Abstract
The motile cilium is a complex organelle that is typically comprised of a 9+2 microtubule skeleton; nine doublet microtubules surrounding a pair of central singlet microtubules. Like the doublet microtubules, the central microtubules form a scaffold for the assembly of protein complexes forming an intricate network of interconnected projections. The central microtubules and associated structures are collectively referred to as the central apparatus (CA). Studies using a variety of experimental approaches and model organisms have led to the discovery of a number of highly conserved protein complexes, unprecedented high-resolution views of projection structure, and new insights into regulation of dynein-driven microtubule sliding. Here, we review recent progress in defining mechanisms for the assembly and function of the CA and include possible implications for the importance of the CA in human health.
Intriguing insights into the assembly, composition, and function of the central microtubules and associated proteins in motile cilia have recently been made. For example, katanin—a protein that severs microtubules—has been implicated in their assembly.
Motile cilia and eukaryotic flagella are characterized by the canonical “9+2” arrangement of microtubules in which nine doublet microtubules surround a central pair of singlet microtubules. These central microtubules and their associated protein projections are collectively referred to as the central apparatus (CA) (Fig. 1). Although there are a few exceptions to the 9+2 arrangement of microtubules, the CA is remarkably well conserved throughout eukaryotes and is thought to have been present in cilia of the last common eukaryotic ancestor (Mitchell 2004). Since researchers first discovered the nearly crystalline arrangement of proteins that form the axoneme, they have tried to answer two fundamental questions: How do these structures assemble? What is their role in motility? Answering these questions has required a sophisticated array of structural, biochemical, genetic, and functional approaches. Here, we review new insights into the structure and composition of the CA, possible mechanisms for assembly, and the role of the CA in regulating ciliary and flagellar motility.
Figure 1.
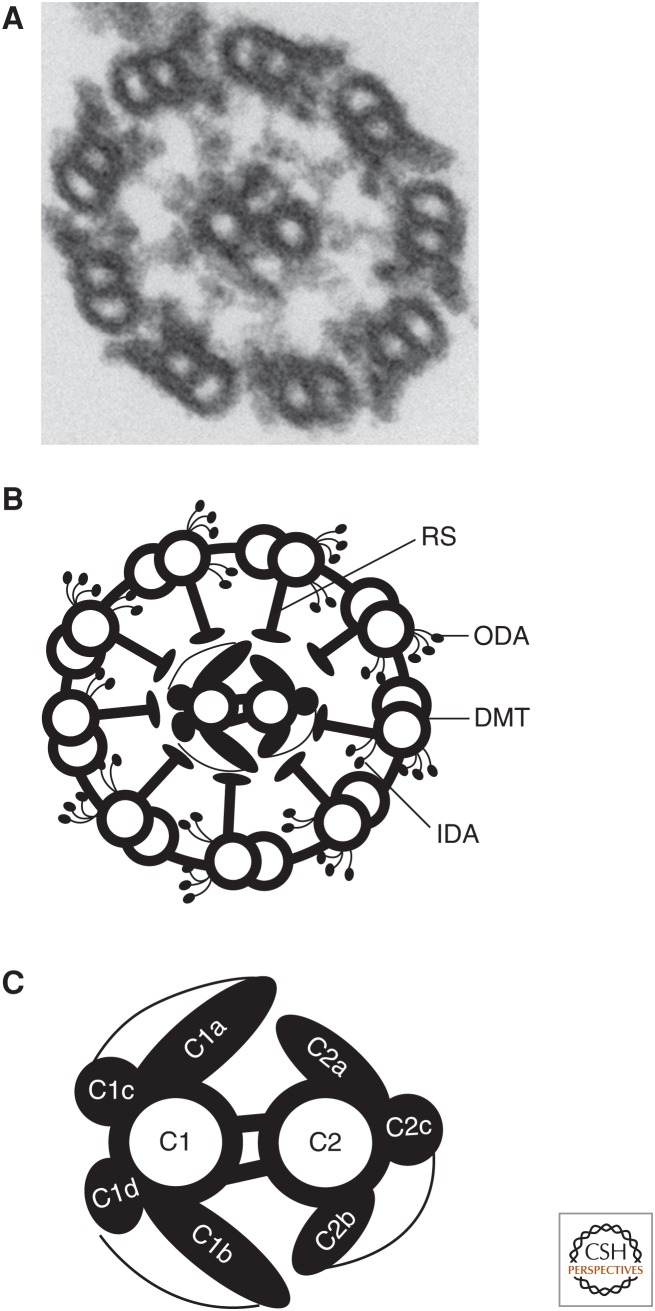
Central apparatus (CA) structure. (A) Transmission electron microscopy (TEM) image of an axoneme transverse section from Chlamydomonas reinhardtii. The central microtubules and their associated projections are surrounded by the outer doublet microtubules. (B) Diagram of axoneme transverse section seen in A. (C) Diagram of CA with projections labeled. DMT, Doublet microtubule; IDA, inner dynein arm; ODA, outer dynein arm; RS, radial spoke.
CENTRAL APPARATUS STRUCTURE
Unlike the nine doublet microtubules, the microtubules of the CA are not continuous with the basal body. The central microtubules extend from a region near the transition zone, elongate beyond the doublet microtubules, and end in a capping structure at the tip of the cilium (Ringo 1967; Dentler and Rosenbaum 1977; Dentler 1984). This cap has not been biochemically characterized, but structurally it contains two major components: a “bead” attached to the membrane, and two plates attached to the central microtubules (Dentler 1984). Each microtubule of the CA is structurally and biochemically distinct (reviewed below) and are referred to as C1 and C2. The portions of the central microtubules that extend beyond the outer doublets are devoid of protein projections (Ringo 1967). In addition to protein projections associated with the surface of the microtubules, the Nicastro laboratory has shown that the internal lumen of the C2 microtubule of Chlamydomonas contains two small densities (Carbajal-Gonzalez et al. 2013), which they refer to as microtubule inner proteins, or MIPs, named MIP-C2a and MIP-C2b. MIP-C2a repeats every 16 nm and is 50 kDa in size, whereas MIP-C2b has an 8-nm periodicity and is only 35 kDa in size. These proteins are not seen in the flagella of sea urchin Strongylocentrotus purpuratus sperm, possibly indicating a specialization unique to Chlamydomonas cilia (Carbajal-Gonzalez et al. 2013).
Advances in electron microscopy (EM) in the 1960s and 1970s opened the door to our current understanding of CA structure. Using both Chlamydomonas and Tetrahymena, early work identified structural differences in the CA projections (Chasey 1969; Hopkins 1970). The microtubule with longer projections was termed C1, and the other C2. The longest projections on C1 are designated C1a and C1b and have a 16-nm repeat period along the length of C1. The prominent projections on C2 (C2a and C2b) have the same 16-nm repeat period. Later work using the newly developed rapid-freeze deep-etch method of EM revealed additional complexities including a sheath-like structure surrounding the CA (Goodenough and Heuser 1985). Advances in digital imaging and image-averaging techniques led to the discovery of smaller projections on both central microtubules: C2c repeating every 16 nm, and C1c and C1d that repeat every 32 nm (Mitchell and Sale 1999; Mitchell 2003b). The central microtubules are held together by a bridging structure that repeats at 16-nm intervals and is likely involved in CA stability (see section on Composition of the Central Apparatus).
Recent application of cryo-electron tomography (cryo-ET) has provided unprecedented views of CA structure and revealed four entirely new protein projections termed C1e, C1f, C2d, and C2e (Fig. 2) (Carbajal-Gonzalez et al. 2013). These new views indicate that the projections actually form a continuous network surrounding the two central microtubules and may explain the sheath structure observed in previous studies (Carbajal-Gonzalez et al. 2013).
Figure 2.
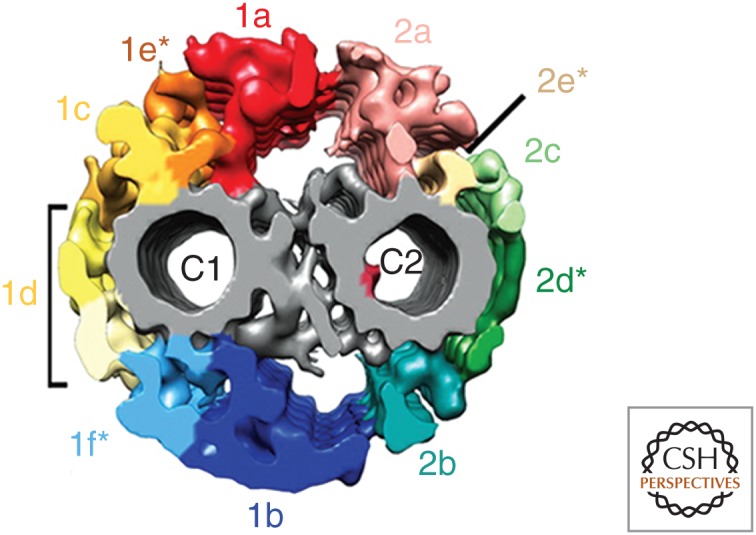
Detailed structural elements of the central apparatus (CA). Isosurface renderings derived from cryo-electron tomography (cryo-ET) of the Chlamydomonas CA viewed in transverse section. The projections form an interconnected network surrounding the two singlet microtubules with connections between C1a and C2a and C1b and C2b. The bridge structure connecting the two microtubules is in gray. MIP-C2a is highlighted within the lumen of the C2 microtubule. The projections labeled C1e, C1f, C2d, and C2e likely correspond to the material previously described as a sheath. (From Carbajal-Gonzalez et al. 2013; reprinted, with permission, from Wiley.)
Finally, the entire CA structure is known to twist in a left-handed helix (Omoto and Kung 1980; Kamiya 1982; Goodenough and Heuser 1985; Mitchell and Nakatsugawa 2004). This helix contains one 360° twist over the length of the Chlamydomonas cilium, whereas in Paramecium the number of twists per cilium length varies (Omoto and Kung 1980; Kamiya 1982). CA twist in Chlamydomonas occurs with or without RS heads, indicating that twist is not due to CA contact with the RSs (Mitchell and Nakatsugawa 2004). In addition, CAs extruded from the axoneme retain a helical structure (Kamiya 1982). The function of this twist is unknown, but it may have a role in regulating motility.
COMPOSITION OF THE CENTRAL APPARATUS
Our current understanding of the composition of the projections is largely based on biochemical approaches and study of Chlamydomonas mutants (for example, Witman et al. 1978; Adams et al. 1981). In one class of mutants, the entire CA fails to assemble (pf15, pf18, pf19, and pf20); these mutants have immotile flagella. The identity of the PF18 gene is unknown; however, the other three loci have been studied extensively. PF20 is a WD repeat-containing protein localized to the bridge structure connecting the two central microtubules and is hypothesized to stabilize the central tubules (Smith and Lefebvre 1997). PF15 and PF19, encode the p80 and p60 subunits, respectively, of the microtubule-severing protein katanin (Dymek et al. 2004; Dymek and Smith 2012) (see section on Assembly of the Central Apparatus for further discussion of PF15 and PF19).
The second class of Chlamydomonas mutants lack subsets of CA projections and includes pf6, cpc1, and pf16. The C1a projection is lacking from pf6 flagella (Dutcher et al. 1984). PF6 encodes a large (240-kDa) protein that contains numerous proline-rich domains (Rupp et al. 2001). Additional biochemical studies led to the discovery of a PF6-containing complex that includes calmodulin (CaM) and four other proteins (Table 1). The PF6 protein likely plays a scaffolding role in the assembly of this complex to form C1a (Wargo et al. 2005).
Table 1.
Proteins associated with the central apparatus
| Structure/localization | Protein | Molecular mass (kDa) | Accession number | Description | References |
|---|---|---|---|---|---|
| C1a projection | PF6a | 240 | AAK38270 | Contains alanine-proline rich domains; ASH domain | Rupp et al. 2001; Wargo et al. 2005 |
| C1a-86 | 86 | AAZ31187 | Contains a PKA RII-like LRR domain | Wargo et al. 2005 | |
| C1a-34 | 34 | AAZ31186 | Contains a coiled-coil region | Wargo et al. 2005 | |
| C1a-32 | 32 | AAZ31185 | Similar to C1a-34 | Wargo et al. 2005 | |
| C1a-18 | 18 | AAZ31184 | Contains MORN domains | Wargo et al. 2005 | |
| Calmodulin | 18 | AAA33083 | Calcium-binding protein | Wargo et al. 2005 | |
| C1b projection | CPC1 | 265 | AAT40992 | Contains EF-hand domain; adenylate kinase domains | Zhang and Mitchell 2004; Mitchell et al. 2005 |
| C1b-350 (FAP42) | 350 | EDP00757 | Contains five guanylate kinase domains; one adenylate kinase domain | Mitchell et al. 2005 | |
| C1b-135 (FAP69) | 135 | EDP06190 | Armadillo repeat-containing protein | Mitchell et al. 2005 | |
| HSP70 | 78 | P25840 | Chaperone/heat shock protein | Zhang and Mitchell 2004; Mitchell et al. 2005 | |
| Enolase | 56 | P13683 | Glycolytic enzyme | Mitchell et al. 2005 | |
| C1d projection | Pcdp1 (FAP221)a | 100 | ADD85929 | Calmodulin-binding protein | DiPetrillo and Smith 2010 |
| FAP54 | 318 | AFG30957 | Contains no known functional domains | DiPetrillo and Smith 2010 | |
| FAP46 | 289 | AFG30956 | Contains no known functional domains | DiPetrillo and Smith 2010 | |
| FAP74 | 204 | ADD85930 | Contains no known functional domains | DiPetrillo and Smith 2010 | |
| C1d-87 | 87 | EDP09774 | WD repeat-containing protein | Brown et al. 2012 | |
| C1 microtubule | PF16a | 57 | AAC49169 | Armadillo repeat-containing protein | Dutcher et al. 1984; Smith and Lefebvre 1996 |
| PP1c | 35 | AAD38850 | Phosphatase | Yang et al. 2000 | |
| C1 kinesin | 110 | Kinesin-like protein | Fox et al. 1994 | ||
| C1–C2 bridge | PF20a | 63 | AAB41727 | WD repeat-containing protein | Smith and Lefebvre 1997 |
| C2b projection | Hydina | 540 | EDP09735 | Contains four ASH domains | Lechtreck and Witman 2007 |
| C2c projection | KLP1 | 96 | P46870 | Kinesin-like protein | Bernstein et al. 1994; Yokoyama et al. 2004 |
| Other | Katanin p60 | 60 | AAF12877 | Catalytic subunit of katanin; AAA ATPase | Dymek and Smith 2012 |
| Katanin p80 | 80 | EDP00085 | Regulatory subunit of katanin; WD repeat-containing protein | Dymek et al. 2004 |
aThese proteins have mammalian homologs that are discussed in the section on Central Apparatus in Mammalian Health and Human Disease.
The C1b projection is lacking in cpc1 flagella (Mitchell and Sale 1999). These mutant flagella often lack portions of the C2b projection as well, possibly indicating structural connections between these two projections (Mitchell and Sale 1999; Zhang and Mitchell 2004). CPC1 is a 265-kDa protein composed of an EF-hand domain and an adenylate kinase domain. Along with CPC1, the C1b projection contains four other identified proteins including the chaperone protein HSP70 and the glycolytic enzyme enolase, which were identified through cosedimentation in sucrose gradients (Table 1) (Mitchell et al. 2005).
Mutations in PF16 cause the disassembly of the C1 microtubule when flagella are demembranated (Dutcher et al. 1984). PF16 is a 57-kDa protein composed of eight continuous armadillo repeat motifs (Smith and Lefebvre 1996, 2000). Armadillo repeats are involved in protein–protein interactions (Peifer et al. 1994), suggesting that PF16 forms complexes that stabilize the C1 microtubule. The pf16 mutant has been invaluable for defining the composition of C1 and C2. Proteins that remain associated with the axoneme in a pf16 mutant are good candidates for C2-associated proteins.
Biochemical approaches have revealed the identities of additional CA-associated proteins. Experiments designed to identify axonemal CaM interacting proteins led to the discovery of a second CaM-containing complex of five proteins associated with C1 (Table 1) (DiPetrillo and Smith 2010). When expression of complex components is reduced, the C1d projection fails to assemble and the cells show uncoordinated flagellar movement (DiPetrillo and Smith 2010). A strain with a mutation in a single complex component has the same phenotype (Brown et al. 2012). Furthermore, the C1d protein FAP221 is homologous to the mouse primary ciliary dyskinesia protein 1 (see section on Central Apparatus in Mammalian Health and Human Disease) (Lee et al. 2008; DiPetrillo and Smith 2010).
Two other proteins are associated with the C1 microtubule. Protein phosphatase 1 was identified in cilia from Paramecium and Chlamydomonas (Friderich et al. 1992; Yang et al. 2000). In addition, a 110-kDa kinesin has been localized to C1 through the use of mutants and polyclonal antibodies (Fox et al. 1994; Johnson et al. 1994). Localization of these proteins to specific projections has not been determined.
Two proteins have been assigned to C2 projections: KLP1 and Hydin. The study that led to the discovery of the 110-kDa C1 kinesin, also led to the identification of KLP1 (Fox et al. 1994; Johnson et al. 1994). KLP1 is tightly bound to the axoneme in a pf16 mutant, thereby localizing it to the C2 microtubule (Bernstein et al. 1994). KLP1 is a member of the kinesin-9 family of proteins and when its expression is knocked down, the C2c projection and a portion of the C2b projection fail to assemble (Yokoyama et al. 2004).
Although many studies in Chlamydomonas have preceded the identification of mammalian homologs (see section on Central Apparatus in Mammalian Health and Human Disease), a study in mammals led to the discovery of Hydin. Mice that have the hy3 mutation in the Hydin gene show hydrocephalus (Davy and Robinson 2003). Furthermore, similar mutations in trypanosomes lead to motility defects (Broadhead et al. 2006). Knockdown of Hydin in Chlamydomonas causes the failure of the C2b projection to assemble and, in some instances, the destabilization of the C1b and C2c projections as well (Lechtreck and Witman 2007). As noted above, the destabilization of the C1b projection due to mutations in CPC1 leads to the reciprocal destabilization of the C2b projection (Mitchell and Sale 1999; Yokoyama et al. 2004; Lechtreck and Witman 2007). Based on its size, Hydin may act as a scaffold for other CA-associated proteins.
ASSEMBLY OF THE CENTRAL APPARATUS
Mutants that fail to assemble the entire CA have contributed to our understanding of CA assembly. Although PF20 has been implicated in stabilizing the central microtubules, katanin may play a direct role in the formation of the central microtubules. Katanin is a hexameric protein composed of an AAA ATPase catalytic subunit (p60, encoded by the PF19 gene) and a regulatory subunit with WD repeat domains (p80, encoded by the PF15 gene) (Hartman et al. 1998; Dymek et al. 2004; Dymek and Smith 2012). Mutations in either subunit yield a central pairless phenotype. Katanin severs microtubules by the binding of its p60 hexamer to tubulin, whereas the p80 subunit potentially targets and enhances this activity (Hartman et al. 1998; McNally et al. 2000). The pf19 mutation inhibits the microtubule-severing activity of the p60 subunit (Dymek and Smith 2012). Therefore, severing activity is necessary for the assembly of the central tubules. It seems paradoxical that the assembly of microtubules requires microtubule-severing activity; however, there is precedent for katanin's involvement in the formation of noncentrosomal microtubule arrays in plants and neurons (Karabay et al. 2004; Yu et al. 2005; Gardiner and Marc 2011). Recall that the central microtubules are not nucleated from the basal body and are also noncentrosomal microtubules.
Several other proteins are linked to central microtubule assembly. Studies in Drosophila identified the basal body protein Bld10p/CEP135 as important for assembly. Bld10p is localized to the central cartwheel structure of the basal body (Carvalho-Santos et al. 2012). In Drosophila, a single microtubule exists before the formation of the central microtubules and is stabilized by Bld10p. Presumably, the stabilization of this microtubule is necessary for the eventual formation of two central microtubules (Carvalho-Santos et al. 2012). It is unknown whether the requirement for Bld10p in CA assembly is unique to Drosophila.
RNA interference has revealed that γ-tubulin and its associated ring complex are necessary for CA assembly in trypanosomes (McKean et al. 2003; Zhou and Li 2015). In addition, in Tetrahymena, central pair formation depends on the glycylation domain of β-tubulin and if this domain is mutated, cilia are short and fail to form central microtubules (Thazhath et al. 2004). Three possible roles for glycylation have been proposed. First, glycylation may be necessary for intraflagellar transport (IFT)-mediated transport of CA proteins (Thazhath et al. 2004). Short doublet microtubules, but not central pair microtubules, assemble in Tetrahymena IFT mutants and in sea urchin eggs in which IFT was inhibited by antibodies (Brown et al. 1999, 2003). Perhaps glycylation is necessary for tubulin’s association with anterograde IFT particles. Second, glycylation may be necessary for central microtubule nucleation at the transition zone (Thazhath et al. 2004). Finally, β-tubulin glycylation may stabilize the growing distal ends of the central microtubules or mediates associations with cap-specific proteins (Thazhath et al. 2004).
Why has evolution favored a pair of singlet central microtubules? The answer may be as simple as space dependence. Chlamydomonas pf14 mutants lack RSs; therefore, the center of the axoneme contains more empty space than in wild-type. Mutant pf14 axonemes have been observed to contain two pairs of central microtubules with identical and correct polarities and complete protein projections (Lechtreck et al. 2013). Another example includes mutations in BLD12. Bld12p is the Chlamydomonas homolog of Sas6 in Caenorhabditis elegans and forms the cartwheel structure of the basal body that establishes ninefold symmetry (Nakazawa et al. 2007). Mutations in BLD12 cause misshaped basal bodies that can manifest as an expansion of the axonemal diameter; in these cases, a corresponding increase in the number of CAs is observed (Nakazawa et al. 2014). These results provide evidence that the number of CAs that assemble may be determined by the allowable space within the axoneme. A key question remains: Why is the CA typically a pair of microtubules as opposed to a single or three or more microtubules?
Assembly and targeting of the CA projections to the central microtubules is also an active area of research. Several ciliary structures, including dynein arm and RS components, form preassembly complexes in the cytoplasm that are then transported into the cilium (Fowkes and Mitchell 1998; Diener et al. 2011; Viswanadha et al. 2014). Do CA proteins assemble in preassembly complexes before targeting to the axoneme? Proteins are transported into the cilium in one of two ways: IFT or diffusion. Are CA projection proteins transported through IFT or diffusion?
These questions have recently been studied in Chlamydomonas flagellar regeneration and dikaryon rescue experiments performed in the Lechtreck laboratory (Lechtreck et al. 2013; Wren et al. 2013). Observations of flagellar regeneration have revealed time-dependent assembly in which the projections assemble onto the central microtubules before dynein arm assembly, despite the observation that the central microtubules form after the outer doublets. Interestingly, the direction of assembly depends on whether the cell is in the process of de novo flagellar assembly (regeneration) or if the CA is assembling in an assembled central pairless axoneme (dikaryon rescue). In regenerating flagella, central microtubule growth begins proximal to the transition zone and continues distally. However, in dikaryon rescue experiments using pf15 or pf19 cells, subdistal microtubule assembly occurred with projection proteins assembling tip to base. These observations indicate that the CA is capable of assembling independent of other structures in the axoneme and that microtubule nucleation can occur from either distal or proximal regions of the flagellum. This laboratory has also shown that PF16 uses IFT to enter the cilium suggesting that other CA proteins may as well (Wren et al. 2013).
CENTRAL APPARATUS FUNCTION
The high degree of CA conservation in motile cilia implies that there is selective pressure for motile cilia to retain the 9+2 microtubule structure. However, is the CA actually necessary for motility? There are examples of motile cilia that lack a CA. For instance, the mature male gametes of the alga Lithodesmium undulatum contain a 9+0 microtubule arrangement and are fully motile (Manton 1966; Carvalho-Santos et al. 2011). The protozoan Lecudina tuzetae and the arthropod Acerentomon microrhinus have more extreme versions of alternate yet motile axonemal structure with 6+0 and 14+0, respectively (Schrevel and Besse 1975; Dallai et al. 2010; Carvalho-Santos et al. 2011). In addition, the nodal cilia present during mammalian development have a 9+0 structure and show a more rotational motility rather than the more planar symmetric and asymmetric waveforms typical of other flagella and cilia (Nonaka et al. 1998).
Examples of motile cilia that lack a CA are rare. Furthermore, in organisms with motile cilia that assemble a CA, the CA is required for motility. As noted above, central pairless mutants of Chlamydomonas show complete flagellar paralysis. In addition, mutations in which CA projections are absent also show aberrant flagellar motility. The pf6 mutant flagella lacking C1a twitch ineffectively (Rupp et al. 2001) and mutants missing only the carboxy terminus of PF6 are paralyzed as well (Goduti and Smith 2012). The flagella of cpc1 mutants beat at 40 Hz instead of 60 Hz and have axonemes that switch waveforms when exposed to high concentrations of calcium (Mitchell and Sale 1999). Mutations affecting C1d cause slow swimming. In addition, the flagella of these cells are uncoordinated and beat at a frequency of ∼30% that of wild-type (DiPetrillo and Smith 2010, 2011). Hydin mutants missing C2b are locked in a position in which one flagellum is oriented along the cell body, whereas the other extends away from it in a “hands up/hands down” conformation and mice missing this projection show cilia stalling (Lechtreck and Witman 2007; Lechtreck et al. 2008). Finally, pf16 flagella are lacking only three proteins yet are completely paralyzed (Dutcher et al. 1984). These findings are not limited to Chlamydomonas. Defects in the mammalian CA, like in Hydin mutants, have also been shown to affect motility (see section on Central Apparatus in Mammalian Health and Human Disease).
Extragenic suppressor mutations that restore motility to paralyzed CA mutants (and RS defective mutants) without restoring the missing CA structures have provided important genetic evidence of a mechanism for the CA’s role in regulating motility (Huang et al. 1982). The suppressor mutations are found in components of the inner and outer dynein arms along with the N-DRC and suggest a regulatory pathway by which the CA regulates dynein activity through the RSs and regulatory complexes located on the doublet microtubules (Huang et al. 1982; Piperno et al. 1992, 1994; Porter et al. 1992, 1994). However, it is important to note that the CA and RS mutants carrying suppressor mutations produce symmetric waveforms of reduced amplitude and beat frequency (Brokaw et al. 1982). This implicates the CA and RSs in the production of proper waveforms necessary for forward swimming.
Despite flagellar paralysis, dynein arms of CA mutants retain their force-generating properties. Using a sliding disintegration assay in which microtubule sliding is uncoupled from flagellar bending, dynein activity can be quantified as microtubule sliding velocity (Summers and Gibbons 1971; Okagaki and Kamiya 1986). Using this assay, pf18 and pf15 mutant axonemes have been shown to have reduced sliding velocities compared with wild-type (Smith 2002b). Evidently, dynein activity is reduced in these mutants. Reduced sliding velocities are also observed for pf16 mutant axonemes showing the importance of the C1 microtubule in regulating dynein. Combining the sliding assay with structural studies has shown that the position of the CA correlates with the position of active doublet sliding (Yoshimura and Shingyoji 1999; Wargo and Smith 2003).
The sliding disintegration assay has also revealed transduction pathways that include second messengers, and kinases and phosphatases anchored to the axoneme. Several studies have shown a link between the CA and regulation of inner dynein arms through axonemal kinases and phosphatases (Smith and Sale 1992; Howard et al. 1994; Habermacher and Sale 1996; Yang and Sale 2000; Kikushima 2009). These studies have been reviewed in Wirschell et al. (2011). A relationship between calcium regulation of motility and the CA has also been shown. For instance, increased intraflagellar calcium concentrations can increase sliding velocity in central pairless mutants, but not pf16 axonemes (Smith 2002a). Studies of sea urchin sperm also show the importance of calcium signaling through the CA in the localized sliding of specific subsets of doublet microtubules (Nakano et al. 2003). Work in Chlamydomonas indicates that these changes are mediated by axoneme-associated CaM (Smith 2002a) and that CaM is associated with both the C1a and C1d projections (Wargo et al. 2005; DiPetrillo and Smith 2009, 2010). These studies provide evidence of a role for the CA in calcium regulation of motility.
Although these studies provide a strong correlation between the CA and regulating microtubule sliding, they do not provide a mechanism for how this regulation is converted to complex ciliary waveforms. Given the location of the CA in relation to the dynein arms, CA signals would likely be mediated by the RSs. This idea was first supported by structural studies of Elliptio gill cilia in which the RS heads make transient contact with the projections of the CA, causing a tilt in the RSs relative to the longitudinal axis of the cilium (Warner and Satir 1974). RS–CA interactions may also occur in protozoan organisms whose CA rotates during bend propagation (Omoto and Kung 1980; Omoto et al. 1999; Mitchell 2003a). Even though this rotation has been shown to be a passive response to flagellar bending, rotation and twist of the CA may provide important positional cues that are transmitted via the spokes (Mitchell and Nakatsugawa 2004).
These studies raise the question of whether the CA provides biochemical or mechanical cues or both to regulate microtubule sliding. What is the relationship between specific protein projections and this regulation? Of the CA projections, C1d is preferentially oriented toward areas of active microtubule sliding (Wargo and Smith 2003; DiPetrillo and Smith 2011). Furthermore, axonemes with expanded diameters show preferential binding between the RSs and specific C1 projections; one site near C1a and one near C1b (Nakazawa et al. 2014). Removing C1a and C1b reveals weaker, yet still seemingly specific, interactions between RS heads and the CA (Nakazawa et al. 2014). These studies indicate that there is specificity in RS–CA interactions; however, a recent study suggests otherwise. By engineering epitope tags on RS heads, Kikkawa’s group rescued motility in pf6 mutants lacking C1a (Oda et al. 2014). The tag extended the RS across the gap creating an artificial interaction that reconstituted motility. These results suggest CA regulation may not require specific CA–RS interactions but may rely on nonspecific mechanosignaling. Further research is required to reconcile these seemingly conflicting studies.
Additional mechanisms for CA regulation of motility have been proposed from mathematical modeling approaches applied to beating cilia and flagella. Mathematical modeling of sperm flagellar bending led to a “geometric clutch” model (Lindemann 1994; Lindemann and Kanous 1995). In this model, the formation of dynein cross-bridges is limited by the space between doublet microtubules. Therefore, only a subset of dynein can be active at any given time. When cross-bridges form, microtubule sliding is induced causing axonemal distortions that result in dynein cross bridges on one side of the axoneme to release and dynein cross-bridges on the opposite side of the axoneme to form (Lindemann and Mitchell 2007). Bending of the flagellum occurs due to switching of cross-bridge formation and release. In this model, the CA and RSs transmit a transverse force during the switch point (Lindemann 2003). However, the force-bearing capacity of the CA is unknown and must be determined to understand how the t-force is distributed in the axoneme (Lindemann 2007). For a recent summary of this model, see Lindemann and Lesich (2015).
One surprising contribution the CA may make in regulating motility is maintaining stable intraciliary ATP levels. The CPC1 protein contains an adenylate kinase domain. CPC1-defective mutants have 64% the beat frequency of wild-type flagella, potentially due to a lack of ATP production from recycled ADP. When mutant axonemes are saturated with ATP in flagellar reactivation assays, beat frequency is returned to 90% that of wild-type (Zhang and Mitchell 2004). Reactivation of beating despite the lack of CPC1 in these flagella indicates that there are potentially other adenylate kinases to be discovered in the axoneme.
THE CENTRAL APPARATUS IN MAMMALIAN HEALTH AND HUMAN DISEASE
One of the most astounding findings in cell biology in the past few decades is the identification of diseases that are caused by ciliary defects known collectively as ciliopathies (Braun and Hildebrandt 2016). These defects can be in either motile or nonmotile cilia. Here, we focus on proteins in motile cilia that when defective cause disease in mammals.
In mammalian motile cilia, three proteins with Chlamydomonas homologs are believed to form a complex: Spag16 (PF20), Spag6 (PF16), and Spag17 (PF6) (Zhang et al. 2005). Each member of this putative “interactome” is essential for proper ciliary motility and mammalian health. The mammalian homolog of PF20, Spag16, occurs in two isoforms: Spag16L, localized to the cilium and Spag16S, localized to the nucleus (Zhang et al. 2006). Spag16 is essential for male fertility by regulating sperm flagellar motility (Zhang et al. 2006). Further studies identified Spag16 as essential for human fertility as well (Zhang et al. 2007b). The mammalian homolog of PF16, Spag6 (Neilson et al. 1999; Zhang et al. 2002), is essential for mammalian health; mice with mutations in Spag6 show hydrocephalus, respiratory distress, infertility, and die 8 weeks after birth (Sapiro et al. 2002). Furthermore, Spag6-deficient mice also have reduced ciliary beat frequency in tracheal epithelial cells and reduced numbers of cilia in both the trachea and brain ependymal cells (Teves et al. 2014). The PF6 homolog, Spag17, is also essential for motility and linked to severe health problems in mice. Spag17-deficient mice show skeletal abnormalities and have enlarged brain ventricles, respiratory distress, and die in an extremely short 12 hours after birth (Teves et al. 2013, 2015). These phenotypes are exacerbated in mice that have both Spag6 and Spag17 mutations (Zhang et al. 2007a). To date, there is no evidence that these three proteins form a complex in Chlamydomonas.
Additional mammalian homologs of Chlamydomonas proteins include the protein FAP221 associated with C1d, which is mammalian Pcdp1, a protein associated with primary ciliary dyskinesia in mice (Lee et al. 2008). More recently, mammalian homologs of other members of C1d have been identified. Mutations in one of these proteins, CFAP54, lead to symptoms associated with primary ciliary dyskinesia such as sperm motility defects and an accumulation of mucus in the lungs (McKenzie et al. 2015). These findings further implicate this small projection as being essential for ciliary motility. Finally, the C2b protein Hydin was first characterized in mice showing hydrocephalus (Davy and Robinson 2003). This hydrocephalus is not due to defects in ciliary length or density in the brain, which are normal in Hydin mutants. Instead, the brain cilia lack C2b causing a motility defect termed “cilia stalling”; this leads to a lack of fluid flow in the brain and hydrocephalus (Lechtreck et al. 2008). (For more information about CA proteins in mammals, see Teves et al. 2016.)
These findings can be extended beyond mouse models to humans. Mutations in projection proteins like Spag16L can cause male infertility, and recessive mutations in Hydin have been linked to primary ciliary dyskinesia in human patients (Zhang et al. 2007b; Olbrich et al. 2012). In addition, there are several accounts of patients with primary ciliary dyskinesia that lack the entire CA (Bautista-Harris et al. 2000; Stannard et al. 2004). Often, the cilia of these patients will show the transposition of one outer doublet microtubule to the center of the axoneme perhaps to function as a CA (Smallman and Gregory 1986; Chilvers et al. 2003; Burgoyne et al. 2014). This particular observation is especially interesting for showing the importance of the CA for the regulation of proper ciliary motility in humans. The locations of the mutations in these patients have not been published. Therefore, it is not known whether they correspond to previously identified CA proteins.
QUESTIONS FOR THE FUTURE
By applying a combination of biochemical, structural, and genetic approaches to understanding how motile cilia assemble and function, investigators continue to peel away the layers of complexity associated with the CA. As we make headway identifying the components of essential complexes, our challenge is to map these complexes onto the intricate CA structures revealed by cryo-ET. In addition, we are only beginning to define the molecular and mechanical mechanisms that couple the CA to regulation of ciliary motility. We need to combine our knowledge of the composition and structure of the CA projections with functional assays that allow for computational and mathematical modeling approaches to elucidate the mechanisms of the CA in regulating motility, including the role of second messengers and other signaling molecules. Finally, despite the intriguing discovery that katanin may play a role in nucleating the central microtubules, there are many questions that remain to be answered about the nucleation of the central microtubules and the assembly of associated structures.
Footnotes
Editors: Wallace Marshall and Renata Basto
Additional Perspectives on Cilia available at www.cshperspectives.org
REFERENCES
- Adams GM, Huang B, Piperno G, Luck DJ. 1981. Central-pair microtubular complex of Chlamydomonas flagella: Polypeptide composition as revealed by analysis of mutants. J Cell Biol 91: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista-Harris G, Julia-Serda G, Rodriguez de Castro F, Santana-Benitez I, Cabrera-Navarro P. 2000. Absence of central microtubules and transposition in the ciliary apparatus of three siblings. Respiration 67: 449–452. [DOI] [PubMed] [Google Scholar]
- Bernstein M, Beech PL, Katz SG, Rosenbaum JL. 1994. A new kinesin-like protein (Klp1) localized to a single microtubule of the Chlamydomonas flagellum. J Cell Biol 125: 1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DA, Hildebrandt F. 2016. Ciliopathies. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a021891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhead R, Dawe HR, Farr H, Griffiths S, Hart SR, Portman N, Shaw MK, Ginger ML, Gaskell SJ, McKean PG, et al. 2006. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature 440: 224–227. [DOI] [PubMed] [Google Scholar]
- Brokaw CJ, Luck DJ, Huang B. 1982. Analysis of the movement of Chlamydomonas flagella: The function of the radial-spoke system is revealed by comparison of wild-type and mutant flagella. J Cell Biol 92: 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Marsala C, Kosoy R, Gaertig J. 1999. Kinesin-II is preferentially targeted to assembling cilia and is required for ciliogenesis and normal cytokinesis in Tetrahymena. Mol Biol Cell 10: 3081–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Fine NA, Pandiyan G, Thazhath R, Gaertig J. 2003. Hypoxia regulates assembly of cilia in suppressors of Tetrahymena lacking an intraflagellar transport subunit gene. Mol Biol Cell 14: 3192–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Dipetrillo CG, Smith EF, Witman GB. 2012. A FAP46 mutant provides new insights into the function and assembly of the C1d complex of the ciliary central apparatus. J Cell Sci 125: 3904–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne T, Lewis A, Dewar A, Luther P, Hogg C, Shoemark A, Dixon M. 2014. Characterizing the ultrastructure of primary ciliary dyskinesia transposition defect using electron tomography. Cytoskeleton 71: 294–301. [DOI] [PubMed] [Google Scholar]
- Carbajal-Gonzalez BI, Heuser T, Fu X, Lin J, Smith BW, Mitchell DR, Nicastro D. 2013. Conserved structural motifs in the central pair complex of eukaryotic flagella. Cytoskeleton 70: 101–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Bettencourt-Dias M. 2011. Evolution: Tracing the origins of centrioles, cilia, and flagella. J Cell Biol 194: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Santos Z, Machado P, Alvarez-Martins I, Gouveia SM, Jana SC, Duarte P, Amado T, Branco P, Freitas MC, Silva ST, et al. 2012. BLD10/CEP135 is a microtubule-associated protein that controls the formation of the flagellum central microtubule pair. Dev Cell 23: 412–424. [DOI] [PubMed] [Google Scholar]
- Chasey D. 1969. Observations on the central pair of microtubules from the cilia of Tetrahymena pyriformis. J Cell Sci 5: 453–458. [DOI] [PubMed] [Google Scholar]
- Chilvers MA, Rutman A, O’Callaghan C. 2003. Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. J Allergy Clin Immunol 112: 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallai R, Mercati D, Bu Y, Yin YW, Callaini G, Riparbelli MG. 2010. The spermatogenesis and sperm structure of Acerentomon microrhinus (Protura, Hexapoda) with considerations on the phylogenetic position of the taxon. Zoomorphology 129: 61–80. [Google Scholar]
- Davy BE, Robinson ML. 2003. Congenital hydrocephalus in hy3 mice is caused by a frameshift mutation in Hydin, a large novel gene. Hum Mol Genet 12: 1163–1170. [DOI] [PubMed] [Google Scholar]
- Dentler WL. 1984. Attachment of the cap to the central microtubules of Tetrahymena cilia. J Cell Sci 66: 167–173. [DOI] [PubMed] [Google Scholar]
- Dentler WL, Rosenbaum JL. 1977. Flagellar elongation and shortening in Chlamydomonas. III: Structures attached to the tips of flagellar microtubules and their relationship to the directionality of flagellar microtubule assembly. J Cell Biol 74: 747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener DR, Yang P, Geimer S, Cole DG, Sale WS, Rosenbaum JL. 2011. Sequential assembly of flagellar radial spokes. Cytoskeleton 68: 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPetrillo C, Smith E. 2009. Calcium regulation of ciliary motility analysis of axonemal calcium-binding proteins. Method Cell Biol 92: 163–180. [DOI] [PubMed] [Google Scholar]
- DiPetrillo CG, Smith EF. 2010. Pcdp1 is a central apparatus protein that binds Ca2+-calmodulin and regulates ciliary motility. J Cell Biol 189: 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPetrillo CG, Smith EF. 2011. The Pcdp1 complex coordinates the activity of dynein isoforms to produce wild-type ciliary motility. Mol Biol Cell 22: 4527–4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher SK, Huang B, Luck DJ. 1984. Genetic dissection of the central pair microtubules of the flagella of Chlamydomonas reinhardtii. J Cell Biol 98: 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymek EE, Smith EF. 2012. PF19 encodes the p60 catalytic subunit of katanin and is required for assembly of the flagellar central apparatus in Chlamydomonas. J Cell Sci 125: 3357–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymek EE, Lefebvre PA, Smith EF. 2004. PF15p is the Chlamydomonas homologue of the Katanin p80 subunit and is required for assembly of flagellar central microtubules. Eukaryotic Cell 3: 870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowkes ME, Mitchell DR. 1998. The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits. Mol Biol Cell 9: 2337–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox LA, Sawin KE, Sale WS. 1994. Kinesin-related proteins in eukaryotic flagella. J Cell Sci 107: 1545–1550. [DOI] [PubMed] [Google Scholar]
- Friderich G, Klumpp S, Russell CB, Hinrichsen RD, Kellner R, Schultz JE. 1992. Purification, characterization and structure of protein phosphatase 1 from the cilia of Paramecium tetraurelia. Eur J Biochem 209: 43–49. [DOI] [PubMed] [Google Scholar]
- Gardiner J, Marc J. 2011. Arabidopsis thaliana, a plant model organism for the neuronal microtubule cytoskeleton? J Exp Botany 62: 89–97. [DOI] [PubMed] [Google Scholar]
- Goduti DJ, Smith EF. 2012. Analyses of functional domains within the PF6 protein of the central apparatus reveal a role for PF6 sub-complex members in regulating flagellar beat frequency. Cytoskeleton 69: 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough UW, Heuser JE. 1985. Substructure of inner dynein arms, radial spokes, and the central pair/projection complex of cilia and flagella. J Cell Biol 100: 2008–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermacher G, Sale WS. 1996. Regulation of flagellar dynein by an axonemal type-1 phosphatase in Chlamydomonas. J Cell Sci 109: 1899–1907. [DOI] [PubMed] [Google Scholar]
- Hartman JJ, Mahr J, McNally K, Okawa K, Iwamatsu A, Thomas S, Cheesman S, Heuser J, Vale RD, McNally FJ. 1998. Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell 93: 277–287. [DOI] [PubMed] [Google Scholar]
- Hopkins JM. 1970. Subsidiary components of the flagella of Chlamydomonas reinhardii. J Cell Sci 7: 823–839. [DOI] [PubMed] [Google Scholar]
- Howard DR, Habermacher G, Glass DB, Smith EF, Sale WS. 1994. Regulation of Chlamydomonas flagellar dynein by an axonemal protein kinase. J Cell Biol 127: 1683–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Ramanis Z, Luck DJ. 1982. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for flagellar function. Cell 28: 115–124. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Haas MA, Rosenbaum JL. 1994. Localization of a kinesin-related protein to the central pair apparatus of the Chlamydomonas reinhardtii flagellum. J Cell Sci 107: 1551–1556. [DOI] [PubMed] [Google Scholar]
- Kamiya R. 1982. Extrusion and rotation of the central-pair microtubules in detergent-treated Chlamydomonas flagella. Prog Clin Biol Res 80: 169–173. [DOI] [PubMed] [Google Scholar]
- Karabay A, Yu W, Solowska JM, Baird DH, Baas PW. 2004. Axonal growth is sensitive to the levels of katanin, a protein that severs microtubules. J Neuroscience 24: 5778–5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikushima K. 2009. Central pair apparatus enhances outer-arm dynein activities through regulation of inner-arm dyneins. Cell Motil Cytoskeleton 66: 272–280. [DOI] [PubMed] [Google Scholar]
- Lechtreck KF, Witman GB. 2007. Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J Cell Biol 176: 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF, Delmotte P, Robinson ML, Sanderson MJ, Witman GB. 2008. Mutations in Hydin impair ciliary motility in mice. J Cell Biol 180: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF, Gould TJ, Witman GB. 2013. Flagellar central pair assembly in Chlamydomonas reinhardtii. Cilia 2: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Campagna DR, Pinkus JL, Mulhern H, Wyatt TA, Sisson JH, Pavlik JA, Pinkus GS, Fleming MD. 2008. Primary ciliary dyskinesia in mice lacking the novel ciliary protein Pcdp1. Mol Cell Biol 28: 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann CB. 1994. A model of flagellar and ciliary functioning which uses the forces transverse to the axoneme as the regulator of dynein activation. Cell Motil Cytoskeleton 29: 141–154. [DOI] [PubMed] [Google Scholar]
- Lindemann CB. 2003. Structural–functional relationships of the dynein, spokes, and central-pair projections predicted from an analysis of the forces acting within a flagellum. Biophys J 84: 4115–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann CB. 2007. The geometric clutch as a working hypothesis for future research on cilia and flagella. Ann NY Acad Sci 1101: 477–493. [DOI] [PubMed] [Google Scholar]
- Lindemann CB, Kanous KS. 1995. “Geometric clutch” hypothesis of axonemal function: Key issues and testable predictions. Cell Motil Cytoskeleton 31: 1–8. [DOI] [PubMed] [Google Scholar]
- Lindemann CB, Lesich KA. 2015. The geometric clutch at 20: Stripping gears or gaining traction? Reproduction 150: R45–R53. [DOI] [PubMed] [Google Scholar]
- Lindemann CB, Mitchell DR. 2007. Evidence for axonemal distortion during the flagellar beat of Chlamydomonas. Cell Motil Cytoskeleton 64: 580–589. [DOI] [PubMed] [Google Scholar]
- Manton I. 1966. Observations on the fine structure of the male gamete of the marine centric diatom Lithodesmium undulatum. J R Microscopical Soc 85: 119–134. [Google Scholar]
- McKean PG, Baines A, Vaughan S, Gull K. 2003. γ-Tubulin functions in the nucleation of a discrete subset of microtubules in the eukaryotic flagellum. Curr Biol 13: 598–602. [DOI] [PubMed] [Google Scholar]
- McKenzie CW, Craige B, Kroeger TV, Finn R, Wyatt TA, Sisson JH, Pavlik JA, Strittmatter L, Hendricks GM, Witman GB, et al. 2015. CFAP54 is required for proper ciliary motility and assembly of the central pair apparatus in mice. Mol Biol Cell 26: 3140–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally KP, Bazirgan OA, McNally FJ. 2000. Two domains of p80 katanin regulate microtubule severing and spindle pole targeting by p60 katanin. J Cell Sci 113: 1623–1633. [DOI] [PubMed] [Google Scholar]
- Mitchell DR. 2003a. Orientation of the central pair complex during flagellar bend formation in Chlamydomonas. Cell Motil Cytoskeleton 56: 120–129. [DOI] [PubMed] [Google Scholar]
- Mitchell DR. 2003b. Reconstruction of the projection periodicity and surface architecture of the flagellar central pair complex. Cell Motil Cytoskeleton 55: 188–199. [DOI] [PubMed] [Google Scholar]
- Mitchell DR. 2004. Speculations on the evolution of 9+2 organelles and the role of central pair microtubules. Biol Cell 96: 691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DR, Nakatsugawa M. 2004. Bend propagation drives central pair rotation in Chlamydomonas reinhardtii flagella. J Cell Biol 166: 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DR, Sale WS. 1999. Characterization of a Chlamydomonas insertional mutant that disrupts flagellar central pair microtubule-associated structures. J Cell Biol 144: 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BF, Pedersen LB, Feely M, Rosenbaum JL, Mitchell DR. 2005. ATP production in Chlamydomonas reinhardtii flagella by glycolytic enzymes. Mol Biol Cell 16: 4509–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano I, Kobayashi T, Yoshimura M, Shingyoji C. 2003. Central-pair-linked regulation of microtubule sliding by calcium in flagellar axonemes. J Cell Sci 116: 1627–1636. [DOI] [PubMed] [Google Scholar]
- Nakazawa Y, Hiraki M, Kamiya R, Hirono M. 2007. SAS-6 is a cartwheel protein that establishes the 9-fold symmetry of the centriole. Curr Biol 17: 2169–2174. [DOI] [PubMed] [Google Scholar]
- Nakazawa Y, Ariyoshi T, Noga A, Kamiya R, Hirono M. 2014. Space-dependent formation of central pair microtubules and their interactions with radial spokes. PLoS ONE 9: e110513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson LI, Schneider PA, Van Deerlin PG, Kiriakidou M, Driscoll DA, Pellegrini MC, Millinder S, Yamamoto KK, French CK, Strauss JF III. 1999. cDNA cloning and characterization of a human sperm antigen (SPAG6) with homology to the product of the Chlamydomonas PF16 locus. Genomics 60: 272–280. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. 1998. Randomization of left–right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95: 829–837. [DOI] [PubMed] [Google Scholar]
- Oda T, Yanagisawa H, Yagi T, Kikkawa M. 2014. Mechanosignaling between central apparatus and radial spokes controls axonemal dynein activity. J Cell Biol 204: 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okagaki T, Kamiya R. 1986. Microtubule sliding in mutant Chlamydomonas axonemes devoid of outer or inner dynein arms. J Cell Biol 103: 1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich H, Schmidts M, Werner C, Onoufriadis A, Loges NT, Raidt J, Banki NF, Shoemark A, Burgoyne T, Al Turki S, et al. 2012. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left–right body asymmetry. Am J Hum Genet 91: 672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto CK, Kung C. 1980. Rotation and twist of the central-pair microtubules in the cilia of Paramecium. J Cell Biol 87: 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto CK, Gibbons IR, Kamiya R, Shingyoji C, Takahashi K, Witman GB. 1999. Rotation of the central pair microtubules in eukaryotic flagella. Mol Biol Cell 10: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M, Berg S, Reynolds AB. 1994. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell 76: 789–791. [DOI] [PubMed] [Google Scholar]
- Piperno G, Mead K, Shestak W. 1992. The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J Cell Biol 118: 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Mead K, LeDizet M, Moscatelli A. 1994. Mutations in the “dynein regulatory complex” alter the ATP-insensitive binding sites for inner arm dyneins in Chlamydomonas axonemes. J Cell Biol 125: 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Power J, Dutcher SK. 1992. Extragenic suppressors of paralyzed flagellar mutations in Chlamydomonas reinhardtii identify loci that alter the inner dynein arms. J Cell Biol 118: 1163–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Knott JA, Gardner LC, Mitchell DR, Dutcher SK. 1994. Mutations in the SUP-PF-1 locus of Chlamydomonas reinhardtii identify a regulatory domain in the β-dynein heavy chain. J Cell Biol 126: 1495–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringo DL. 1967. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J Cell Biol 33: 543–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp G, O’Toole E, Porter ME. 2001. The Chlamydomonas PF6 locus encodes a large alanine/proline-rich polypeptide that is required for assembly of a central pair projection and regulates flagellar motility. Mol Biol Cell 12: 739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapiro R, Kostetskii I, Olds-Clarke P, Gerton GL, Radice GL, Strauss IJ. 2002. Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol Cell Biol 22: 6298–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrevel J, Besse C. 1975. A functional flagella with a 6+0 pattern. J Cell Biol 66: 492–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallman LA, Gregory J. 1986. Ultrastructural abnormalities of cilia in the human respiratory tract. Hum Pathol 17: 848–855. [DOI] [PubMed] [Google Scholar]
- Smith EF. 2002a. Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase. Mol Biol Cell 13: 3303–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EF. 2002b. Regulation of flagellar dynein by the axonemal central apparatus. Cell Motil Cytoskeleton 52: 33–42. [DOI] [PubMed] [Google Scholar]
- Smith EF, Lefebvre PA. 1996. PF16 encodes a protein with armadillo repeats and localizes to a single microtubule of the central apparatus in Chlamydomonas flagella. J Cell Biol 132: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EF, Lefebvre PA. 1997. PF20 gene product contains WD repeats and localizes to the intermicrotubule bridges in Chlamydomonas flagella. Mol Biol Cell 8: 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EF, Lefebvre PA. 2000. Defining functional domains within PF16: A central apparatus component required for flagellar motility. Cell Motil Cytoskeleton 46: 157–165. [DOI] [PubMed] [Google Scholar]
- Smith EF, Sale WS. 1992. Regulation of dynein-driven microtubule sliding by the radial spokes in flagella. Science 257: 1557–1559. [DOI] [PubMed] [Google Scholar]
- Stannard W, Rutman A, Wallis C, O’Callaghan C. 2004. Central microtubular agenesis causing primary ciliary dyskinesia. Am J Respir Crit Care Med 169: 634–637. [DOI] [PubMed] [Google Scholar]
- Summers KE, Gibbons IR. 1971. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc Natl Acad Sci 68: 3092–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves ME, Zhang Z, Costanzo RM, Henderson SC, Corwin FD, Zweit J, Sundaresan G, Subler M, Salloum FN, Rubin BK, et al. 2013. Sperm-associated antigen-17 gene is essential for motile cilia function and neonatal survival. Am J Respir Cell Mol Biol 48: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves ME, Sears PR, Li W, Zhang Z, Tang W, van Reesema L, Costanzo RM, Davis CW, Knowles MR, Strauss JF III, et al. 2014. Sperm-associated antigen 6 (SPAG6) deficiency and defects in ciliogenesis and cilia function: Polarity, density, and beat. PLoS ONE 9: e107271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves ME, Sundaresan G, Cohen DJ, Hyzy SL, Kajan I, Maczis M, Zhang Z, Costanzo RM, Zweit J, Schwartz Z, et al. 2015. Spag17 deficiency results in skeletal malformations and bone abnormalities. PLoS ONE 10: e0125936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves ME, Nagarkatti-Gude DR, Zhang Z, Strauss JF III. 2016. Mammalian axoneme central pair complex proteins: Broader roles revealed by gene knockout phenotypes. Cytoskeleton 73: 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thazhath R, Jerka-Dziadosz M, Duan J, Wloga D, Gorovsky MA, Frankel J, Gaertig J. 2004. Cell context-specific effects of the β-tubulin glycylation domain on assembly and size of microtubular organelles. Mol Biol Cell 15: 4136–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanadha R, Hunter EL, Yamamoto R, Wirschell M, Alford LM, Dutcher SK, Sale WS. 2014. The ciliary inner dynein arm, I1 dynein, is assembled in the cytoplasm and transported by IFT before axonemal docking. Cytoskeleton 71: 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargo MJ, Smith EF. 2003. Asymmetry of the central apparatus defines the location of active microtubule sliding in Chlamydomonas flagella. Proc Natl Acad Sci 100: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargo MJ, Dymek EE, Smith EF. 2005. Calmodulin and PF6 are components of a complex that localizes to the C1 microtubule of the flagellar central apparatus. J Cell Sci 118: 4655–4665. [DOI] [PubMed] [Google Scholar]
- Warner FD, Satir P. 1974. The structural basis of ciliary bend formation. Radial spoke positional changes accompanying microtubule sliding. J Cell Biol 63: 35–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirschell M, Yamamoto R, Alford L, Gokhale A, Gaillard A, Sale WS. 2011. Regulation of ciliary motility: Conserved protein kinases and phosphatases are targeted and anchored in the ciliary axoneme. Arch Biochem Biophys 510: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman GB, Plummer J, Sander G. 1978. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J Cell Biol 76: 729–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren KN, Craft JM, Tritschler D, Schauer A, Patel DK, Smith EF, Porter ME, Kner P, Lechtreck KF. 2013. A differential cargo-loading model of ciliary length regulation by IFT. Curr Biol 23: 2463–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Sale WS. 2000. Casein kinase I is anchored on axonemal doublet microtubules and regulates flagellar dynein phosphorylation and activity. J Biol Chem 275: 18905–18912. [DOI] [PubMed] [Google Scholar]
- Yang P, Fox L, Colbran RJ, Sale WS. 2000. Protein phosphatases PP1 and PP2A are located in distinct positions in the Chlamydomonas flagellar axoneme. J Cell Sci 113: 91–102. [DOI] [PubMed] [Google Scholar]
- Yokoyama R, O’Toole E, Ghosh S, Mitchell DR. 2004. Regulation of flagellar dynein activity by a central pair kinesin. Proc Natl Acad Sci 101: 17398–17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Shingyoji C. 1999. Effects of the central pair apparatus on microtubule sliding velocity in sea urchin sperm flagella. Cell Struct Funct 24: 43–54. [DOI] [PubMed] [Google Scholar]
- Yu W, Solowska JM, Qiang L, Karabay A, Baird D, Baas PW. 2005. Regulation of microtubule severing by katanin subunits during neuronal development. J Neurosci 25: 5573–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Mitchell DR. 2004. Cpc1, a Chlamydomonas central pair protein with an adenylate kinase domain. J Cell Sci 117: 4179–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Sapiro R, Kapfhamer D, Bucan M, Bray J, Chennathukuzhi V, McNamara P, Curtis A, Zhang M, Blanchette-Mackie EJ, et al. 2002. A sperm-associated WD repeat protein orthologous to Chlamydomonas PF20 associates with Spag6, the mammalian orthologue of Chlamydomonas PF16. Mol Cell Biol 22: 7993–8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Jones BH, Tang W, Moss SB, Wei Z, Ho C, Pollack M, Horowitz E, Bennett J, Baker ME, et al. 2005. Dissecting the axoneme interactome: The mammalian orthologue of Chlamydomonas PF6 interacts with sperm-associated antigen 6, the mammalian orthologue of Chlamydomonas PF16. Mol Cell Proteomics 4: 914–923. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Kostetskii I, Tang W, Haig-Ladewig L, Sapiro R, Wei Z, Patel AM, Bennett J, Gerton GL, Moss SB, et al. 2006. Deficiency of SPAG16L causes male infertility associated with impaired sperm motility. Biol Reprod 74: 751–759. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tang W, Zhou R, Shen X, Wei Z, Patel AM, Povlishock JT, Bennett J, Strauss JF 3rd. 2007a. Accelerated mortality from hydrocephalus and pneumonia in mice with a combined deficiency of SPAG6 and SPAG16L reveals a functional interrelationship between the two central apparatus proteins. Cell Motil Cytoskeleton 64: 360–376. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zariwala MA, Mahadevan MM, Caballero-Campo P, Shen X, Escudier E, Duriez B, Bridoux AM, Leigh M, Gerton GL, et al. 2007b. A heterozygous mutation disrupting the SPAG16 gene results in biochemical instability of central apparatus components of the human sperm axoneme. Biol Reprod 77: 864–871. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Li Z. 2015. γ-Tubulin complex in Trypanosoma brucei: Molecular composition, subunit interdependence and requirement for axonemal central pair protein assembly. Mol Microbiol 98: 667–680. [DOI] [PMC free article] [PubMed] [Google Scholar]