Abstract
Endocrine disruptors are critical environmental exposures that influence health and can promote epigenetic transgenerational inheritance of disease and abnormal physiology. Advances in 2015 included analyses of the effects of endocrine disruptors on human disease, further examples of endocrine disruptors promoting transgenerational behavioural effects, insights into effects of endocrine disruptors on epigenetic programming of primordial germ cells and the finding that endocrine disruptors can transgenerationally promote genetic mutations.
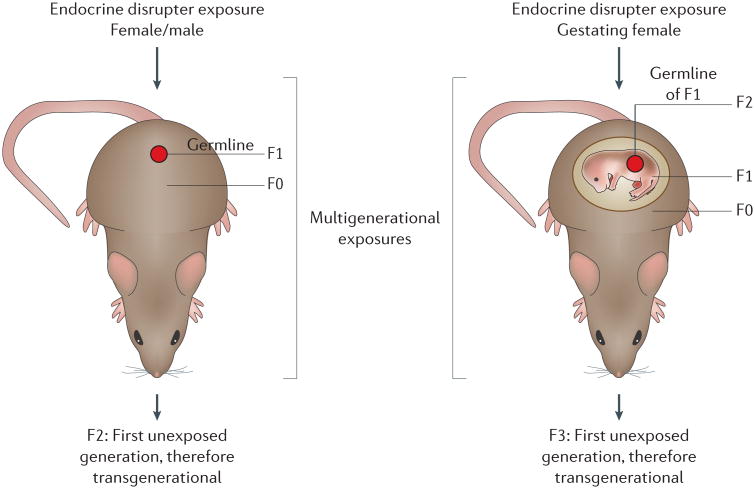
Environmental compounds that alter and/or disrupt normal endocrine hormone signalling at the receptor or signal transduction level are termed endocrine disruptors. The first endo crine disruptors studied and shown to promote abnormal physiology and disease were diethyl stilbestrol (DES) and dichlorodiphenyltrichloroethane (DDT)1. The number of known endocrine disruptors has since expanded dramatically to include compounds such as bisphenol A (BPA) and phthalates; natural compounds, such as genistein from plants, can also act as endocrine disruptors1. Over the past several decades, research on endocrine disruptors has improved our under standing of the molecular and physiological actions of these agents on human health1. In addition to the direct effects of exposure on an individual, molecular alterations to the germ line can promote effects on subsequent generations. As most exposures to endocrine disruptors do not promote genetic mutations, these generational effects are mediated via epigenetic mech anisms. When the effects of an endocrine disruptor alters the epigenetic programming of the germ line, these changes are transmitted between generations in the absence of direct exposure (FIG. 1), an effect termed epigenetic transgenerational inheritance1.
Figure 1. Endocrine-disruptor-induced epigenetic transgenerational inheritance.
Schematic representation of environmental exposure and affected generations.
The initial observation of epigenetic transgenerational inheritance involved the endocrine disruptor vinclozolin, an antiandrogenic agricultural fungicide2. Vinclozolin and the pesticide methoxychlor promote the epi genetic transgenerational inheritance of reduced male fertility2. A large number of other endocrine disruptors and environ mental exposures have now also been shown to promote epigenetic transgenerational inheritance of disease and abnormal physiology in a wide variety of species from plants to humans1. This process involves epigenetic alterations of the germ line that can include DNA methylation, non-coding RNAs, histone modifications and alterations in chromatin structure. The effects range from reproductive and behavioural effects to obesity1. This nongenetic form of inheritance has altered our understanding of the molecular control of disease aetiology and evolution. Here, I focus on advances in 2015 involving endocrine disruptors and epigenetic transgenerational inheritance.
A large epidemiology study published in 2015 by Kalfa et al.3 extended the findings of a previous study reporting the effects of phthalates on humans4 by demonstrating the associ ation of human hypospadias with prenatal exposure to a variety of endocrine disruptors. This French study examined a cohort of 300 consecutive children without a genetic defect and found that after control for genetic mutation, parental occupational and environ mental exposure to chemical products increased the risk of hypospadias in children3. Although Kalfa and colleagues focused on exposure in children, a future consideration is if such effects might influence epigenetic transgenerational inheritance mechanisms.
Another advance in 2015 further documented the generational effect of endocrine disruptors on brain development and behaviour. Quinnies and colleagues demonstrated the transgenerational actions of the phthalate di-(2-ethylhexyl)phthalate (DEHP) on levels of stress hormones and behaviour5. Gestating mice were exposed to DEHP during fetal gonadal sex determination and the subsequent third generation (F3) had altered stress hormone levels (corticosterone), pitui tary gene expression and behaviour in both male and female mice5. A number of previous studies have demonstrated the trans generational actions of endocrine disruptors on behaviour1, and a recent review6 focusing on the neuro science of the phenomena supports the concept that epigenetic mechanisms might inform us about the transgenerational inheritance of behavioural traits that are increasingly being reported.
In considering the molecular mechanisms underlying endocrine-disruptor-induced epigenetic transgenerational inheritance of disease and phenotypic variation, the germ line transmission of epigenetic information between generations in the absence of continued exposure is critical1 (FIG. 1). The original observations suggested that DNA methylation alterations in the sperm were crucial2; noncoding RNAs and histone modifications have, subsequently, also been shown to be involved1. Epigenetic reprogramming of the germ line primarily involves the primordial germ cell (PGC) development period and later stages of gametogenesis. Brieno-Enriquez and colleagues exposed gestating female mice to vinclozolin to produce epigenetic transgenerational inheritance of testicular cell apoptosis and abnormalities7. This study confirmed the observations and transgenerational phenotypes previously observed in a rat model2. Brieno-Enriquez et al. extended the previous observations with an analysis of PGCs and identified alterations in epigenetic programming and gene expression that are critical to PGC development (such as those in Blimp1). Although the global DNA methylation analysis used was insufficient to assess specific DNA methylation sites, this study7 demonstrated interesting alterations in noncoding RNAs such as miR-23b and miR-21. Brieno-Enriquez and colleagues also showed that vinclozolin promotes epigenetic transgenerational inheritance of abnormalities in male testes and alters PGC noncoding RNA programming. A supporting study provided a major resource for epigenetic alterations during development of the human germ line epigenome8. This study identified a critical role for the Blimp-1 pathway, DNA methylation reprogramming and gene expression alterations that occur during normal develop ment of PGCs and the subsequent germ line8. Specific DNA methylation sites that escaped DNA methylation erasure, termed ‘escapees’, were also identified and support a role for altered germ line DNA methylation in epigenetic transgenerational inheritance8. This supporting study provides additional mechanistic insights into the study of Brieno-Enriquez and colleagues7. Although science today has a strong reductionist view that tends to choose one process over another, the epigenetic control of transgenerational inheritance involves the integrated actions of DNA methylation, noncoding RNAs and histone modifications. These epigenetic processes are so interlinked that they must be viewed as integrated rather than disconnected. The observation that PGCs and the developing germ line undergo major epigenetic programming, which can be transgenerationally altered by endocrine disruptors7, was a significant advance in 2015.
Endocrine-disruptor-induced epigenetic transgenerational inheritance of germ line epimutations has a critical role in this form of nongenetic inheritance1. Previous studies have shown that susceptibility to genetic mutations is increased by epigenetic alterations such as CpG methylation that promotes C to T conversions (point mutations), DNA methylation that influences repeat element copy number variation (CNV) and transposable element movement, and histone modifications and DNA methylation that alter chromosome translocation breakpoints. The role of genetics in epigenetic transgenerational inheritance is, thus, important. In 2015, my colleagues and I showed that vinclozolin promotes epigenetic transgenerational inheritance of genetic mutations in sperm (that is, CNV)9. In the directly exposed F1 generation, no change in CNV was seen, but in the F3 generation a significant increase in CNV was observed9. Transgenerational alterations in the epigenome, therefore, increase genetic instability and promote genetic mutation and variation. Transgenerational mechanisms and phenotypes will probably involve a combination of epigenetics and genetics, as genetics and epigenetics cannot be separated9. Further studies are now needed to elucidate this process, which might have a critical role in environmentally influenced disease aetiology and evolution.
The effects of endocrine disruptors on epigenetic programming during development provides a molecular mechanism for the development of disease later in life1. In the event that germ line epigenetic programming is altered, this change can lead to environmentally induced epigenetic transgenerational inheritance of disease and phenotypic variation. Although the majority of environmental exposures are not endocrine disruptors, in today's society, endocrine disruptors are an important source of contamination. The advances in 2015 discussed here support critical effects of endocrine disruptors on human health and in inducing epigenetic transgenerational inheritance, and increase our understanding of the molecular processes involved. This form of nongenetic inheritance that is environmentally responsive affects all of biology from disease aetiology to evolution.
Key advances.
Prenatal exposure to endocrine disruptors is associated with human hypospadias3
Epigenetic transgenerational inheritance of behavioural abnormalities is induced by the phthalate di-(2-ethylhexyl)phthalate5
Vinclozolin induces epigenetic transgenerational inheritance of primordial germ cell epigenetic programming via noncoding RNAs and alterations in gene expression7
Endocrine disruptors induce epigenetic transgenerational inheritance of genetic mutations in sperm9
Acknowledgments
The author's work is supported by grants from the NIH and the Templeton Foundation.
Footnotes
Competing interests statement: The author declares no competing interests.
References
- 1.Skinner MK. Endocrine disruptor induction of epigenetic transgenerational inheritance of disease. Mol Cell Endocrinol. 2014;398:4–12. doi: 10.1016/j.mce.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalfa N, et al. Is hypospadias associated with prenatal exposure to endocrine disruptors? A French collaborative controlled study of a cohort of 300 consecutive children without genetic defect. Eur Urol. 2015;68:1023–1030. doi: 10.1016/j.eururo.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinnies KM, Doyle TJ, Kim KH, Rissman EF. Transgenerational effects of di-(2-ethylhexyl) phthalate (DEHP) on stress hormones and behavior. Endocrinology. 2015;156:3077–3083. doi: 10.1210/EN.2015-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dias BG, Maddox SA, Klengel T, Ressler KJ. Epigenetic mechanisms underlying learning and the inheritance of learned behaviors. Trends Neurosci. 2015;38:96–107. doi: 10.1016/j.tins.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brieno-Enriquez MA, et al. Exposure to endocrine disruptor induces transgenerational epigenetic deregulation of microRNAs in primordial germ cells. PLoS ONE. 2015;10:e0124296. doi: 10.1371/journal.pone.0124296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang WW, et al. A unique gene regulatory network resets the human germline epigenome for development. Cell. 2015;161:1453–1467. doi: 10.1016/j.cell.2015.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skinner MK, Guerrero-Bosagna C, Haque MM. Environmentally induced epigenetic transgenerational inheritance of sperm epimutations promote genetic mutations. Epigenetics. 2015;10:762–771. doi: 10.1080/15592294.2015.1062207. [DOI] [PMC free article] [PubMed] [Google Scholar]