-square.
Total fragile sites = 332.
Total fragile sites = 612.
P < 0.05.
P < 0.01.
P < 0.001, two-tailed test.
In cells grown in Medium 199 compared with those grown in RPMI 1640 with FUdR, chromosome 3 had the highest number of fragile sites or lesions in both culture conditions (81 of 301 sites or 26.9% for Medium 199 and 232 of 1,118 sites or 20.8% for RPMI 1640 with FUdR). The second most common chromosome with fragile sites or lesions in cells grown in Medium 199 was chromosome number 16 (42 of 301 sites or 14.0%) and chromosome number 2 (104 of 1,118 sites or 9.3%) for RPMI 1640 with FUdR. The third most common chromosome with fragile sites or lesions in cells grown in Medium 199 was chromosome 1 (24 of 301 sites or 8.0%) and chromosome 6 (98 of 1,118 sites or 8.8%) for RPMI 1640 with FUdR (Table 2). Similarly, in cells grown in RPMI 1640 with BrdU, the chromosome with the most fragile sites or lesions was number 16 (64 of 612 sites or 10.5%) followed by chromosome 4 (56 of 612 sites or 9.2%). Chromosomes 9 and 10 (55 of 612 sites or 9.0%) were tied for third (Table 3).
The most common chromosome fragile site in cells grown in Medium 199 was 3p14, which accounted for 23.8% of the 332 total sites from 117 mentally retarded males. 16q23 was second, accounting for 9.3% of sites. The most common fragile site in cells grown in RPMI 1640 with BrdU was 16q22, which accounted for 9.8% of the 612 sites from the 121 mentally retarded males. Fragile sites 10q25 and 12q24 each accounted for 5.1% of the fragile sites in RPMI 1640 with BrdU. The most common chromosome fragile site in cells grown in RPMI 1640 with FUdR was 3p14, which accounted for 16.8% of the 1,118 total sites (n = 165). 6q26 was second (6.1% of sites) and was followed by 16q23 (4.7%) (Tables 4 and 5).
Table 4.
Comparison of most common fragile sites between Medium 199 and RPMI 1640 with fluorodeoxyuridine in mentally retarded males.
| Chromosome Number | Medium 1991
|
RPMI 1640 with FUdR2
|
||
|---|---|---|---|---|
| Band Most Commonly Involved (No. Seen) | Sites Represented by Band (%) | Band Most Commonly Involved (No. Seen) | Sites Represented by Band (%) | |
| 1 | p22 (8) | 2.7 | p22 (21) | 1.9 |
| 2 | q33 (3)a | 1.0 | q31 (24)a | 2.1 |
| 3 | p14 (71) | 23.6 | p14 (188) | 16.8 |
| 4 | q31 (4) | 1.3 | q31 (12) | 1.1 |
| 5 | q31 (10) | 3.3 | q31 (36) | 3.2 |
| 6 | q26 (7) | 2.3 | q26 (68) | 6.1 |
| 7 | q32 (9) | 3.0 | q32 (42) | 3.8 |
| 8 | q22 (3) | 1.0 | q22 (16) | 1.4 |
| 9 | q13 (3)a | 1.0 | q32 (11)a | 1.0 |
| 10 | q23 (4)a | 1.3 | q22 (7)a | 0.6 |
| 11 | q13 (4)a | 1.3 | q23 (7)a | 0.6 |
| 12 | q24 (4)a | 1.3 | q13 (6)a | 0.5 |
| 13 | q21 (3)a | 1.0 | q34 (5)a | 0.4 |
| 14 | q24 (4) | 1.3 | q24 (22) | 2.0 |
| 15 | q21 (1) | 0.3 | q21 (6) | 0.5 |
| q22 (1)a,b | ||||
| q24 (1)b | ||||
| 16 | q23 (25) | 8.3 | q23 (53) | 4.7 |
| 17 | p12 (1)a | 0.3 | q23 (4) | 0.4 |
| q23 (1)b | ||||
| 18 | q21 (2)a | 0.7 | q22 (8)a | 0.7 |
| 19 | — | — | q13 (5) | 0.4 |
| 20 | p11(1) | 0.3 | p11 (5) | 0.4 |
| 21 | — | — | q21 (2) | 0.2 |
| q22 (2)b | ||||
| 22 | q12(1) | 0.3 | q12 (3) | 0.3 |
| X | p22 (2) | 0.7 | p22 (14) | 1.2 |
| q22 (2)b | ||||
| Y | — | — | q12 (1) | 0.1 |
Total cells = 5,096.
Total cells = 4,738, FUdR is fluorodeoxyuridine.
Most common fragile site but differs for each chromosome number between the two culture conditions.
Tied for the most common fragile site for each chromosome.
Table 5.
Comparison of most common fragile sites between medium 199 and RPMI 1640 with bromodeoxyuridine in mentally retarded males.
| Chromosome Number | Medium 1991
|
RPMI 1640 with BrdU2
|
||
|---|---|---|---|---|
| Band Most Commonly Involved (No. Seen) | Sites Represented by Band (%) | Band Most Commonly Involved (No. Seen) | Sites Represented by Band (%) | |
| 1 | p22 (8)a | 2.4 | q25 (8)a | 1.3 |
| 2 | q13 (4) | 1.2 | q13 (8) | 1.3 |
| q31 (8)b | ||||
| 3 | p14 (79) | 23.8 | p14 (14) | 2.3 |
| q27 (14)b | ||||
| 4 | q31 (5)a | 1.5 | p16 (11)a | 1.8 |
| 5 | q31 (10)a | 3.0 | q15 (12)a | 2.4 |
| 6 | q26 (8) | 2.4 | q26 (6) | 1.0 |
| 7 | q32 (10)a | 3.0 | q22 (9)a | 1.5 |
| 8 | q22 (4) | 1.2 | q22 (4) | 0.6 |
| q24 (4)b | ||||
| 9 | q13 (3) | 0.9 | q13 (23) | 3.8 |
| 10 | q23 (5)a | 1.5 | q25 (31)a | 5.1 |
| 11 | q13 (4)a | 1.2 | p13 (8)a | 1.3 |
| 12 | q24 (4) | 1.2 | q24 (31) | 5.1 |
| 13 | q21 (3) | 0.9 | q21 (5) | 0.8 |
| 14 | q24 (5) | 1.5 | q24 (19) | 3.1 |
| 15 | q21 (1) | 0.3 | q26 (2)a | 0.3 |
| q22 (1) | ||||
| q24 (1)a,b | ||||
| 16 | q23 (31)a | 9.3 | q22 (60)a | 9.8 |
| 17 | p12 (1) | 0.3 | q22 (2)a | 0.3 |
| q23 (1)a,b | ||||
| 18 | q21 (3) | 0.9 | q21 (3) | 0.5 |
| 19 | — | — | p13 (2) | 0.3 |
| 20 | p11 (1) | 0.3 | — | — |
| 21 | — | — | — | — |
| 22 | q12 (1) | 0.3 | q12 (1) | 0.2 |
| X | p22 (2) | 0.6 | q22 (9) | 1.5 |
| q22 (2) | ||||
| q24 (2)b | ||||
| Y | — | — | q12 (7) | 1.1 |
Total cells = 6,009.
Total cells = 5,846, BrdU is bromodeoxyuridine.
Most common fragile site but differs for each chromosome number between the two culture conditions.
Tied for the most common fragile site for each chromosome.
In addition, significant differences (P < 0.05) were observed in the frequency of fragile sites in Medium 199 compared with a similar number of cells grown in RPMI 1640 with FUdR for chromosomes 3, 6, and 16. An increased percentage of sites were observed for chromosome 3 (band 3p14) for Medium 199 while increases were observed in RPMI 1640 with FUdR for chromosomes 6 (band 6q26) and 16 (band 16q23) (Tables 2 and 4). Significant differences (P < 0.05) in the frequency of fragile sites in Medium 199 compared with a comparable number of cells grown in RPMI 1640 with BrdU were observed for chromosomes 1, 3, 4, 9, 10, and 12 (Tables 3 and 5). An increased percentage of sites was observed for chromosome 1 (band 1p22) and chromosome 3 (band 3p14) for Medium 199 while increases were observed in RPMI 1640 with BrdU for chromosomes 4 (band 4p16), chromosome 9 (band 9q13), chromosome 10 (band 10q25) and chromosome 12 (band 12q24) (Tables 4 and 5).
The distribution of fragile sites or lesions on chromosomes is nonrandom and are preferentially located in light G bands (Hecht, 1988a; Sutherland and Sommers, 1988; Hecht, 1988b). To confirm these observations in mentally retarded males, we examined the location of the fragile sites in cells grown in Medium 199, RPMI 1640 with FUdR, and RPMI 1640 with BrdU. The number of fragile sites or lesions observed on each chromosome and the relative length of each chromosome (Paris Conference, 1971) for each culture condition were analyzed statistically with the Chi-square test (Table 6).
Table 6.
Summary data comparing relative length and number of fragile sites for each culture condition.
| Chromosome Number | Relative Length1 | Fragile Sites from Medium 199 χ2 | Fragile Sites from RPMI 1640 with FUdR χ2 | Fragile Sites from RPMI 1640 with BrdU χ2 |
|---|---|---|---|---|
| 1 | 8.0 | 24 (0.01) | 87 (0.01) | 25 (8.92)b |
| 2 | 7.6 | 18 (0.68) | 104 (1.75) | 40 (0.49) |
| 3 | 6.4 | [81] (96.22)c | [232] (89.24)c | 41 (0.02) |
| 4 | 6.0 | 9 (3.60) | 47 (3.19) | [56] (5.18)a |
| 5 | 5.8 | 15 (0.16) | 70 (0.12) | 48 (0.19) |
| 6 | 5.6 | 12 (0.91) | [98] (7.38)b | 29 (0.40) |
| 7 | 5.1 | 18 (0.20) | [85] (5.09)a | 32 (0.00) |
| 8 | 47 | 8 (1.90) | 31 (5.00)a | 17 (3.19) |
| 9 | 4.6 | 11 (0.29) | 35 (2.71) | [55] (11.70)c |
| 10 | 4.4 | 13 (0.01) | 30 (412)a | [55] (13.07)c |
| 11 | 4.5 | 10 (0.53) | 31 (4.07)a | 20 (1.19) |
| 12 | 4.4 | 8 (1.40) | 28 (5.21)a | 41 (3.57) |
| 13 | 3.1 | 6 (0.66) | 18 (4.55)a | 17 (0.05) |
| 14 | 2.9 | 6 (0.42) | 31 (0.00) | 27 (2.16) |
| 15 | 2.7 | 3 (2.27) | 16 (3.66) | 4 (7.38)b |
| 16 | 3.2 | [42] (47.89)c | [86] (19.40)c | [64] (34.42)c |
| 17 | 3.2 | 2 (4.89)a | 14 (8.53)b | 8 (4.87)a |
| 18 | 2.9 | 5 (0.95) | 22 (1.58) | 7 (4.59)a |
| 19 | 2.6 | 0 (6.71)b | 9 (9.39)b | 3 (8.41)b |
| 20 | 2.4 | 1 (4.21)a | 7 (10.32)b | 0 (13.32)c |
| 21 | 1.5 | 0 (3.35) | 5 (5.18)a | 0 (7.71)b |
| 22 | 1.5 | 1 (1.72) | 5 (5.18)a | 1 (5.61)a |
| X | 4.7 | 8 (1.91) | 26 (8.24)b | 15 (4.60)a |
| Y | 2.0 | 0 (4.86)a | 1 (17.73)c | 7 (1.21) |
Relative length measurements (% of total haploid length), χ2 is Chi-square. The [ ] indicates the number of fragile sites or lesions that are significantly over represented compared to relative chromosome length. The other significant differences depict under representations.
P < 0.05.
P < 0.01.
P < 0.001, two-tailed test.
A significant over representation of fragile sites in relation to the length of the chromosome was seen in Medium 199 for chromosomes 3 and 16 but significantly under represented for chromosomes 17, 19, 20, and Y. A significant over representation of fragile sites was seen in RPMI 1640 with FUdR for chromosomes 3, 6, 7, and 16 but significantly under represented for chromosomes 8, 10, 11, 12, 13, 17, 19, 20, 21, 22, X, and Y. A significant over representation of fragile sites was seen in RPMI 1640 with BrdU for chromosomes 4, 9, 10, and 16 but significantly under represented for chromosomes 1, 15, 17, 18, 19, 20, 21, 22, and X.
One hundred three (34%) of the chromosome bands (excluding acrocentric short arms) based on mid metaphase chromosome idiograms (Paris Conference, 1971) contained fragile sites or lesions in cells grown in Medium 199 and 203 bands (66%) contained fragile sites or lesions in a comparable number of cells grown in RPMI 1640 with FUdR. These reported idiograms (Paris Conference, 1971) were chosen because they reflect the approximate average band length of the cytogenetic preparations and include relative lengths calculated for each chromosome for comparison with the cytogenetic data in our study. One hundred forty five (47%) of the 306 bands contained fragile sites in cells grown in RPMI 1640 with BrdU. Thus, the distribution of fragile sites is nonrandom in our mentally retarded males (Fig. 1 and 2).
Fig. 1.
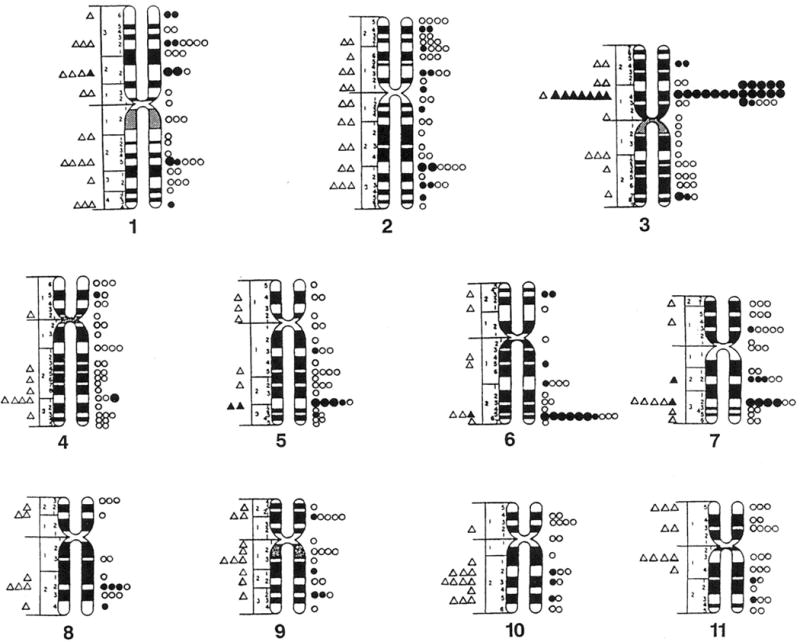
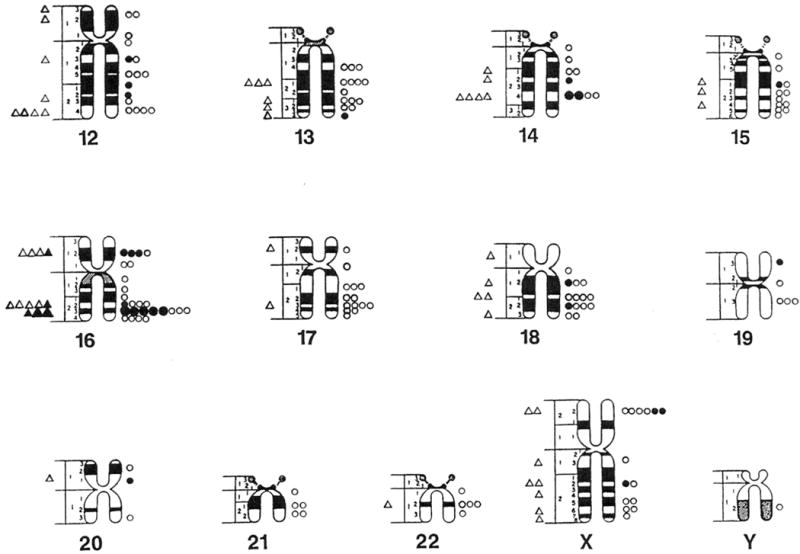
Location and distribution of fragile sites recorded on standard mid-metaphase chromosome ideograms (Paris Conference 1971). Fragile sites from cells grown in Medium 199 are represented by triangles (small open = 1 site, small closed = 5 sites, large closed = 10 sites). Fragile sites from cells grown in RPMI 1640 with fluorodeoxyuridine (FudR) are represented by circles (small open = 1 site, small closed = 5 sites, large closed = 10 sites).
Fig. 2.
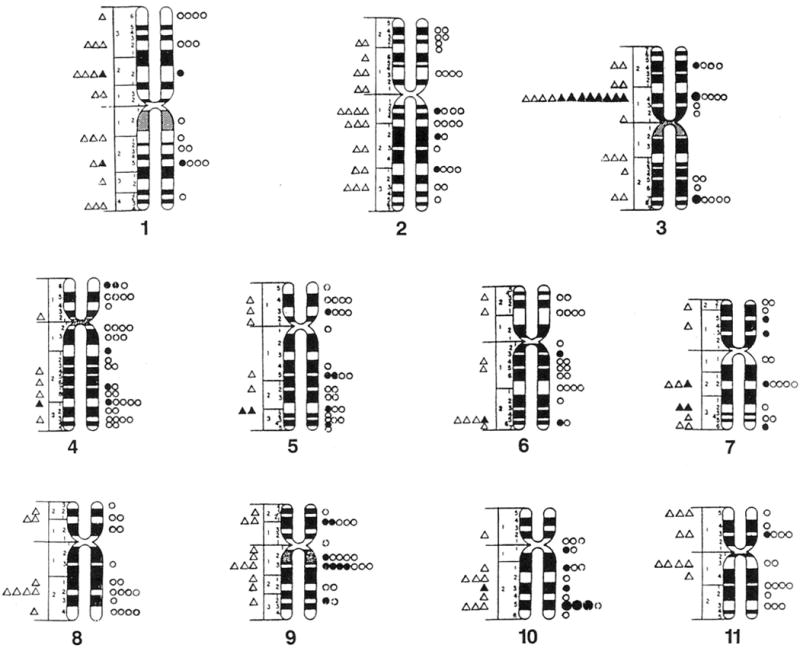
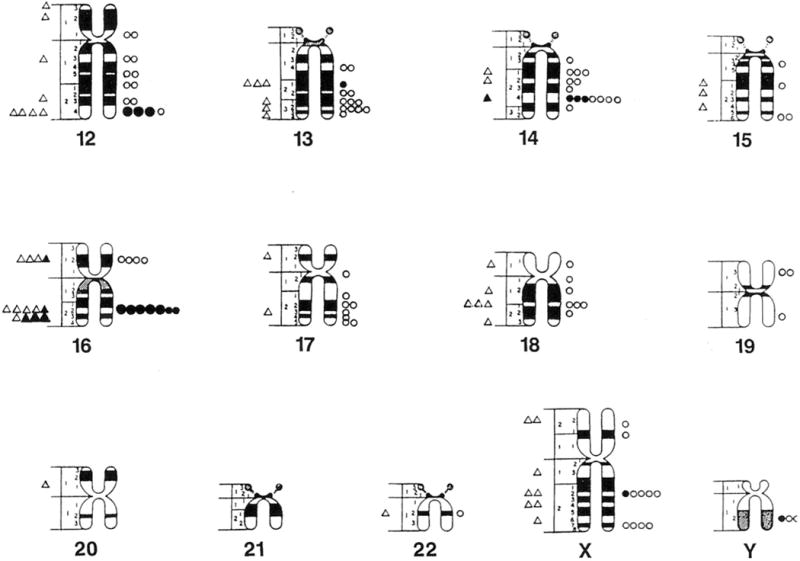
Location and distribution of fragile sites recorded on standard mid-metaphase chromosome ideograms (Paris Conference 1971). Fragile sites from cells grown in Medium 199 are represented by triangles (small open = 1 site, small closed = 5 sites, large closed = 10 sites). Fragile sites from cells grown in RPMI 1640 with bromodeoxyuridine (BrdU) are represented by circles (small open = 1 site, small closed = 5 sites, large closed =10 sites).
Discussion
A detailed analysis of fragile site frequency and distribution in cells grown in three different culture conditions was undertaken in a relatively large number of males with mental retardation of unknown etiology. The paucity of data comparing fragile site frequency and distribution in cells grown in different culture conditions of mentally retarded males in the medical literature was a major reason for undertaking this descriptive study.
In our earlier study (Butler et al., 1988; Butler, 1990), no significant differences in the percentage of cells with chromosome lesions were found in cells grown in Medium 199 from 37 control subjects with normal intelligence (3368 cells; 6.8 ± 5.6%; range = 0–21%) and 44 mentally retarded individuals (2329 cells; 5.4 ± 4.3%; range = 0–20%). In addition, significantly more chromosome lesions were observed in cells grown in RPMI medium with FUdR compared with Medium 199 for both control and mentally retarded individuals (Butler, 1990). The 3p14 and 16q23 sites were the most common autosomal locations for chromosome lesions in cells grown in folate deficient culture conditions from the 37 control individuals (Butler et al., 1988). The present larger study of mentally retarded individuals analyzed the fragile site frequency and distribution in cells grown in three different fragile site culture conditions. The objective was to identify new or under reported fragile sites in these patients that were inducible by varying the culture conditions.
Cells grown in folate-deficient culture conditions (Medium 199) showed the lowest percentage of cells with fragile sites (5.8%) while RPMI 1640 with FUdR had the highest percentage (17.7%). The observation of enhanced fragile site expression using FUdR agrees with earlier data from three groups of subjects (individuals with mental retardation and with or without the fragile X syndrome and individuals with normal intelligence) (Butler, 1990). Statistically, more sites were induced by either FUdR or BrdU than in Medium 199 alone. More overall sites were induced with FUdR compared with Medium 199 but statistically significantly different for only chromosome 6 (band 6q26) while a greater percentage of sites were seen in Medium 199 for chromosomes 3 (band 3p14) and 16 (band 16q23). More sites were induced with BrdU for chromosomes 4, 9, 10 and 12. These findings confirm those reported previously, (Sutherland and Hecht, 1985; Rao et al., 1988; Butler et al., 1990; Butler et al., 1988; Butler, 1990) although the 9q13 site induced by BrdU appears to represent a new or underreported BrdU site.
Most sites found in Medium 199 also were seen in RPMI 1640 with FUdR. However, seven chromosomes did not demonstrate the identical chromosome band as the most common site (Table 4). In addition, most sites observed in Medium 199 also were observed in RPMI 1640 with BrdU, but nine chromosomes did not demonstrate the identical chromosome band as the most common site (Table 5). Thus, a similarity was seen between the induction of fragile sites between Medium 199 and RPMI 1640 with FUdR or BrdU but differences were seen in fragile site expression as well as the appearance of different sites. Our study confirms that several fragile sites can be induced by more than one culture condition even though the mechanisms for the induction are different (Glover, 1981; Butler et al., 1990) (Table 7). Band 3p14 was the most common site in cells grown in Medium 199 and RPMI 1640 with FUdR but was tied for only sixth place in cells grown in RPMI 1640 with BrdU. In addition, this fragile site appears to be the most common site previously reported, regardless of the culture conditions in the general population or in nonspecific mentally retarded individuals (Sutherland and Hecht, 1985; Kahkonen, 1988; Butler et al., 1988; Rao et al., 1988; Mavrou et al., 1991). A similar overlap in the induction of fragile sites has been observed previously by Glover (1985) and Rao et al. (1988), and indicates inherent fragility at these sites regardless of the inducing agent for expression. Eight fragile sites accounting for at least 2% of the total number of sites were observed in cells grown in Medium 199 from our mentally retarded males. These eight sites accounted for 48% of all fragile sites. Seven sites accounting for at least 2% of the total sites were observed in cells grown in RPMI 1640 with FUdR. These seven sites accounted for 39% of all fragile sites. Eight fragile sites accounting for at least 2% of the total sites were observed in cells grown in RPMI 1640 with BrdU. These eight sites accounted for 34% of all fragile sites. However, if a 4% frequency is used as a cut-off for delineating fragile sites from random breakage as others have suggested (Jacobs et al., 1980; Howard-Peebles, 1981; Rao et al., 1988), then only two chromosome lesions (bands 3p14 and 16q23) would be interpreted as fragile sites in cells grown in Medium 199, four sites (3p14, 6q26, 16q23, 7q32) in RPMI 1640 with FUdR, and four sites (16q22, 12q24, 10q25, 9q13) in RPMI 1640 with BrdU. Thus, there is a nonrandom distribution of sites in all three culture conditions in our study and also reported previously by the author and other investigators using controls and individuals with mental retardation (Sutherland and Hecht, 1985; Petit et al., 1986; Kahkonen 1988; Butler et al., 1988; Rao et al., 1988).
Table 7.
Summary of fragile sites observed in the three culture conditions using two percent or more as a criteria for inclusion.
| Culture Condition | Fragile Site | Sites Observed | % of Total Sites | # and (%) of Patients with Sites | Average % per Patient | % Range per Patient |
|---|---|---|---|---|---|---|
| Medium 199 | 1p22 | 8 | 2.7 | 6 (6.0) | 2.3 | 2.0–4.0 |
| (n = 100) | 3p14* | 71 | 23.6 | 46 (46.0) | 2.9 | 1.0–10.0 |
| 5q31 | 10 | 3.3 | 9 (9.0) | 2.2 | 2.0–4.0 | |
| 6q26 | 7 | 2.3 | 7 (7.0) | 2.0 | 1.7–2.3 | |
| 7q32 | 9 | 3.0 | 9 (9.0) | 2.0 | 1.6–2.0 | |
| 16p12** | 8 | 2.7 | 5 (5.0) | 2.7 | 1.8–6.0 | |
| 16q22 | 9 | 3.0 | 9 (9.0) | 2.0 | 1.0–3.8 | |
| 16q23 | 25 | 8.3 | 22 (22.0) | 2.6 | 1.0–6.0 | |
| RPMI 1640 + FUdR1 | 2q31 | 24 | 2.2 | 21 (12.7) | 3.9 | 1.3–10.5 |
| (n = 165) | 3p14* | 188 | 16.8 | 94 (57.0) | 7.1 | 3.4–24.0 |
| 5q31 | 36 | 3.2 | 24 (14.5) | 4.4 | 1.3–12.0 | |
| 6q26 | 68 | 6.1 | 51 (31.0) | 7.9 | 0.8–16.0 | |
| 7q32 | 42 | 3.8 | 36 (21.8) | 3.5 | 0.8–6.7 | |
| 14q24 | 22 | 2.0 | 21 (12.7) | 3.2 | 1.6–4.3 | |
| 16q23 | 53 | 4.7 | 35 (21.2) | 5.0 | 1.9–20.0 | |
| RPMI 1640 + BrdU2 | 3p14* | 14 | 2.3 | 12 (10.0) | 2.7 | 1.0–7.0 |
| (n = 121) | 3q27 | 14 | 2.3 | 14 (11.6) | 2.0 | 2.0–4.0 |
| 9p21*** | 13 | 2.1 | 9 (7.4) | 2.9 | 2.0–8.0 | |
| 9q13 | 23 | 3.8 | 12 (10.0) | 3.4 | 1.9–8.0 | |
| 10q25*** | 31 | 5.1 | 14 (11.6) | 3.6 | 2.0–14.0 | |
| 12q24*** | 31 | 5.1 | 13 (10.7) | 4.7 | 2.0–16.0 | |
| 14q24 | 19 | 3.1 | 15 (12.4) | 2.1 | 2.0–2.6 | |
| 16q22 | 60 | 9.8 | 13 (10.7) | 11.2 | 2.0–48.0 |
FUdR is fluorodeoxyuridine.
BrdU is bromodeoxyuridine.
Common to all culture conditions.
Recognized folate sensitive site (Hecht et al., 1990).
Recognized BrdU fragile site (Hecht et al., 1990).
In our study of folate-sensitive fragile sites in cells grown in Medium 199 from males with mental retardation, we found a larger percentage of these retarded patients with fragile sites than reported in the general population. A number of surveys of fragile sites under low folate conditions have been reported (Quack et al., 1978; Guichaoua et al., 1982; Sutherland, 1982; Sutherland and Hecht, 1985; Petit et al., 1986; Kahkonen et al., 1986; Kahkonen, 1988; Hecht et al., 1988; Mavrou et al., 1991), although there is a paucity of studies comparing the location and frequency of fragile sites induced by different culture conditions in individuals with mental retardation as reported in the present study. Interestingly, newborn surveys tend to give a relatively low yield of folate sensitive sites (Sutherland, 1982; Sutherland and Hecht, 1985) possibly due to the frequent use of maternal vitamin supplements before delivery. If the supplement contains folic acid, then its transfer to the fetus transplacentally may suppress cytologic expression of folate-sensitive sites. None-the-less, folate-sensitive autosomal fragile sites are relatively rare. For example, Petit et al., (1986) summarized the literature and found 85 ascertainments of autosomal folate-sensitive sites in 33,237 persons (4 of 3,090, or 0.13% of newborns; 61 of 28,973, or 0.21% of patients from routine cytogenetic testing and 20 of 1,174, or 1.7% of nonspecific patients with mental retardation). From published surveys the incidence of autosomal folate sensitive fragile sites for newborns was about 1 in 775; about 1 in 475 patients presenting for routine cytogenetic testing and about 1 in 60 nonspecific mentally retarded patients. The range of individual surveys of newborns or patients presenting for routine cytogenetic testing was 0.10% to 0.60% (Sutherland, 1982), while the range for individuals with mental retardation was 0.91% (Sutherland, 1982) to 3.21% (Petit et al., 1986). Thus, Sutherland (1982) reported that the incidence of specific folate-sensitive autosomal fragile sites (2q11, 9q32, 10q23) in patients with mental retardation was seven-fold greater than among randomly selected newborns. In addition, Petit et al. (1986) reported that the incidence of autosomal folate-sensitive fragile sites was more than 20-fold greater than in newborns. They reported that the fragile site at band 3p14 (57.6% of all fragile sites) occurred most frequently in their 405 individuals with nonspecific mental retardation, followed by 6q26 (30.9%), 10q23 (5.8%), 2q11 (2.8%), 16q22 (2.2%), and 9q32 (0.7%). Mavrou et al. (1991) also reported that 3p14 was the most frequent fragile site using folate-deficient culture conditions in their survey of 96 children with mental retardation and accounted for 53% of all fragile site ascertainments followed by 6q26 (23%). In comparison with our study of mentally retarded males with cells grown in folate-deficient culture conditions (Medium 199), 3p14 also was the most common site (23.6% of all fragile sites) as similarly reported in our laboratory for fragile X syndrome males and males of normal intelligence (Butler et al., 1990), followed by 16q23 (7.0%), 5q31 (3.3%), 7q32 (3.0%), 16q22 (3.0%), 1p22 (2.7%), 16p12 (2.7%), and 6q26 (2.3%).
Petit et al. (1986) reported a total concurrence of the 10q23 folate-sensitive site with the 3p14 common site in 8 patients in their study of 405 mentally retarded individuals. Their study indicated total concurrence of the 3p14 and 10q23 fragile sites. However no biological significance could be assigned, and they proposed that this occurrence may reflect observer bias. If a fragile site is seen, the observer may look more intensively for another site. Interestingly, a lack of concurrence was seen in fragile sites 10q23 and 3p14 in the 6 patients in our study whose cells were grown in Medium 199 and found to have a 10q23 site. Only one of the 6 patients with a 10q23 site had a 3p14 site. There also appears to be no consistent concurrence of other fragile sites in the patients analyzed.
In the present study only one site (3p14 in cells grown in RPMI 1640 with FUdR and seen in 57% of the patients) could be classified as common or found in greater than 50% of the population (Hecht, 1986). The next most prevalent site was 6q26, found in cells grown in RPMI 1640 with FUdR (31%), followed by 16q23 in cells grown in Medium 199 (22%) (Table 7). Sites other than 3p14 were polymorphic (1–50% of the population) or rare (<1% of the population) (Hecht, 1986). In addition, 301 fragile sites or lesions were recorded on 103 of a total of 306 chromosome bands in Medium 199, 1,118 sites were recorded on 203 bands in RPMI 1640 with FUdR, and 612 sites were recorded on 145 bands in RPMI 1640 with BrdU. Thus, a clustering of sites is seen in the chromosomes in all three culture conditions, and more sites were induced by RPMI 1640 with FUdR and RPMI 1640 with BrdU than in Medium 199 alone.
In summary, the frequency and distribution of fragile sites have been reviewed as well as significant differences described between fragile site location and expression using three different culture conditions in a population of males with mental retardation of unknown cause. There are clear differences in the frequency and location of fragile sites in mentally retarded males that are dependent on specific culture conditions. Some sites are more susceptible to certain inducing agents and over- and under-representation of sites does occur; although site 3p14 was the most common in all culture conditions. Clustering of sites is seen in the chromomsomes in all three culture conditions. The BrdU site at 9q13 was seen in ten percent of our mentally retarded males and appears to represent a new or underreported BrdU site.
Acknowledgments
I acknowledge the support of the Tennessee Department of Mental Health and Retardation. I thank the patients and staff at Clover Bottom Developmental Center, Nashville for their cooperation. I thank A. Allen, K. King, D. N. Singh and L. Hedges for technical assistance and N. Morris at the Kennedy Center, Vanderbilt University, for artistic assistance.
Literature Cited
- Branda RF, Arthur DC, Woods WG, Danzl TJ, KING RA. Folate metabolism and chromosomal stability in the fragile X syndrome. Am J Med. 1984;77:602–611. doi: 10.1016/0002-9343(84)90349-8. [DOI] [PubMed] [Google Scholar]
- Butler MG. No significant relationship between age and frequency of chromosome lesions in mentally retarded individuals with or without the fragile X syndrome. Hum Genet. 1990;84:216–217. doi: 10.1007/BF00208948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Allen GA, Haynes JL, Clark SJ. Chromosome lesions which could be interpreted as “fragile sites” on the distal end of Xq. Am J Med Genet. 1990;37:250–253. doi: 10.1002/ajmg.1320370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Allen GA, Haynes JT, Singh DN, Watson MS, Breg WR. Anthropometric comparison of mentally retarded males with and without the fragile X syndrome. Am J Med Genet. 1991a;38:260–268. doi: 10.1002/ajmg.1320380220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Mangrum T, Gupta R, Singh D. A 15 item checklist for screening mentally retarded males for the fragile X syndrome. Clin Genet. 1991b;39:347–354. doi: 10.1111/j.1399-0004.1991.tb03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Pratesi R, Vnencak-Jones C. Molecular genetic screening in cytogenetically normal mentally retarded males with manifestations of fragile X syndrome. Am J Med Genet. 1994;51:315–316. doi: 10.1002/ajmg.1320510406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Joseph GM, Rames LJ, Cacheiro N, Lozzio CB. Chromosomal breakage in control and fragile X subjects using folate-deficient culture conditions. Human Genet. 1988;78:383. doi: 10.1007/BF00291743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Singh DN. Clinical and cytogenetic survey of institutionalized mentally retarded patients with emphasis on the fragile X syndrome. J Intellect Disabil Res. 1993;37:131–142. doi: 10.1111/j.1365-2788.1993.tb00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Pratesi R, Watson MS, Breg WR, Singh DN. Anthropometric and craniofacial patterns in mentally retarded males with emphasis on the fragile X syndrome. Clin Genet. 1993;44:129–138. doi: 10.1111/j.1399-0004.1993.tb03863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover TW. FUdR induction of the X chromosome fragile site: evidence for the mechanism of folic acid and thymidine inhibition. Am J Hum Genet. 1981;33:234–242. [PMC free article] [PubMed] [Google Scholar]
- Guichaoua M, Mattei MG, Mattei JF, Giraud F. Aspects genetiques des sites fragiles autosomiques. A propos de 40 cas. J Genet Hum. 1982;30:183–197. [PubMed] [Google Scholar]
- Hagerman RJ, Amir K, Cronister A. The fragile X checklist. Am J Med Genet. 1991;38:283–287. doi: 10.1002/ajmg.1320380223. [DOI] [PubMed] [Google Scholar]
- Hecht F. Rare polymorphic and common fragile sites: a classification. Hum Genet. 1986;74:207–208. doi: 10.1007/BF00282099. [DOI] [PubMed] [Google Scholar]
- Hecht F. Fragile sites, cancer chromosome breakpoints, and oncogenes all cluster in light G bands. Cancer Genet Cytogenet. 1988a;31:17–24. doi: 10.1016/0165-4608(88)90005-2. [DOI] [PubMed] [Google Scholar]
- Hecht F. Fragile sites update. Cancer Genet Cytogenet. 1988b;31:125–128. doi: 10.1016/0165-4608(88)90021-0. [DOI] [PubMed] [Google Scholar]
- Hecht F, Tajara EH, Lockwood DH, Sandberg AA, Hecht BK. New common fragile sites. Cancer Genet Cytogenet. 1988;33:1–9. doi: 10.1016/0165-4608(88)90042-8. [DOI] [PubMed] [Google Scholar]
- Hecht F, Ramesh KH, Lockwood DH. A guide to fragile sites on human chromosomes. Cancer Genet Cytogenet. 1990;44:37–45. doi: 10.1016/0165-4608(90)90195-g. [DOI] [PubMed] [Google Scholar]
- Howard-Peebles PN. Chromosome banding in X-linked mental retardation. Lancet. 1981;I:4940. [Google Scholar]
- Jacobs PA, Glover TW, Mayer M, Fox P, Gerard JW, Dunn HG, Herbst DS. X-linked mental retardation. A study of seven families. Am J Med Genet. 1980;7:471–489. doi: 10.1002/ajmg.1320070408. [DOI] [PubMed] [Google Scholar]
- Kahkonen M. Population cytogenetics of folate-sensitive fragile sites. I. Common sites. Hum Genet. 1988;80:344–348. doi: 10.1007/BF00273649. [DOI] [PubMed] [Google Scholar]
- Kahkonen M, Leisti J, Thoden CJ, Autio S. Frequency of rare fragile sites among mentally subnormal schoolchildren. Clin Genet. 1986;30:234–238. doi: 10.1111/j.1399-0004.1986.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Mavrou A, Syrrou M, Tsenghi C, Metaxotou C. Autosomal folate sensitive fragile sites in normal and mentally retarded individuals in Greece. Am J Med Genet. 1991;38:437–439. doi: 10.1002/ajmg.1320380259. [DOI] [PubMed] [Google Scholar]
- Paris Conference (1971) Standardization in human cytogenetics. Birth Defects Orig Arti Ser. 1972;8:1–46. [PubMed] [Google Scholar]
- Petit P, Fryns JP, Van den Berghe H, Hecht F. Population cytogenetics of autosomal fragile sites. Clin Genet. 1985;29:96–100. doi: 10.1111/j.1399-0004.1986.tb01229.x. [DOI] [PubMed] [Google Scholar]
- Quack B, Nantois Y, Mottet J, Noel B. Lacune stereotypee constitutionelle des chromosomes humains. J Genet Hum. 1978;26:55–67. [PubMed] [Google Scholar]
- Rao PN, Heerema NA, Palmer CG. Fragile sites induced by FudR, caffeine, and aphidicolin. Hum Genet. 1988;78:21–16. doi: 10.1007/BF00291228. [DOI] [PubMed] [Google Scholar]
- Sanfilippo S, Neri G, Tedeschi B, Carlo-Stella N, Triolo O, Serra A. Chromosomal fragile sites: preliminary data of a population survey. Clin Genet. 1983;24:295. [Google Scholar]
- Steinbach P, Barbi G, Boller T. On the frequency of telomeric chromosomal changes induced by culture conditions suitable for fragile X expression. Hum Genet. 1982;61:160–162. doi: 10.1007/BF00274209. [DOI] [PubMed] [Google Scholar]
- Sutherland GR. Heritable fragile sites on human chromosomes. VIII. Preliminary population cytogenetic data on the folic-acid-sensitive fragile sites. Am J Hum Genet. 1982;34:452–458. [PMC free article] [PubMed] [Google Scholar]
- Sutherland GR, Hecht F. Fragile sites on human chromosomes. Oxford University Press; New York, New York: 1985. [Google Scholar]
- Sutherland GR, Sommers RN. No statistical association between common fragile sites and nonrandom chromosome breakpoints in cancer cells. Can Genet Cytogenet. 1988;31:9–15. doi: 10.1016/0165-4608(88)90004-0. [DOI] [PubMed] [Google Scholar]
- Vekemens M, Popovich B, Rosenblatt D, Monroe P. Chromosomal breakage in normal and fragile X subjects using low folate culture conditions. J Med Genet. 1993;20:404–407. doi: 10.1136/jmg.20.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Reidy J, Chen ATL. Chromosome fragility in folic acid-deficient medium. Am J Hum Genet. 1982;34:153A. [Google Scholar]


