Abstract
Background
Leukotriene receptor antagonists have been used to prevent virus-induced asthma exacerbations in autumn. Its efficacy, however, might differ with age and sex.
Objective
This study aimed to investigate whether pranlukast added to usual asthma therapy in Japanese children during autumn, season associated with the peak of asthma, reduces asthma exacerbations. It was also evaluated the effect of age and sex on pranlukast's efficacy.
Methods
A total of 121 asthmatic children aged 1 to 14 years were randomly assigned to receive regular pranlukast or not according to sex, and were divided in 2 age groups, 1–5 years and 6–14 years. The primary outcome was total asthma score calculated during 8 weeks by using a sticker calendar related to the days in which a child experienced a worsening of asthma symptoms. This open study lasted 60 days from September 15 to November 14, 2007.
Results
Significant differences in pranlukast efficacy were observed between sex and age groups. Boys aged 1 to 5 years had the lower total asthma score at 8 weeks (p = 0.002), and experienced fewer cold episodes (p = 0.007). There were no significant differences between pranlukast and control group in total asthma score at 8 weeks (p = 0.35), and in the days in which a child experienced a worsening of asthma symptoms (p = 0.67).
Conclusion
There was a substantial benefit of adding pranlukast to usual therapy in asthmatic children, especially in boys aged 1 to 5 years, during autumn season.
Keywords: Age, Asthma, Child, Exacerbations, Sex, Pranlukast
INTRODUCTION
Prevalence of asthma in developed countries is high, asthma exacerbations are associated with an increased rate of hospitalizations and account for a substantial burden of social morbidity and economic costs [1]. Asthma exacerbations have been reduced by regular treatment with inhaled corticosteroids (ICS) or in combination with ICS/long-acting β2-agonists (LABAs). However, the regular treatment with ICS has shown to be ineffective in the treatment of asthma episodes induced by viral infection [2]. Viral infections have been identified as the most frequent triggers of exacerbations in children and adults [3,4]. These viral-induced asthma exacerbations in children, especially during September, have been reported in several countries [5]. Therefore, prevention of viral-induced asthma exacerbations need a different assessment and treatment. Leukotriene receptor antagonists (LTRAs) have been demonstrated to prevent respiratory viral-induced asthma exacerbations in children [6,7,8]. Differences in this efficacy, however, were shown to be related to age and sex in Johnston et al. [7]'s study in a Canadian population, which demonstrated a greater benefit from montelukast administration especially on boys aged 2 to 5 years than in older boys, whereas there was nonsignificant effect in younger girls. Although these differences in efficacy may be dependent on asthma phenotypes [9], it is not known whether LTRAs efficacy is influenced or not according to age, sex, or asthma phenotypes in different geographical area and races.
Thus, the present study investigated whether pranlukast, a LTRA added to usual therapy of asthmatic children reduces exacerbations during autumn season in a Japanese population, and focused on the differences in pranlukast's efficacy according to age and sex.
MATERIALS AND METHODS
Study design
This was a randomized, open study conducted at multiple clinical sites in Chiba, Japan. This investigation was approved by the Research Ethics Board of Chiba University, Chiba (approval number: 631). Written informed consent was obtained from parents of all the subjects and child assent when appropriate.
Setting and participants
Study participants were recruited between July and August 2007 through advertising and from clinical practices in Chiba, Japan. Asthma was diagnosed by primary care doctors based on the Japanese pediatric guidelines for the treatment and management of bronchial asthma 2005 [10]. Entry criteria included children 1 to 14 years old, physician-diagnosed asthma needing a rescue inhaler in the last year, with a history of asthma exacerbations associated with apparent respiratory viral infections. Patients who had been treated with LTRA were included after 14-day washout period. Exclusion criteria were children who had significant cardiorespiratory comorbidity, were using regular oral corticosteroid, or had an asthma exacerbation in the month before treatment with pranlukast started. Clinical history, demographic, and current-therapy information was obtained at recruitment by using a questionnaire.
Randomization
After enrollment in August, participants were randomly assigned to a pranlukast group and control group according to sex, within age groups 1–5 and 6–14 years. In the pranlukast group, participants were instructed to take pranlukast (7 mg/kg), twice daily, in addition to their usual asthma therapy. In the control group, participants did not take additional medicine including placebo. Patients who had respiratory symptoms and/or problems with diary recording during observation were excluded from the study.
Measurement of asthma status and cold episodes
We prepared a calendar (Supplemental material) for the period September 15 to November 14, 2007, with a supply of blue, green, yellow, orange, red and purple stickers, 1 of each color printed with the date of each study day, similar as Johnston et al. [7]'s report.
We evaluated asthma status by 2 indices, “total asthma score” and “number of days with worse asthma symptoms” by using the stickers. Total asthma score in this study was evaluated as follows, a blue sticker (score, 0) was applied on days when a child had no asthma symptoms; a green sticker (score, 1) indicated mild asthma symptoms; a yellow sticker (score, 2) indicated symptoms that were worse than usual or needed extra asthma medication, and an orange sticker (score, 3) was applied if a child`s breathing symptoms required an unscheduled visit to a physician or treatment with oral corticosteroids. Days with worse asthma symptoms were defined as those with either an orange or yellow sticker.
We also evaluated cold episodes, days with cold symptoms and those with fever. A purple sticker was applied on days that a child had symptoms of a cold, and a red sticker was applied on days that a child had a fever > 38℃. A “cold” was defined by the presence of >2 consecutive purple stickers indicating days with cold symptoms. At least 5 days with no cold symptoms were required before a subsequent new cold was identified.
Frequency of use of asthma medications, such as ICS, short-acting and long-acting β2-agonists was obtained by questionnaire. Calendars were returned by participants and reported stickers were verified. The stickers were also useful to verify the adherence to treatment. Questionnaire data were double-entered by different operators.
Outcomes
The primary outcome was total asthma score during 8 weeks, calculated by daily stickers. The secondary outcomes were days with worse asthma symptoms, number of colds, and days with fever. We also evaluated these outcomes according to sex, and age groups 1–5 and 6–14 years.
Statistical analysis
The combined numbers of yellow and orange calendar stickers for each child, irrespective of whether cold symptoms (purple sticker) were present, was expressed as a percentage of the total blue, green, yellow, orange stickers applied for each child. Mann-Whitney test was used to compare overall rankings between treatment with or without pranlukast and was stratified according to age group and sex.
Comparisons of the baseline characteristics of the study groups, the secondary outcome of unscheduled physician visits, and cold frequencies were conducted by using chi-square and Mann-Whitney U-test. Multiple regression analysis was used to examine the relationship between age and sex, pranlukast and ICS in total asthma score, and the number of cold episodes. All statistical analyses were conducted using SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA). The threshold for significance was p < 0.05.
RESULTS
Study subjects
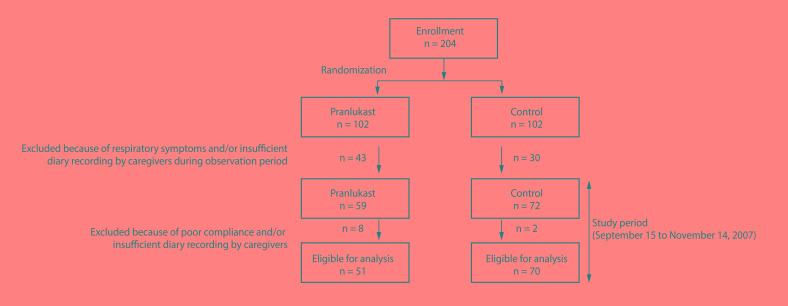
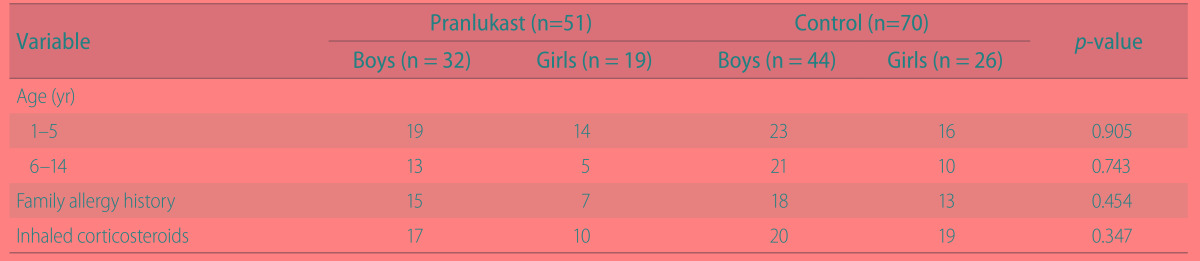
In August 2007, two hundred four children with asthma were enrolled and randomly assigned to pranlukast or control group (each 102 subjects). Forty-three children assigned to pranlukast group and 30 children assigned to control group were excluded because of respiratory symptoms and/or insufficient diary recording during observation period, and finally 121 children (59 in pranlukast group and 72 in control) started the study from September 15. Eight subjects in pranlulast and 2 subjects in control were excluded because of poor compliance and/or insufficient diary recording by caregivers, 51 subjects in pranlulast group and 70 subjects in control group were eligible for the final analyses (Fig. 1). There were no clinically important baseline differences between groups according to medical history or prescribed asthma treatments (Table 1). Fifty-four percent (54%) of children were prescribed ICS. There were no children prescribed with a combination product (ICS with LABA).
Fig. 1. Patients flow diagram.
Table 1. Subject demographics.
Compliance
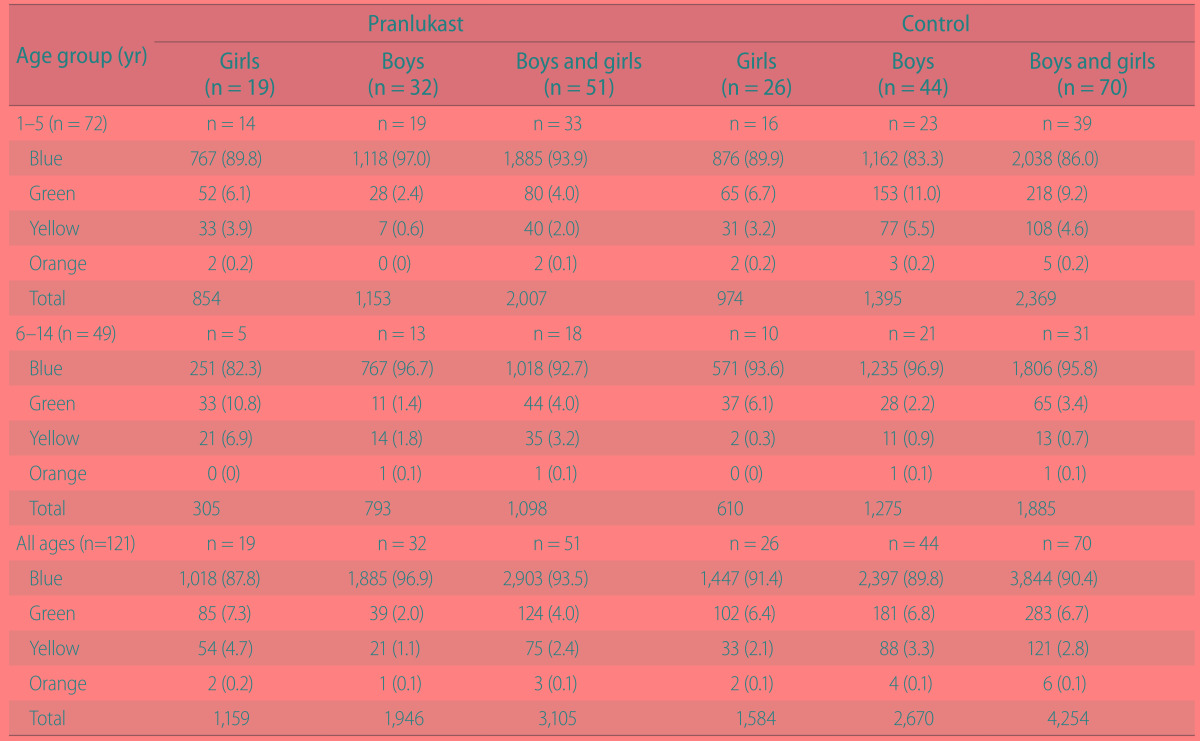
Total number of daily asthma symptom-calendar stickers among pranlukast and control groups was shown in Table 2. A total of 7,359 (99.7%) of a possible 7,381 daily stickers were applied during the study. 5 days were left blank by children on pranlukast and 16 by children on the control group. Compliance with the therapy was 99.8% for pranlukast and 99.6% for control group.
Table 2. Total number of daily asthma symptom-calendar stickers among children given pranlukast compared to controls according to age group and sex.
Blue indicates no asthma symptoms; green, usual asthma symptoms; yellow, asthma symptoms worse than usual or needed extra asthma medication; orange, child's breathing symptoms required an unscheduled physician visit or administration of oral corticosteroid.
Adverse events
There were no children who discontinued study medication because of adverse events.
Univariate analysis
Outcomes
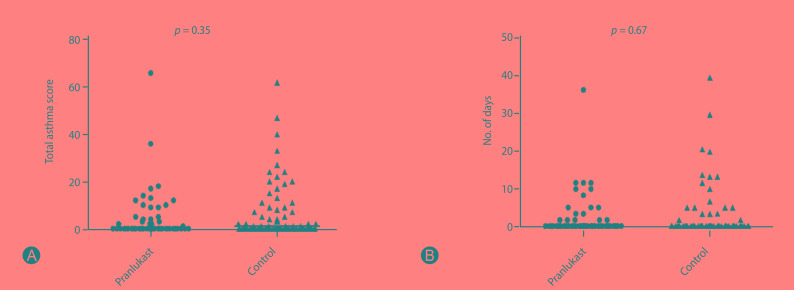
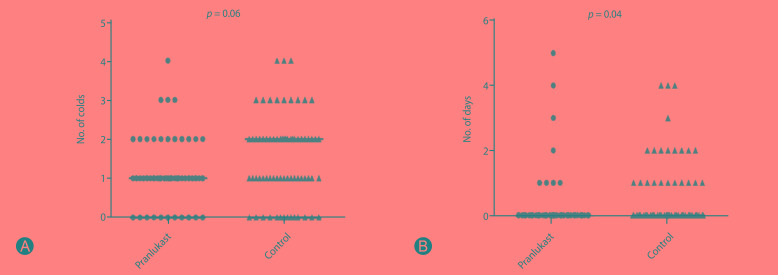
There were no differences in total asthma score on 8 weeks of treatment between pranlukast and control groups (p = 0.35), and in the number of days with worse asthma symptoms (p = 0.67) (Fig. 2). Fig. 3 shows a tendency of higher number of colds in the control group compared to the pranlukast group (p = 0.06), and children taking pranlukast experienced fewer days with fever compared with control group (p = 0.04).
Fig. 2. Differences between pranlukast and control groups in total asthma score (A) and number of days with worse asthma symptoms (B). No significant differences were observed between pranlukast and control groups.
Fig. 3. Differences between pranlukast and control groups in number of colds (A) and number of days with fever (B). The pranlukast group presented a decreasing trend of number of colds and lower number of days with fever than controls.
Outcomes according to age and sex
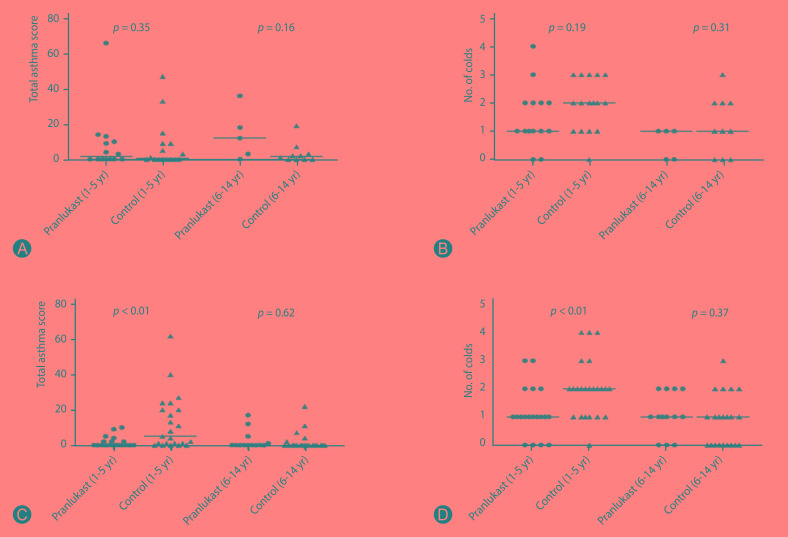
There were significant differences in the effect of pranlukast on asthma morbidity between sex and age groups. Among 1- to 5-year-old boys, in comparison to controls, pranlukast group had lower total asthma score on 8 weeks (p < 0.01), and experienced fewer number of colds (p < 0.01) (Fig. 4).
Fig. 4. (A, B) Girls: differences between pranlukast and control groups in total asthma score (A) and number of colds (B). (C, D) Boys: differences between pranlukast and control groups in total asthma score (C) and number of colds (D). One- to 5-year-old boys in the pranlukast group had a significant lower total asthma score and also less number of cold episodes than control group.
Multivariable analysis
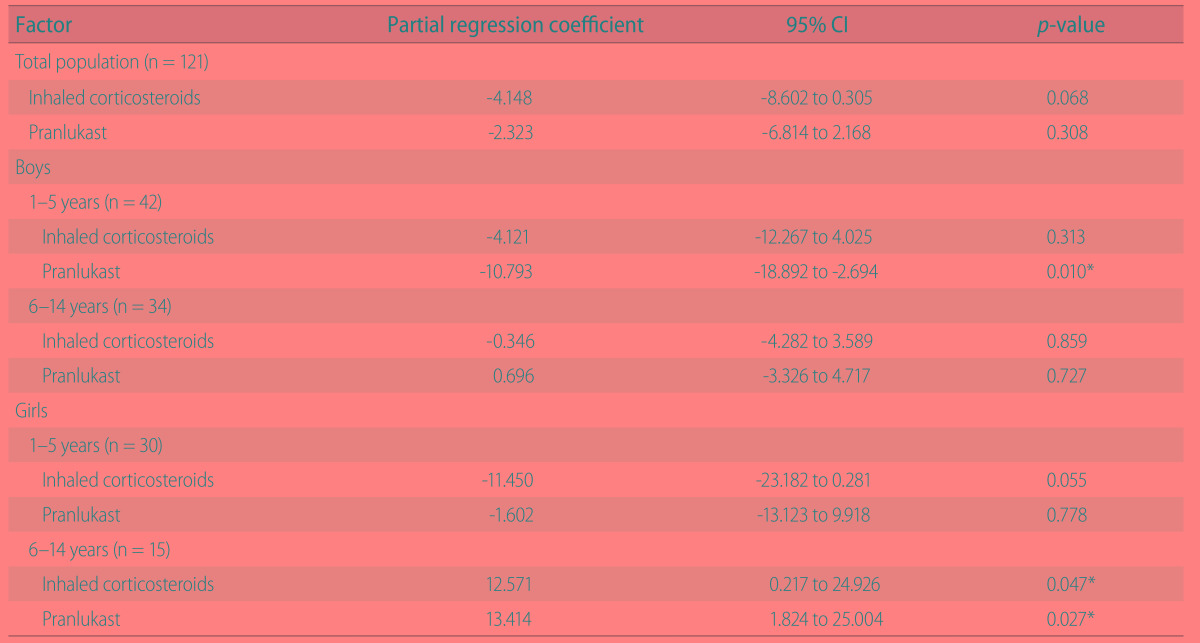
Total asthma score
Significant differences in the multiple regression analysis were observed when evaluated the effect of pranlukast on age and sex (Table 3). In boys, pranlukast reduced significantly total asthma score among 1- to 5-year-olds (p = 0.010), but did not reduce it among 6- to 14-year-olds. On the other hand, in girls pranlukast did not affect total asthma score among 1- to 5-year-olds, but increased total asthma score among 6- to 14-year-olds (p = 0.027).
Table 3. Multiple regression analysis for the effect of pranlukast on total asthma score stratified by sex and age.
CI, confidence interval.
*p < 0.05 statistically significant.
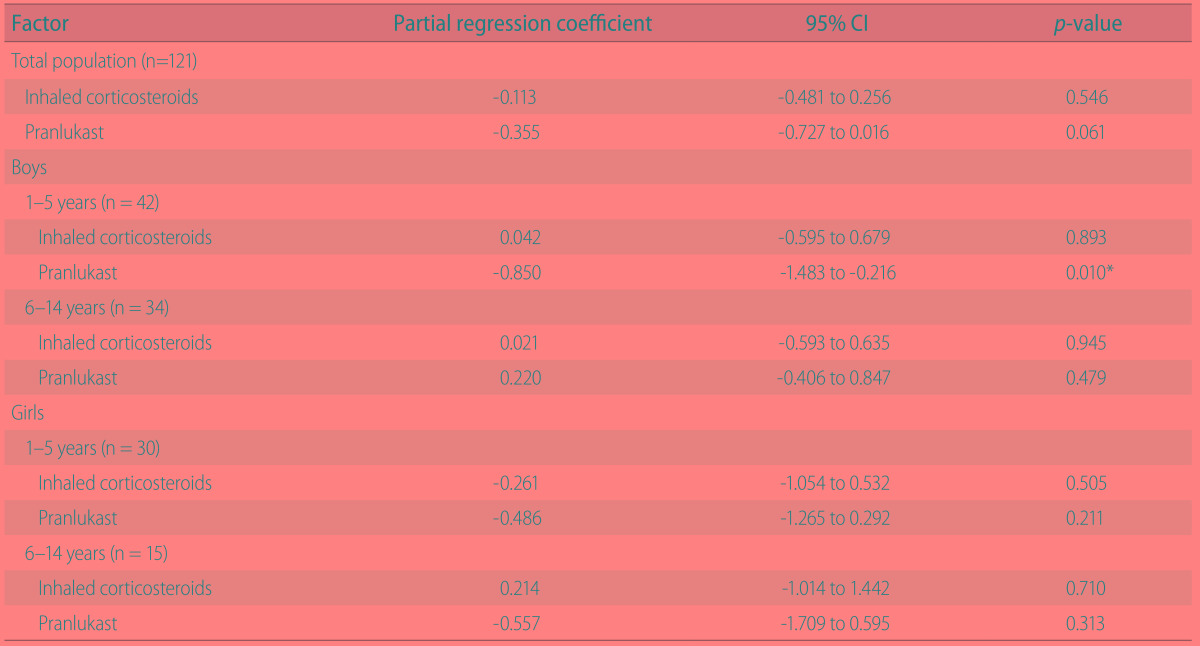
Number of colds
Sixty cold episodes were reported in the pranlukast group and 107 cases in the control group. A significant reduction in the number of cold episodes was observed in 1- to 5-year-old boys who were treated with pranlukast (p < 0.001) (Table 4).
Table 4. Multiple regression analysis for the effect of pranlukast on number of colds stratified by age and sex.
CI, confidence interval.
*p < 0.05 statistically significant.
DISCUSSION
This study demonstrated that pranlukast administration was associated with the reduction of asthma exacerbations in autumn especially in 1- to 5-year-old boys. Moreover, this population of children required less number of unscheduled visits to a physician. On the contrary, pranlukast had no significant effect in reducing asthma symptoms in 6- to 14-year-olds, in both boys and girls. In general, participants taking pranlukast had lower number of cold episodes and fever days, and using ICS or not did not affect the results.
Pranlukast reduced total asthma score in young asthmatic boys, but not in girls. These results are congruent with those reported by Johnston et al. [7]. In contrast, it was also found pranlukast did not reduce total asthma score in 6- to 14-year-old girls, this finding is different from Johnston et al. [7]'s report. In their study, it was shown LTRAs reduced asthma symptoms in 6- to 14-year-old girls. This difference may come from the small numbers of older girls entry in our study (n = 15), and 3 of 5 older girls in the pranlukast group had asthma exacerbation during the study period. Multiple regression analysis showed that taking not only pranlukast but also ICS increased total asthma score in older girls.
It is difficult to explain the difference in effectiveness of LTRAs by age and sex, although there are some reports that correlated the effectiveness of LTRAs with single nucleotide polymorphisms in different races [11,12]. In regard to the effect of age, some reports showed LTRAs was effective to viral induced asthma exacerbations especially in a lower age, Bisgaard et al. [6] reported montelukast reduces asthma exacerbations induced by viral infection in 2- to 5-year-old children with intermittent asthma. Robertson et al. [8] reported that for children with intermittent asthma, a course of parent-initiated montelukast at the onset of an upper respiratory tract infection or asthma symptoms results in a modest reduction in health care resource utilization symptoms. In the present study, the number of cold episodes was lower in 1- to 5-year-old children given pranlukast in addition to regular therapy, compared to 6- to 14-year-old children. It seems the age-related difference in the effect of pranlukast was affected by the number of cold episodes. These viral infections that induced asthma exacerbations, have been shown to have a different sex response [13]. This result may represent differences in pranlukast's efficacy according to age and sex in younger children. However, it could not explain the differences observed in older girls.
In 1- to 5-year-old boys, pranlukast group had better score, and less number of cold episodes than the control group. In 6- to 14-year-old girls, pranlukast group had a higher, but not significant asthma score, and similar number of cold episodes compared to control group. There has been little evidence LTRA reduced the number of upper respiratory infections. Kozer et al. [14] reported montelukast did not reduce the severity and opportunity of upper respiratory tract infection in 1- to 5-year healthy children. On the other hand, Horiguchi et al. [15] reported that LTRA reduced the symptoms of cold in adult asthma. Respiratory infections with rhinovirus and respiratory syncytial virus are associated with recurrent wheezing illness [16,17], and increased local leukotriene C4 levels [18]; by inhibiting this leukotriene increase, LTRAs might reduce the symptoms of cold, but not the onset of cold, although this mechanism is not well elucidated.
Both, our study and Johnston et al.'s report demonstrated 1- to 5-year-old boys given LTRAs in addition to regular therapy had less total asthma score and less number of cold episodes during the annual autumn asthma epidemic period; therefore, LTRAs have a special effect on a particular subgroup of childhood asthma, which should be studied further.
The limitations of this study included the open label design, and the small number of older girls. Nonetheless, the present study suggests that LTRAs reduces asthma symptoms in 1- to 5-year-old asthmatic Japanese boys, and additional studies are needed to evaluate these findings.
In conclusion, the present investigation showed pranlukast's efficacy differs according to age and sex. Addition of pranlukast to regular treatment during autumn is effective especially in young asthmatic boys.
ACKNOWLEDGEMENTS
We express our gratitude to all the children/families that participated in our study.
SUPPLEMENTARY MATERIAL
Supplementary material can be found via http://www.apallergy.org/src/sm/apallergy-7-10-s001.pdf.
Asthma calendar
References
- 1.Korhonen K, Reijonen TM, Remes K, Malmström K, Klaukka T, Korppi M. Reasons for and costs of hospitalization for pediatric asthma: a prospective 1-year follow-up in a population-based setting. Pediatr Allergy Immunol. 2001;12:331–338. doi: 10.1034/j.1399-3038.2001.0o085.x. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger M. Treatment strategies for viral respiratory infection-induced asthma. J Pediatr. 2003;142(2 Suppl):S34–S38. doi: 10.1067/mpd.2003.24. [DOI] [PubMed] [Google Scholar]
- 3.Caramori G, Ito K, Contoli M, Di Stefano A, Johnston SL, Adcock IM, Papi A. Molecular mechanisms of respiratory virus-induced asthma and COPD exacerbations and pneumonia. Curr Med Chem. 2006;13:2267–2290. doi: 10.2174/092986706777935159. [DOI] [PubMed] [Google Scholar]
- 4.Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011;128:1165–1174. doi: 10.1016/j.jaci.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston NW, Sears MR. Asthma exacerbations. 1: epidemiology. Thorax. 2006;61:722–728. doi: 10.1136/thx.2005.045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisgaard H, Zielen S, Garcia-Garcia ML, Johnston SL, Gilles L, Menten J, Tozzi CA, Polos P. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med. 2005;171:315–322. doi: 10.1164/rccm.200407-894OC. [DOI] [PubMed] [Google Scholar]
- 7.Johnston NW, Mandhane PJ, Dai J, Duncan JM, Greene JM, Lambert K, Sears MR. Attenuation of the September epidemic of asthma exacerbations in children: a randomized, controlled trial of montelukast added to usual therapy. Pediatrics. 2007;120:e702–e712. doi: 10.1542/peds.2006-3317. [DOI] [PubMed] [Google Scholar]
- 8.Robertson CF, Price D, Henry R, Mellis C, Glasgow N, Fitzgerald D, Lee AJ, Turner J, Sant M. Short-course montelukast for intermittent asthma in children: a randomized controlled trial. Am J Respir Crit Care Med. 2007;175:323–329. doi: 10.1164/rccm.200510-1546OC. [DOI] [PubMed] [Google Scholar]
- 9.Almqvist C, Worm M, Leynaert B working group of GA2LEN WP 2.5 Sex. Impact of sex on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63:47–57. doi: 10.1111/j.1398-9995.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 10.Morikawa A, Nishima S Japanese Society of Pediatric Allergy and Clinical Immunology. New Japanese pediatric guidelines for the treatment and management of bronchial asthma. Pediatr Int. 2007;49:1023–1031. doi: 10.1111/j.1442-200X.2007.02462.x. [DOI] [PubMed] [Google Scholar]
- 11.Asano K, Shiomi T, Hasegawa N, Nakamura H, Kudo H, Matsuzaki T, Hakuno H, Fukunaga K, Suzuki Y, Kanazawa M, Yamaguchi K. Leukotriene C4 synthase gene A(-444)C polymorphism and clinical response to a CYS-LT(1) antagonist, pranlukast, in Japanese patients with moderate asthma. Pharmacogenetics. 2002;12:565–570. doi: 10.1097/00008571-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Telleria JJ, Blanco-Quiros A, Varillas D, Armentia A, Fernandez-Carvajal I, Jesus Alonso M, Diez I. ALOX5 promoter genotype and response to montelukast in moderate persistent asthma. Respir Med. 2008;102:857–861. doi: 10.1016/j.rmed.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Muenchhoff M, Goulder PJ. Sex differences in pediatric infectious diseases. J Infect Dis. 2014;209(Suppl 3):S120–S126. doi: 10.1093/infdis/jiu232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozer E, Lotem Z, Elgarushe M, Torgovicky R, Cohen R, Cohen HA, Berkovitch M. RCT of montelukast as prophylaxis for upper respiratory tract infections in children. Pediatrics. 2012;129:e285–e290. doi: 10.1542/peds.2011-0358. [DOI] [PubMed] [Google Scholar]
- 15.Horiguchi T, Ohira D, Kobayashi K, Hirose M, Miyazaki J, Kondo R, Tachikawa S. Clinical evaluation of leukotriene receptor antagonists in preventing common cold-like symptoms in bronchial asthma patients. Allergol Int. 2007;56:263–267. doi: 10.2332/allergolint.O-06-473. [DOI] [PubMed] [Google Scholar]
- 16.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF., Jr Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, Kimpen JL; Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. 42.e1. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 18.Gentile DA, Fireman P, Skoner DP. Elevations of local leukotriene C4 levels during viral upper respiratory tract infections. Ann Allergy Asthma Immunol. 2003;91:270–274. doi: 10.1016/S1081-1206(10)63529-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Asthma calendar