Abstract
A double-stranded RNA (dsRNA) mycovirus was detected in malformed fruiting bodies of Pleurotus ostreatus strain ASI2792, one of bottle cultivated commercial strains of the edible oyster mushroom. The partial RNA-dependent RNA polymerase (RdRp) gene of the P. ostreatus ASI2792 mycovirus (PoV-ASI2792) was cloned, and a cDNA sequences alignment revealed that the sequence was identical to the RdRp gene of a known PoSV found in the P. ostreatus strain. To investigate the symptoms of PoV-ASI2792 infection by comparing the isogenic virus-free P. ostreatus strains with a virus-infected strain, isogenic virus-cured P. ostreatus strains were obtained by the mycelial fragmentation method for virus curing. The absence of virus was verified with gel electrophoresis after dsRNA-specific virus purification and Northern blot analysis using a partial RdRp cDNA of PoV-ASI2792. The growth rate and mycelial dry weight of virus-infected P. ostreatus strain with PoV-ASI2792 mycovirus were compared to those of three virus-free isogenic strains on 10 different media. The virus-cured strains showed distinctly higher mycelial growth rates and dry weights on all kinds of experimental culture media, with at least a 2.2-fold higher mycelial growth rate on mushroom complete media (MCM) and Hamada media, and a 2.7-fold higher mycelial dry weight on MCM and yeastmalt-glucose agar media than those of the virus-infected strain. These results suggest that the infection of PoV mycovirus has a deleterious effect on the vegetative growth of P. ostreatus.
Keywords: Curing, Isogenic strain, Mycovirus, Pleurotus ostreatus, Viral symptom
Mycoviruses (fungal viruses) have been reported in filamentous fungi and yeast for more than 50-years [1], and the presence of viruses in fungi is today recognized as not ‘exceptional’ but ‘common’ [2,3]. Viral infection occurs in all major taxa pertaining to the fungi kingdom. Although single-stranded RNA (ssRNA) mycoviruses are still detected with significant frequency, double-stranded RNA (dsRNA) genomes hold a large majority in characterized fungal mycoviruses. As a general rule, infection with a mycovirus does not cause phenotypic changes in the fungal host, and if anything, mycovirus remains latent or cryptic. Occasionally, however, a mycoviral infection can lead to substantial morphological and physiological changes, including changes in growth rate, colony morphology, spore production, pigmentation, and virulence-related phenotypes in fungal cells and tissues [2,4].
Pleurotus ostreatus cultivation has been occasionally damaged by viral infections of cultivated oyster mushroom. The symptoms of viral infection in P. ostreatus are revealed by reduced mycelial growth, delayed fruiting body formation, decreased fruiting body yield, and malformed fruiting body. dsRNA mycoviruses from P. ostreatus in Korea and China were isolated and characterized [5,6,7,8,9,10,11,12]. P. ostreatus viral diseases have been associated with several reported mycoviruses such as oyster mushroom spherical virus [11], oyster mushroom isomeric virus (OMIV) I and II [9,12], and so on [10]. However, investigations of fungus-mycovirus interactions have been hampered by the difficulty of virus curing and the lack of simple methods for artificial inoculation of mycoviruses.
Virus-free and virus-infected isogenic lines, which have identical genetic backgrounds, have been established to explore direct mycovirus-fungal host interactions because the diverse genetic backgrounds of fungal strains could dissimilarly respond to the same mycovirus. Although curing methods of virus-infected fungi have been attempted using cycloheximide or ribavirin treatment, hyphal tip cut, single spore or other subculture, protoplast regeneration, or incubation with temperature stress, these methods have not always resulted in mycovirus curing. Further, these stresses do damage to fungal growth as circumstances require, thus, these virus-cured strains are unsuitable for the comparison group to research on fungus-mycovirus interactions [13,14,15,16,17]. The appropriate virus-curing method was previously developed to research fungal symptoms in response to viral infection using the mycelial fragmentation method followed by single colony isolation [18,19].
In the present study, a dsRNA mycovirus was discovered from dysmorphic fruting bodies in P. ostreatus strain (ASI No. 2792) and the partial viral sequence was identified as the RNA-dependent RNA polymerase (RdRp) coding gene. Virus-cured isolates of P. ostreatus ASI2792 strain infected with PoV-ASI2792 dsRNA mycovirus were obtained and compared to their isogenic virus-infected strain to determine the range of the phenotypic variants in the fungal host P. osreatus caused by a dsRNA PoV-ASI2792.
MATERIALS AND METHODS
Fungal strains and growth condition
The malformed fruiting bodies of P. ostreatus ASI2792 strain were collected from a commercial mushroom farm in Pohang, Gyeongbuk, Korea. Internal organization of mushroom pileus were sliced aseptically with a sharp razor and cultured on potato dextrose agar (PDA) plates in darkness at 25℃ for 8 days. By this time the medium edge of the petriplate became covered with a white mycelial mat. All fungal cultures were fundamentally maintained on PDA plates with subculture and a mini culture for dsRNA purification [20] using mycelial agar-blocks (5 mm in diameter). The fungal strains were cultured on various media to examine radial growth and mycelial density in the same method.
dsRNA isolation
dsRNA miniprep method for the detection and purification [21,22] was applied with the slight modified method. The P. ostreatus isolates were cultured on top of cellophane-overlay, the mycelia were taken off from the overlays, and transferred to mortars containing nitrogen gas. The frozen mycelia were ground to a fine powder, and the dsRNA was purified from 450mg of frozen mycelial powder via whatman CC41 cellulose column (Whatman, Little Chalfont, UK). The dsRNA was dissolved in 40 µL RNase-free water. Individual dsRNA fragments were detected by electrophoresis on 1.0% agarose gels in TAE buffer (40 mM Tris-acetate, 1 mM EDTA, pH 8.0), visualized by ethidium bromide staining, and confirmed by dsRNA-specific RNase III and RNase A degradation [20].
Cloning and sequence analysis of the partial RdRp gene
To obtain a cDNA clone that corresponded to the mycovirus RdRp, cDNA synthesis was conducted as described procedure using a cDNA synthesis kit (Promega, Fitchburg, WI, USA) and reverse transcription-PCR (RT-PCR) was performed as described previously [18]. The primers were used (PV-RDRP forward, 5′-CCN NTN CAY TTT RYN GA-3′ and PV-RDRP reverse, 5′-SWR TCN ARN SWY TGN GT-3′). Degenerate primers specific for consensus nucleotide sequence corresponding to the conserved motifs of the known P. ostreatus mycovirus RdRps were designed and the PCR amplicon was cloned into a pGEM-T vector (Promega). The inserted DNA fragments of positive bacterial clones were sequenced using the dideoxynucleotide method and universal primers.
The dsRNA sequence of the RdRp region was aligned with similar sequences using the program Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) [23] and sequence similarity searches were conducted using the NCBI BLAST program (https://www.ncbi.nlm.nih.gov/blast/) [24].
Virus curing and Northern blot analysis
The mycelial fragmentation method for virus curing [21] was applied with the slight modified method. To cure mycoviruses from the P. ostreatus strain, after the mycelia infected with virus were prolongedly incubated in a PDA plate, the mycelia were obtained by scraping the 5-week-old plates with a glass rod in the 8 mL sterile water and filtering them through a nylon membrane filter (41 µm; Millipore, Billerica, MA, USA). Then, old mycelial fragments that lacked dsRNA segments were spread on PDA plates and cultured in darkness at 25℃ for 5 days. Virus-cured lines were selected by RT-PCR and Northern blot analysis using each purified dsRNA from fungal colonies.
Northern blot analysis for the detection of dsRNA was conducted as described previously [20]. Purified viral dsRNAs were separated on a 0.7% agarose gel in TAE buffer, denatured, and neutralized by the appropriate solution. The RNA was transferred to hybond-N+ nylon membranes (Amersham, Amersham, UK) via capillary blotting, and then cross-linked to the membrane by UV irradiation. Labeling, hybridization, and autoradiography were carried out as described previously [20].
Determination of growth rate and mycelial density
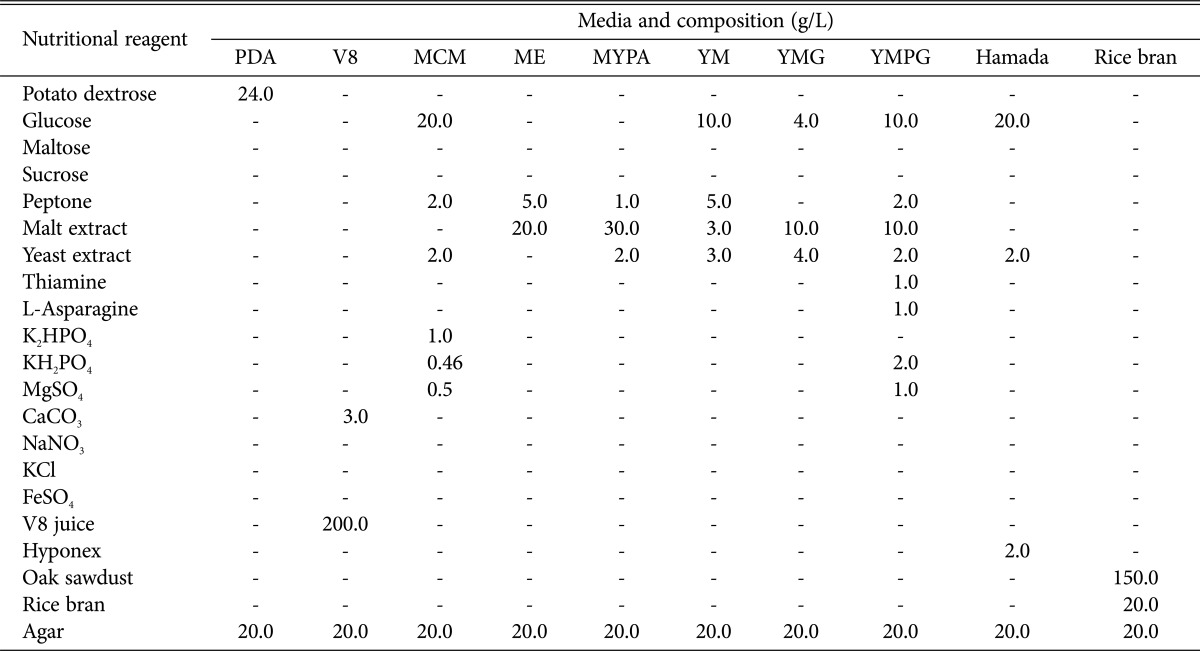
To compare growth rates and mycelial masses were measured as previously described [19]. The growth rate and mycelial dry weight of the selected virus-cured P. ostreatus lines were analyzed with those of the virus-infected P. ostreatus line using 10 different media (Table 1) [25]. Radial growth on each plate was evaluated every other day for 8 days by measuring the diameter of the colonies that originated from an inoculated agar plug (5-mm diameter) taken from the edge of an actively growing colony.
Table 1. Composition and concentration of culture media in this study.
PDA, potato dextrose agar; MCM, mushroom complete media; ME, malt extract agar; MYPA, malt-yeast-peptone agar; YM, yeast-malt agar; YMG, yeast-malt-glucose agar; YMPG, yeast-malt-peptone-glucose agar.
In order to assess the mycelial mass, P. ostreatus strains were cultured on top of cellophane overlays for 8 days, the mycelia were taken off from the overlays, and then dried at 70℃ for 12 hr. The mycelial dry mass of each fungus was weighed in a balance.
Statistical analyses
Statistical analyses were conducted with a one-way ANOVA using SPSS ver. 15 (SPSS, Chicago, IL, USA), with significant differences between P. ostreatus strains measured by the magnitude of the F value at p < 0.01 to determine the viral effects on both the growth rate and mycelial mass in various media. When a significant F value was obtained, the significance of the effects of the strains was determined by Duncan's multiple range tests at p < 0.01.
RESULTS AND DISCUSSION
Detection and identification of dsRNA from diseased P. ostreatus
Fruiting bodies of the healthy-looking P. ostreatus strains show typical phenotypes of oyster mushroom, such as a long and thick stipe, and a middle slate-gray pileus with a low funnel-shape [6,16]. Abnormal mycelial cells were obtained from the malformed fruiting body of P. ostreatus ASI2792 strain, comparing with a normal fruiting body, and were maintained on PDA with periodic transfer as described in Materials and Methods. The symptoms of mycoviruses infection in P. ostreatus are reduced mycelial growth, fruiting body abnormalities, and decreased fruiting body yield [9,10,11,12,16]. For that reason, a dsRNA purified with CC41 was visualized by 1.0% agarose gel electrophoresis and was confirmed to be resistant to DNase and RNase A at a high-salt concentration, but disappeared upon exposure to dsRNA-specific RNase A at low-salt concentrations to verify the presence of about 8.0-kb dsRNA fragment (data not shown). In addition, PoV-ASI2792 (the P. ostreatus ASI2792 mycovirus examined in this study) dsRNA was also detected when either the stipes or pilus were directly used as sources of cell tissues.
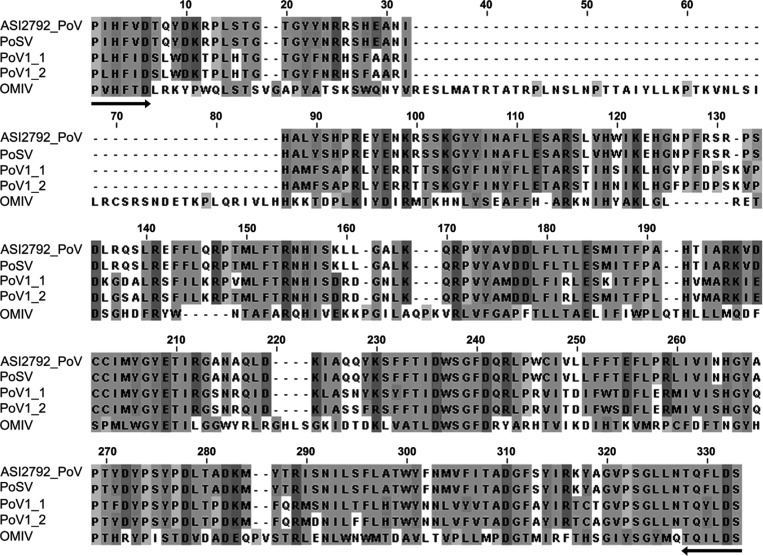
The 724-bp cDNA fragment that corresponded to the partial RdRp of the mycovirus in PoV-ASI2792 was amplified using degenerated primers derived from the conserved motifs of the deposited RdRp of P. ostreatus mycovirus in the GenBank database (PoSV, KX976461; PoV1-1, ACX43952.1; PoV1-2, YP227355.1; OMIV II, AAP74192.1), cloned into a pGEM T easy vector, and sequenced with universal primers. A sequence analysis of the clone revealed that it showed the 100.0% identity with the recently released sequences of an RdRp of the P. ostreatus strain PoSV mycovirus (unpublished data), which caused a mushroom disease. It also showed a wide range of similarity, with 68.2%, 66.3%, and 28.2% identity at the amino acid level to the RdRps of the P. ostreatus strain PoV1-2, PoV1-1, and OMIV II mycoviruses, respectively [7,8,9]. An alignment of conserved RdRp sequence motifs for the above-mentioned mycoviruses with that of PoV-ASI2792 is shown in Fig. 1. An abnormal P. ostreatus ASI2792 strain was infected with PoV-ASI2792, and the mycovirus was similar to established mycoviruses, that are known to cause a malformed fruiting body and an abnormal mycelial growth [7,8], except OMIV II mycovirus [9]. Mycoviruses of other mushrooms also cause severe mushroom diseases leading to a significant decrease in fruiting body yield and/or product values of mushroom. La France isometric virus [1] and mushroom bacilliform virus [26] were found in Agaricus bisporus, Pleurotus eryngii spherical virus is discovered in P. eryngii [27], and Lentinula edodes spherical virus [28] and Lentinula edodes mycovirus HKB are discovered in L. edodes [29]. These results indicate the possibility that PoV-ASI2792 of this study caused malformed fruiting bodies and abnormal mycelial growth.
Fig. 1. Alignment of the predicted amino acid sequences of the partial RNA-dependent RNA polymerase (RdRp) from Pleurotus ostreatus ASI2792 mycovirus (ASI2792-PoV) with other P. ostreatus mycoviruses. The amino acid sequences of P. ostreatus PoSV mycovirus (PoSV) (GenBank accession No. KX976461), P. ostreatus Chinese PoV1 mycovirus (PoV1-1) (GenBank accession No. ACX43952.1), P. ostreatus Korean PoV1 mycovirus (PoV1-2) (GenBank accession No. YP227355.1), and P. ostreatus OMIV mycovirus (OMIV II) (GenBank accession No. AAP74192.1) were aligned and the amino acid identities were shaded using Jalview 2 [31]. Identical amino acid residues are indicated by black boxes, whereas similar amino acids are indicated by gray boxes. Conserved regions used for primer design are marked by arrows.
Curing of PoV-ASI2792 virus from P. ostreatus
It is uncertain whether mycoviruses of mushrooms are the immediate cause of abnormalities because they are occasionally detected in asymptomatic mycelia and fruiting bodies. Furthermore, whether the presence of a specific mycovirus is closely associated with the vegetative characteristics of mycelia and formation of fruiting bodies has been investigated [30]. Preparation of virus-free isogenic lines is essential to verifing fungal symptoms of PoV-ASI2792 infection by comparison of isogenic virus-free P. ostreatus strains with a virus-infected strain. For pair-wise comparisons of PoV-ASI2792 virus-infected and virus-free lines, based on the mycelial fragmentation method [21], we intended to cure P. ostreatus ASI2792 strain infected with PoV-ASI2792 virus in order to establish virus-infected and its virus-free isogenic strain.
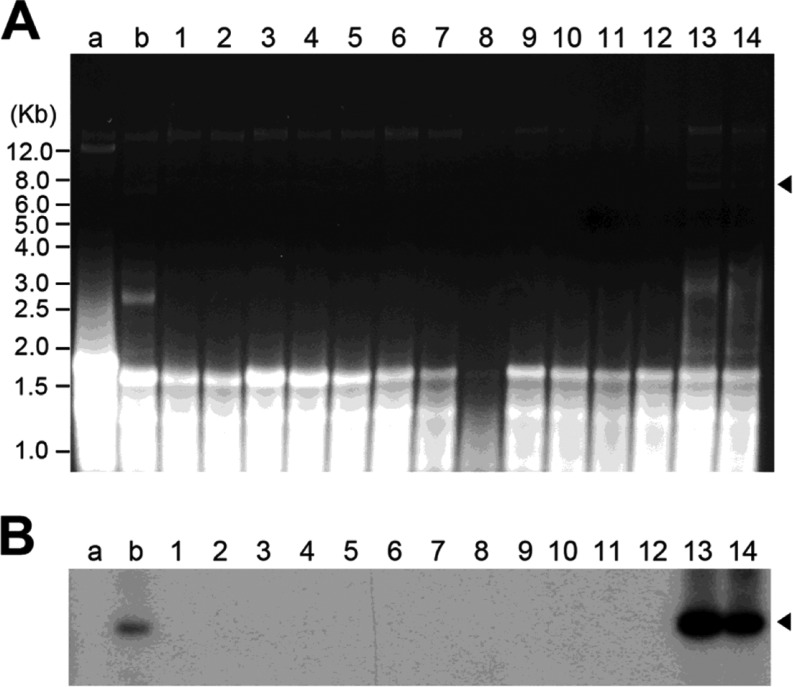
The mycelium of the P. ostreatus strain infected with PoV-ASI2792, which had not been treated with any chemical agent, was scraped to form a suspension of hyphal fragments consisting of one to five cells, that were then was spread on plates. The hyphal growth rates of the virus-cured strains increased slightly relative to that of the original P. ostreatus and L. edodes strains [8,17,19]. Thus, a total of 80 single-isolated colonies were obtained, and 14 of those were selected on the basis of their normal phenotypes, there were always some hyphal fragments that grew better than the parental virus-infected strain. The absence of dsRNA mycovirus of virus-cured lines was based on electrophoretic banding pattern and Northern blot analysis of purified dsRNA by conventional CC41 cellulose chromatography. As shown in Fig. 2, more than 80% of healthy-looking isolates from mycelial fragments did not contain the dsRNA. Confirmations of virus-curing were performed at least five times for each preparation of mycelial fragments. In addition, the absence of dsRNA was confirmed by several detection methods, and cured-isolates showed no mycovirus recurrence over many subcultures. The virus-cured lines of the P. ostreatus ASI2792 strain were successfully isolated from old mycelia of abnormally formed fruiting bodies.
Fig. 2. A, Ethidium bromide-stained agarose gel of dsRNA. Lanes a and b contain dsRNA extracts from two representative strains of Lentinula edodes FMRI0339 mycovirus (LeV-FMRI0339) and Pleurotus ostreatus ASI2792 mycovirus (ASI2792-PoV) with the characteristic 12-kb viral genome of LeV and approximately 8.0-kb viral genome of ASI2792-PoV, respectively. Lanes 1 to 14 contain dsRNA extracts from 14 isolates obtained by the mycelia fragmentation method described in “Materials and Methods”; B, Northern blot analysis of the corresponding gel with the dsRNA using a partial cDNA clone (792 bp) encoding the RNA-dependent RNA polymerase (RdRp) protein in the PoV-ASI2792 as a probe. Arrowheads indicate the mycovirus genome segment from PoV-ASI2792.
Characterization and comparison of PoV-ASI2792-infected P. ostreatus strain and virus-cured strains
Infection with a mycovirus occasionally causes severe morphological and physiological changes in fungi, such as changes in growth rate, colony morphology, spore production, pigmentation, and virulence-related phenotypes [2,4]. Viral infection of P. otreatus are also revealed reduced mycelial growth, delayed fruiting body formation, decreased fruiting body yield, and malformed fruiting bodies [5,8,9,10,12]. However, virus-free and virus-infected isogenic lines, which have identical genetic backgrounds, are needed to investigation of mycovirus-fungal host interactions because noncoincidence genetic backgrounds of fungal strains can lead to different symptoms in response to infection with the same mycovirus [19,30]. According to pair-wise comparison of isogenic strains, we sought to preferable determine the interaction between PoV-ASI2792 infection and vegetative characteristics of P. ostreatus.
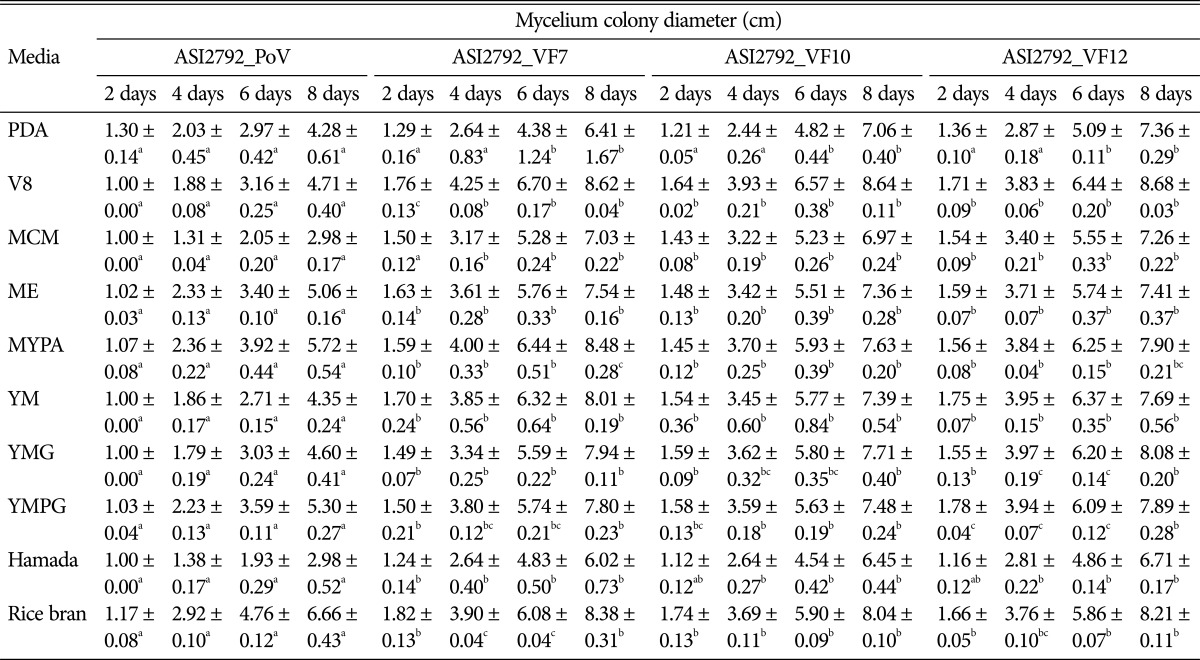
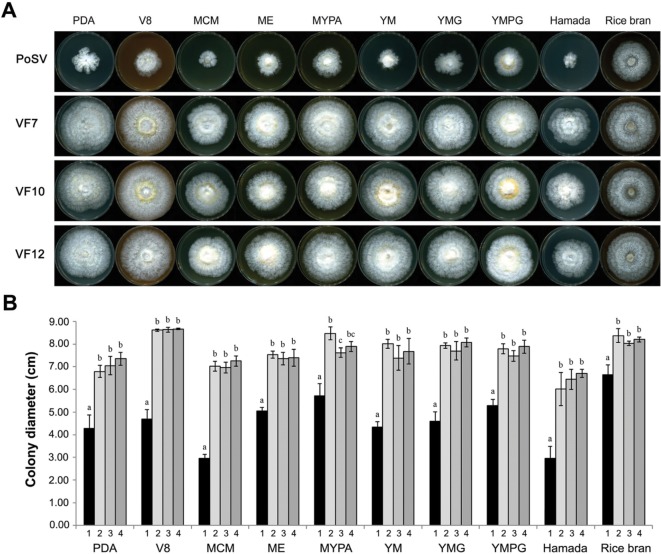
Three virus-cured lines, P. ostreatus AS2792-VF7, P. ostreatus AS2792-VF10, and P. ostreatus AS2792-VF12 were selected based on their normal phenotypes for further analysis. The mycovirus PoV-ASI2792 never reappear in any cured lines, which were transferred to new media at 8-day intervals for 4 mon. Moreover, there has been no sign of virus resurrection during subculture for more than three years. The growth rate and mycelial dry weight of the PoV-ASI2792-infected P. ostreatus strain were compared with those of three virus-free isogenic strains grown on 10 different media (PDA, V8, mushroom complete media [MCM], malt extract agar [ME], malt-yeast-peptone agar [MYPA], yeast-malt agar [YM], yeast-malt-peptone-glucose agar [YMG], Hamada, and rice bran) that were suitable for analysis of vegetative growth in mushrooms [25]. Colony diameters were measured at 2, 4, 6, and 8 days of culture to compare growth rates between virus-infected and virus-free lines. The colony diameters at 8-day-old cultures, representing the cultivation time required to cover more than 95% of the culture plate, which had the fastest-growing, and the mycelial dry weight were measure as described in “Materials and Methods” section.
Concerning the growth rate, virus-cured lines exhibited mycelial colony diameters greater than that of the virus-infected control strain on all media (Table 2, Fig. 3). All virus-cured lines, P. ostreatus AS2792-VF7, P. ostreatus AS2792-VF10, and P. ostreatus AS2792-VF12, showed a significant increase in colony diameter compared to that of the P. ostreatus control strain in all media tested. Colony diameters of the three virus-cured lines cultured on MCM and Hamada media for 8 days were greater, 2.4-fold and 2.2-fold, respectively, than those of the virus-infected strain in all media. In V8, YM, YMG, PDA, ME, and yeast-malt-peptone-glucose agar (YMPG) media, the virus-cured lines statistically showed 1.9-, 1.8-, 1.7-, 1.6-, 1.5-, and 1.5-fold larger colony diameters than the virus-infected control strain, respectively. Even in the MYPA and rice bran media, P. ostreatus showed an increase of over 25% in colony diameters (Fig. 3). In addition, the cured strains of P. ostreatus, AS2792-VF7, -VF10, and -VF12 strains showed statistically significant increases in colony diameters compared to that of the PoV-ASI2792-infected P. ostreatus strain throughout the cultivation period (2~8 days), except for in PDA medium on day 2. However, even in the PDA medium, P. ostreatus colony diameters increased over 30% after 4 days of culture (Table 2). These virus-cured strains were showed a significantly higher mycelial growth rate than other P. ostreatus virus-free strains reported previously [8,17], and these results were statistically significant. Taken together, these results indicate that PoV-ASI2792 led to abnormal vegetative growth in P. ostreatus.
Table 2. Radial growth rate of virus-infected and virus-cured strains on different culture media.
Data are the means ± standard deviations from five replications in three independent experiments. Mean separation by Duncan's multiple range test at p < 0.01.
PDA, potato dextrose agar; MCM, mushroom complete media; ME, malt extract agar; MYPA, malt-yeast-peptone agar; YM, yeast-malt agar; YMG, yeast-malt-glucose agar; YMPG, yeast-malt-peptone-glucose agar.
Fig. 3. A, Colony morphology of Pleurotus ostreatus virus-cured lines grown on different culture media. The parental strain P. ostreatus ASI2792, which was infected with a mycovirus (PoV-ASI2792) and three P. ostreatus virus-cured lines (VF7, VF10, and VF12) were grown on solid media (PDA, V8, MCM, ME, MYPA, YM, YMG, YMPG, Hamada, and rice bran). Strains were photographed 7 days after center point inoculation; B, Analysis of mycelial growth rates of P. ostreatus virus-cured lines on the various culture media. Number 1 to 4 indicate the virus-infected P. ostreatus ASI2792 strain (PoV, black bar) and three virus-cured P. ostreatus ASI2792 strains (VF7, VF10, and VF12, gray bars), respectively. Data are the means ± standard deviations from five replications in three independent experiments. Error bars represent the standard deviation from five replicates in three independent experiments. Mean separation by Duncan's multiple range test at p < 0.01. PDA, potato dextrose agar; MCM, mushroom complete media; ME, malt extract agar; MYPA, malt-yeast-peptone agar; YM, yeast-malt agar; YMG, yeast-malt-glucose agar; YMPG, yeast-malt-peptone-glucose agar.
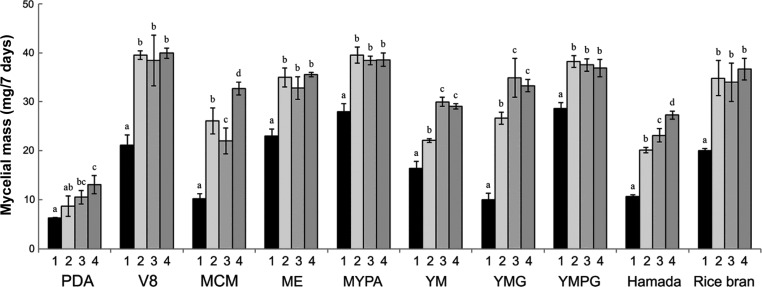
Concerning the mycelial dry weight, in all 10 media, the average mass of the virus-infected P. ostreatus strain was less than those of the three virus-cured lines (Fig. 4). All virus-cured strains, P. ostreatus AS2792-VF7, AS2792-VF10, and AS2792-VF12 definitely showed considerable increase in mycelial dry weight relative to the virus-infected strain in all media as a result of their growth rates. The mycelial densities of virus-cured lines cultured on YMG medium for 8 days were the highest in all media, with densities approximately 3.2-fold higher than that of the virus-infected strain. The MCM medium secondarily showed that the virus-cured lines had 2.7-fold greater mass than the P. ostreatus control strain. In the Hamada, V8, rice bran, PDA, YM, and ME media, the virus-cured lines showed 2.2-, 1.9-, 1.8-, 1.7-, 1.6-, and 1.5-fold greater mass, respectively, than the virus-infected strain, and even in the YMPG and MYPA media, the virus-cured lines showed an increase in mycelial mass of over 30% (Fig. 4). These results confirmed that PoV-ASI2792 caused abnormal vegetative growth in P. ostreatus, as indicated by colony diameter. This comparison of mycelial mass between control and virus-cured strains the first reported for P. ostreatus.
Fig. 4. Mycelial dry weights of Pleurotus ostreatus virus-cured lines on 10 different culture media. Number 1, virus-infected P. ostreatus ASI2792 (black bar); numbers 2~4, virus-cured P. ostreatus ASI2792-VF7, P. ostreatus ASI2792-VF10, and P. ostreatus ASI2792-VF12, respectively (gray bars). Data are the means ± standard deviations from five replications in three independent experiments. Mean separation by Duncan's multiple range test at p<0.05. PDA, potato dextrose agar; MCM, mushroom complete media; ME, malt extract agar; MYPA, malt-yeast-peptone agar; YM, yeast-malt agar; YMG, yeast-malt-glucose agar; YMPG, yeast-malt-peptone-glucose agar.
In addition, we observed that infection of PoV-ASI2792 affected colony pigmentation in P. ostreatus since mycovirus was able to cause a deficiency of pigmentation in fungi [2]. The virus-infected strain produced little or no pigment, with the exception of P. ostreatus strains grown in two culture media (MYPA and YMPG). However, the virus-cured lines produced yellowish pigment in the center of colonies grown in the PDA, V8, MCM, ME, and YM culture media, were especially observed the greatest pigmentation in the 2 media (V8 and YM) (Fig. 3A). These results indicate that infection of PoV-ASI2792 also affect pigmentation of the mycelial culture in P. ostreatus.
In this report, we describe P. ostreatus infection with PoV-ASI2792 that was cured by mycelial fragmentation followed by single colony isolation. The PoV-ASI2792 cured-fungal strains resulted in significantly higher growth rates and greater mycelial masses on all analyzed media, relative to those of the virus-infected fungal strain. In addition, they showed stronger pigmentation than the virus-infected strain on several media. In conclusion, infection of PoV-ASI2792 led to severe effect on the vegetative growth of P. ostreatus. Pair-wise comparisons of isogenic virus-cured and virus-infected strains were used to investigate the interactions between a mushroom host and a mycovirus. Therefore, investigations to explore the effects of PoV-ASI2792 on fruiting body initiation and production are assured in the coming research. We are currently examining the deleterious effect of PoV-ASI2792 on fruiting body formation and yield in P. ostreatus.
ACKNOWLEDGEMENTS
This work was carried out with the support of the “Cooperative Research Program for Agricultural Science & Technology Development (Project No. PJ009998042016)”, Rural Development Administration, Republic of Korea. We appreciate the kind gifts of P. ostreatus strains from Dr. H.S. Lee, Gyeongsang National University, Chinju, Korea.
References
- 1.Hollings M. Viruses associated with a die-back disease of cultivated mushroom. Nature. 1962;196:962–965. [Google Scholar]
- 2.Dawe AL, Nuss DL. Hypoviruses and chestnut blight: exploiting viruses to understand and modulate fungal pathogenesis. Annu Rev Genet. 2001;35:1–29. doi: 10.1146/annurev.genet.35.102401.085929. [DOI] [PubMed] [Google Scholar]
- 3.Pearson MN, Beever RE, Boine B, Arthur K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol Plant Pathol. 2009;10:115–128. doi: 10.1111/j.1364-3703.2008.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro M, Kramer K, Valdivia L, Ortiz S, Castillo A. A double-stranded RNA mycovirus confers hypovirulence-associated traits to Botrytis cinerea. FEMS Microbiol Lett. 2003;228:87–91. doi: 10.1016/S0378-1097(03)00755-9. [DOI] [PubMed] [Google Scholar]
- 5.Kim YJ, Kim JY, Kim JH, Yoon SM, Yoo YB, Yie SW. The identification of a novel Pleurotus ostreatus dsRNA virus and determination of the distribution of viruses in mushroom spores. J Microbiol. 2008;46:95–99. doi: 10.1007/s12275-007-0171-y. [DOI] [PubMed] [Google Scholar]
- 6.Lee JK, Lee KH, Shim H, Yang JS, Kim GH, Kong WS, Yoo YB, Kim DH, Kim D, Lee S. A new double-stranded RNA mycovirus from Pleurotus ostreatus (ASI 2504) Plant Pathol J. 2006;22:68–74. [Google Scholar]
- 7.Lim WS, Jeong JH, Jeong RD, Yoo YB, Yie SW, Kim KH. Complete nucleotide sequence and genome organization of a dsRNA partitivirus infecting Pleurotus ostreatus. Virus Res. 2005;108:111–119. doi: 10.1016/j.virusres.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Qiu L, Li Y, Liu Y, Gao Y, Qi Y, Shen J. Particle and naked RNA mycoviruses in industrially cultivated mushroom Pleurotus ostreatus in China. Fungal Biol. 2010;114:507–513. doi: 10.1016/j.funbio.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Ro HS, Lee NJ, Lee CW, Lee HS. Isolation of a novel mycovirus OMIV in Pleurotus ostreatus and its detection using a triple antibody sandwich-ELISA. J Virol Methods. 2006;138:24–29. doi: 10.1016/j.jviromet.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Van der Lende TR, Harmsen MC, Go SJ, Wessel JG. Double-stranded RNA mycoviruses in mycelium of Pleurotus ostreatus. FEMS Microbiol Lett. 1995;125:51–56. doi: 10.1111/j.1574-6968.1995.tb07334.x. [DOI] [PubMed] [Google Scholar]
- 11.Yu HJ, Lim D, Lee HS. Characterization of a novel single-stranded RNA mycovirus in Pleurotus ostreatus. Virology. 2003;314:9–15. doi: 10.1016/s0042-6822(03)00382-9. [DOI] [PubMed] [Google Scholar]
- 12.Yu H, Lee J, Lee NJ, Kim S, Gang E, Bae D, Chang M, Lee H. Identification of three isomeric viruses from Pleurotus ostreatus. J Huazhong Agric Univ. 2004;23:150–156. [Google Scholar]
- 13.Carroll K, Wickner RB. Translation and M1 double-stranded RNA propagation: MAK18 = RPL41B and cycloheximide curing. J Bacteriol. 1995;177:2887–2891. doi: 10.1128/jb.177.10.2887-2891.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrero N, Zabalgogeazcoa I. Mycoviruses infecting the endophytic and entomopathogenic fungus Tolypocladium cylindrosporum. Virus Res. 2011;160:409–413. doi: 10.1016/j.virusres.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Romo M, Leuchtmann A, García B, Zabalgogeazcoa I. A totivirus infecting the mutualistic fungal endophyte Epichloë festucae. Virus Res. 2007;124:38–43. doi: 10.1016/j.virusres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Kwon YC, Jeong DW, Gim SI, Ro HS, Lee HS. Curing viruses in Pleurotus ostreatus by growth on a limited nutrient medium containing cAMP and rifamycin. J Virol Methods. 2012;185:156–159. doi: 10.1016/j.jviromet.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Zhang CH, Liu YM, Qi YC, Gao YQ, Shen JW, Qiu LY. Comparisons of different methods for virus-elimination of edible fungi. Bing Du Xue Bao. 2010;26:249–254. [PubMed] [Google Scholar]
- 18.Kim JM, Yun SH, Park SM, Ko HG, Kim DH. Occurrence of dsRNA mycovirus (LeV-FMRI0339) in the edible mushroom Lentinula edodes and meiotic stability of LeV-FMRI0339 among monokaryotic progeny. Plant Pathol J. 2013;29:460–464. doi: 10.5423/PPJ.NT.03.2013.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JM, Song HY, Choi HJ, Yun SH, So KK, Ko HK, Kim DH. Changes in the mycovirus (LeV) titer and viral effect on the vegetative growth of the edible mushroom Lentinula edodes. Virus Res. 2015;197:8–12. doi: 10.1016/j.virusres.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Park SM, Kim JM, Chung HJ, Lim JY, Kwon BR, Lim JG, Kim JA, Kim MJ, Cha BJ, Lee SH, et al. Occurrence of diverse dsRNA in a Korean population of the chestnut blight fungus, Cryphonectria parasitica. Mycol Res. 2008;112(Pt 10):1220–1226. doi: 10.1016/j.mycres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Kim JM, Jung JE, Park JA, Park SM, Cha BJ, Kim DH. Biological function of a novel chrysovirus, CnV1-BS122, in the Korean Cryphonectria nitschkei BS122 strain. J Biosci Bioeng. 2013;115:1–3. doi: 10.1016/j.jbiosc.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Morris TJ, Dodds JA, Hillman B, Jordan RL, Lommel SA, Tamaki SJ. Viral specific dsRNA: diagnostic value for plant virus disease identification. Plant Mol Biol Rep. 1983;1:27–30. [Google Scholar]
- 23.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. NCBI BLAST: a better web interface. Nucleic Acids Res. 2008;36:W5–W9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun DR, Chai JK. The cultivate characteristics and the wood rotting ability and type of the Kuehneromyces mutabilis Sing. et A. H. Smith. J Mushroom Sci Prod. 2004;2:192–199. [Google Scholar]
- 26.Tavantzis SM, Romaine CP, Smith SH. Purification and partial characterization of a bacilliform virus from Agaricus bisporus: a single-stranded RNA mycovirus. Virology. 1980;105:94–102. doi: 10.1016/0042-6822(80)90159-2. [DOI] [PubMed] [Google Scholar]
- 27.Ro HS, Kang EJ, Yu JS, Lee TS, Lee CW, Lee HS. Isolation and characterization of a novel mycovirus, PeSV, in Pleurotus eryngii and the development of a diagnostic system for it. Biotechnol Lett. 2007;29:129–135. doi: 10.1007/s10529-006-9206-4. [DOI] [PubMed] [Google Scholar]
- 28.Won HK, Park SJ, Kim DK, Shin MJ, Kim N, Lee SH, Kwon YC, Ko HK, Ro HS, Lee HS. Isolation and characterization of a mycovirus in Lentinula edodes. J Microbiol. 2013;51:118–122. doi: 10.1007/s12275-013-2351-2. [DOI] [PubMed] [Google Scholar]
- 29.Magae Y. Molecular characterization of a novel mycovirus in the cultivated mushroom, Lentinula edodes. Virol J. 2012;9:60. doi: 10.1186/1743-422X-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rytter JL, Royse DJ, Romaine CP. Incidence and diversity of double-stranded RNA in Letinula edodes. Mycologia. 1991;83:506–510. [Google Scholar]
- 31.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2: a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]