Abstract
Culinary mushroom Pleurotus pulmonarius has been popular in Asian countries. In this study, the anti-oxidant, cholinesterase, and inflammation inhibitory activities of methanol extract (ME) of fruiting bodies of P. pulmonarius were evaluted. The 1,1-diphenyl-2-picryl-hydrazy free radical scavenging activity of ME at 2.0 mg/mL was comparable to that of butylated hydroxytoluene, the standard reference. The ME exhibited significantly higher hydroxyl radical scavenging activity than butylated hydroxytoluene. ME showed slightly lower but moderate inhibitory activity against acetylcholinesterase (AChE) and butyrylcholinesterase than galantamine, a standard AChE inhibitor. It also exhibited protective effect against cytotoxicity to PC-12 cells induced by glutamate (10~100 µg/mL), inhibitory effect on nitric oxide (NO) production and inducible nitric oxide synthase protein expression in lipopolysaccharide-stimulated RAW 264.7 macrophages, and carrageenan-induced paw edema in a rat model. High-performance liquid chromatography analysis revealed the ME of P. pulmonarius contained at least 10 phenolic compounds and some of them were identified by the comparison with known standard phenolics. Taken together, our results demonstrate that fruiting bodies of P. pulmonarius possess antioxidant, anti-cholinesterase, and inflammation inhibitory activities.
Keywords: Anti-cholinesterase, Anti-inflammation, Antioxidant, Cytotoxicity, Pleurotus pulmonarius
The metabolic processes in the human body will produce free radicals or reactive oxygen species (ROS) such as superoxide anion, hydroxyl radical, and hydrogen peroxide. These ROS are capable of oxidizing bio-molecules including lipids, proteins, DNA, and RNA. Furthermore, they play crucial roles in the development of degenerative diseases such as arthritis, asthma, atherosclerosis, cancer, cirrhosis, dementia, and Parkinson's disease. Although all living organisms possess defense systems to mitigate oxidative damage, these defense systems are not sufficient enough to protect the body against oxidative damages [1]. Thus, screening new antioxidants from natural sources including mushrooms is crucial. 1,1-Diphenyl-2-picryl-hydrazy (DPPH) is a free radical that is stable at room temperature. It has a purple color in methanol solution. DPPH is reduced in the presence of antioxidant molecule, giving rise to a yellowish color. Antioxidants are molecules that can inhibit or quench free radical reactions and delay or inhibit cellular damage. Antioxidants can be categorized as enzymatic and non-enzymatic antioxidants. Enzymatic antioxidant works by breaking down and removing free radicals and converts dangerous oxidative products to hydrogen peroxide (H2O2) and then to water in a multi-step process, while non-enzymatic antioxidant works by interrupting free radical chain reactions and donating hydrogen atom to a radical, thereby scavenging free radicals [2]. Lipid peroxidation is an oxidative degradation process of lipids. It is mainly generated by ROS such as hydroxyl radical, hydrogen peroxide, and singlet oxygen. These ROS can readily attack polyunsaturated fatty acids and initiate a self-propagating chain reaction by stealing electrons from lipids in cell membranes, resulting in cell damage and deterioration of biological systems. Lipid peroxidation is a critical step in the pathogenesis of several diseases including atherosclerosis, asthma, Parkinson's disease, and kidney failure [3]. Hydroxyl radicals are considered as one of the rapid initiators of the lipid peroxidation process. They can eliminate hydrogen atoms from polyunsaturated fatty acid or from each carbon atom of the sugar moiety of DNA, thus causing oxidative damage to DNA. These effects have been implicated in mutagenesis, carcinogenesis, and aging [4].
Acetylcholinesterase (AChE) is one of the main cholinesterase in the body. It catalyzes the breakdown of acetylcholine and other choline esters functioning as neurotransmitters. AChE is mainly found at neuromuscular junctions where it can terminate synaptic transmission. High levels of AChE in neuromuscular junctions can cause neurological disorders [5]. An AChE inhibitor is a chemical that prevents acetylcholine from breaking down by AChE enzyme, thereby increasing both the level and the duration of action of neurotransmitter acetylcholine [6]. AChE inhibitors are the most important prescription drugs that medicate early symptoms of Alzheimer's disease (AD). To improve cognitive symptoms of AD, AChE inhibitors such as donepezil, galantamine, huperzine A, physostigmine, and tacrine have been developed. However, these medicines have side effects, including vomiting, diarrhea, loss of body weight, insomnia, and nausea [7]. Thus, it is important to search for new AChE inhibitors from natural products without causing side effect.
Inflammation is considered localized complex biological response as a result of infection from pathogens, irritation caused by thermal heat, UV light, or ionizing radiation, and tissue injury. Recently, non-steroidal anti-inflammatory drugs (NSAIDs) such as indomethacin, aspirin, piroxicam, ibuprofen, and diclofenac are widely used for the treatment of inflammatory symptoms. However, administration of NSAIDs in a long-term may give rise to significant side effects [8]. Therefore, it is necessary to develop good and effective anti-inflammatory products from natural sources. Carrageenan-induced acute rat paw edema assay has been widely used to screen in vivo anti-inflammatory activity. The time course of carrageenan-induced paw edema development in rats is generally characterized by biphasic step [9]. The first phase of inflammation take places within half an hour after carrageenan administration is mainly due to the release of histamine and serotonin, whereas the second phase of inflammation involves kinin and bradykinin mediated by prostaglandins. The first phase of inflammation is characterized by an increasing in outward movement of vascular permeability and cellular infiltration of fluid and proteins into extracellular species, whereas prostaglandin release is responsible for edema formation in the second phase [10].
Mushrooms have been used as good nutritious foods since they are rich in carbohydrates, proteins, free amino acids, vitamins, and different essential mineral elements [11,12]. In the literature, more than 300 species of mushrooms with various therapeutic activities have been listed as folk medicines. They are rich in many bioactive metabolites with high medicinal values, including polysaccharides, polyphenols, flavonoids, terpenoids, ergosterols, and volatile organic compounds [13,14]. Therefore, mushrooms have shown various biological activities including immunity-stimulating, antitumor, antimicrobial, antioxidant, anti-diabetic, anti-hyperlipidemic, anti-hypercholesterolemic, hepatoprotectice, and anti-inflammatory activities [15,16]. Among mushrooms belonging to genus Pleurotus, several species including P. ostreatus, P. eryngii, P. ferulae, and P. citrinopileatus have been studied for their therapeutic potentials due to their antimicrobial, antitumor, hypoglycemic, hypotensive, and anti-inflammatory activities [17].
Culinary mushroom P. pulmonarius is commonly known as Indian oyster or lung oyster mushroom prefers to grow in warm weather. It utilizes various lignocellulosic materials, and it is very popular in Asian countries [18]. Recently, P. pulmonarius has become commercially available as an important culinary mushroom in Korea. Although P. pulmonarius possesses good sources of dietary nutrients and other valuable medicinal components [19,20], the therapeutically beneficial effects of P. pulmonarius have not been thoroughly studied. Therefore, the objective of this study was to investigate the antioxidant, anti-cholinesterase, and anti-inflammation activities of methanol extract (ME) of P. pulmonarius fruiting bodies. In addition, its protection against cytotoxicity of PC-12 cells induced by glutamate was determined in this study. Moreover, the profile of phenolic compounds present in ME of fruiting bodies of this mushroom was analyzed.
MATERIALS AND METHODS
Chemicals and reagents
Anti-inducible nitric oxide synthase (iNOS) antibody and enhanced chemiluminescence kit were obtained from Santa Cruz Biotechnology Co. (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Amersham Bioscience Co. (Buckinghamshire, UK), respectively. All other chemicals and solvents used for experiments were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA).
Animals
Sprague Dawley female rats (5-week-old, 155~165 g) were obtained from Daehan-Biolink Inc. (Eumseong, Korea). Rats were kept in polypropylene cages at 23 ± 2℃ and 50~60% relative humidity with 12 hr light and dark cycles. They were provided free access to water and food (standard rat chow). Rats were acclimated in the animal house for 1 wk before experiments. The experimental design and protocols were approved by the Animal Ethics Committee of the Incheon National University.
Mushroom and extract preparation
The fruiting bodies of P. pulmonarius were obtained from Mushroom Research Institute, Geonggi Agricultural Research and Extension Service, Korea. Air dried (45℃ for 48 hr) fresh fruiting bodies were finely pulverized. In this study, ME of P. pulmonarius was used for evaluating physiologically beneficial activities because ME from other mushrooms contained higher concentration of phenolic compounds and exhibited significantly higher antioxidant, xanthine oxidase and tyrosinase inhibitory effects compared with the hot water extract [21]. To prepare mushroom extract, 15 g of the sample powder in 300 mL of methanol (80%) were kept in shaker (120rpm) for 24hr at 25℃. The powder mixture in methanol solution was filtered. The residue was extracted with 300 mL of methanol (80%) two more times as described above. Methanol in the extract solution was removed using a rotary evaporator under reduced pressure at 45℃. Water remained in the extract was evaporated by freeze-dry.
Antioxidant assay
DPPH radical scavenging activity
Antioxidant effect of ME of P. pulmonarius fruiting bodies was assessed by using DPPH assay [22] with slight modifications. Briefly, 1 mL of DPPH (0.1 mM) in methanol was mixed with 1 mL of various ME concentrations (0.125, 0.25, 0.5, 1.0, and 2.0 mg/mL). The mixture was vortexed and incubated for 30 min at room temperature in the dark. The absorbance of the mixture was determined at wavelength of 517 nm using a UV-vis spectrophotometer. The following formula was employed to determine the DPPH radical scavenging activity:
| Inhibition (%) = [(Acontrol − Asample)/Acontrol] × 100, |
where Acontrol was the absorbance value of the control (containing all reagents without the test sample) and Asample was the absorbance value of the test sample. Butylatedydroxytoluene (BHT) was used as the positive control.
Inhibitory activity against lipid peroxidation
The inhibitory activity of ME of P. pulmonarius fruiting bodies against lipid peroxidation was determined using a previously described method [23] with minor modification. Briefly, egg yolk homogenate (250 µL of 10%, v/v) and 50 µL of ME were added to a test tube. The final volume was adjusted to 500 µL with distilled water. Twenty-five mictoliters of FeSO4 (0.07M) was supplemented to the above mixture and incubated at room temperature for 30 min. After the incubation, 750 µL of acetic acid (20%, pH 3.5) and equal volume of thiobarbituric acid (TBA, 0.8%) in sodium dodecyl sulphate (1.1%) with 25 µL of trichloroacetic acid (TCA, 20%) were added. The mixture was vortexed and incubated in 100℃ water bath for 60 min. After cooling down to room temperature, 3.0mL of 1-butanol was added to the each tube to extract the organic phase. The solution was centrifuged at 3,000 rpm for 10 min. The absorbance value of the supernatant was measured at wavelength of 532 nm with a spectrophotometer. The percentage of lipid peroxidation inhibition was determined using the following formula:
| Inhibition (%) = [(Acontol − Asample)/Acontrol] × 100, |
where Acontrol was the absorbance value of the control without any test sample and Asample was the absorbance value of the test sample. BHT was used as positive control.
Hydroxyl radical (OH−) scavenging activity
Hydroxyl radical (OH−) scavenging effect of the ME P. pulmonarius fruiting bodies was investigated using published method [24] with minor modifications. Briefly, 500 µL of various concentrations of ME (0.125, 0.25, 0.5. 1.0, and 2.0 mg/mL) were incubated with a solution containing 100 µL of 2.8 mM 2-deoxyribose dissolved in phosphate buffer (10 mM, pH 7.4), 200 µL of FeCl3, (200 µM), 1.04 µM ethylenediaminetetraacetic acid (1 : 1 v/v), 100 µL of H2O2 (1.0 mM), and 100 µL of ascorbic acid (1.0 mM). The extent of deoxyribose degradation was measured after adding 1.0 mL of 1% TBA and 1.0 mL of 1% TCA followed by incubation at 100℃ for 20 min. The absorbance value was measured on an UV-Vis spectrophotometer at wavelength of 532 nm. The percent of inhibition on hydroxyl radical was calculated using the following formula:
| Inhibition (%) = [(Acontol − Asample)/Acontrol] × 100, |
where Acontrol was the absorbance value of the control without any test sample and Asample was the absorbance value of the test sample. BHT was used as positive control.
High-performance liquid chromatography (HPLC) identification and quantification of phenolic compounds
Twenty phenolic compound standards were purchased from Sigma-Aldrich Co.. The preparation of samples for analysis of phenolic compounds followed the procedures of Kim et al. [25]. Briefly, HPLC system 2695 of Alliance (Waters, Milford, MA, USA) was employed for analyzing phenolic compounds present in the ME of P. pulmonarius fruiting bodies. Reverse phase column (XSELECT CSH; 3.5 µm × 150 mm × 4.6 mm i.d.) with temperature kept at 40℃ was used for chromatographic separation. Injection volume was 20 µL. Mobile phase and gradient followed those of Im et al. [26]. Photodiode array detector (Waters 2988) was employed for identifying and quantifying phenolic compounds present in the extract of P. pulmonarius fruiting bodies at wavelength of 280 nm.
Cholinesterase inhibitory activity.
AChE inhibitory activity
Anti-AChE activity of ME was investigated using the method of Ellman et al. [27]. Acetylthiocholine iodide was used as the substrate while electric eel AChE (Sigma-Aldrich Co.) was employed as the reference enzyme. Briefly, 120 µL of sodium phosphate buffer (0.1 mM, pH 8.0), 30 µL of various concentration of ME (0.063, 0.125, 0.25, 0.5, and 1.0 mg/mL) dissolved in methanol, and 30 µL of AChE (3 U/mL) were supplemented and kept for 30 min at 25℃. Then 10 µL of 0.5 mM 5,5′-dithio(bis-2-nitrobenzoic) acid (DTNB) was added. The reaction was started by the addition of 10 µL of acetylthiocholine iodide (0.71 mM). As a result of the reaction, acetylthiocholine iodide was hydrolyzed to thiocholine and acetate by the AChE enzyme. Thiocholine then reacted with DTNB to form yellow colored 5-thio-2-nitrobenzoate anion. The yellow color intensity was measured at wavelength of 412 nm in 96-well microplate reader. The AChE inhibitory activity (%) was calculated using the following formula:
| AChE inhibitory activity (%) = [(Acontrol − Asample)/Acontrol] × 100, |
where Acontrol was the enzyme activity of the control and Asample was the enzyme activity of the test sample. Galantamine was used as the positive control.
Butyrylcholinesterase (BChE) inhibitory activity
BChE inhibition activity was performed using the method of Orhan et al. [28] with slight modifications. Butyrylcholine iodide was used as a substrate while horse serum BChE was employed as the reference enzyme. Briefly, 120 µL of Na2HPO4 (100 mM, pH 8.0), 30 µL of varying concentration of ME dissolved in methanol, and 30 µL of BChE (0.35 U/mL) was supplemented and kept at 25℃ for 30 min. After adding 10 µL of DTNB (0.5 mM), the reaction was started by adding 10 µL of substrate (0.2 mM). The absorbance value was obtained at wavelength of 412 nm on a microplate reader. BChE inhibitory activity (%) was calculated using the following formula:
| BChE inhibitory activity (%) = [(Acontrol − Asample)/Acontrol] × 100, |
where Acontrol was the enzyme activity of the control and Asample was the enzyme activity of the test sample. Galantamine was used as the positive control.
Protective activity of ME for PC-12 cells against glutamate-induced cytotoxicity
The protective activity of ME for PC-12 cells against glutamate-induced cytotoxicity was performed using the method of Ma et al. [29] with slight modifications. Briefly, PC-12 cells were cultured in RPMI 1640 media supplemented with 5% (v/v) fetal bovine serum, 10% (v/v) horse serum, 100 U/mL penicillin, and 100 U/mL streptomycin at 37℃ in 5% CO2 atmosphere. Cells were seeded into 96-well culture plate at a density of 1 × 105 cells/well and incubated for 24 hr at 37℃. The medium was then replaced with fresh media containing 10mM of glutamate. After 12 hr of culturing, PC-12 cells were treated with various concentrations of ME (5, 10, 20, 40, and 100 µg/mL) followed by incubation for 24 hr. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [28] was employed to determine PC-12 cell viability. Briefly, MTT (10 µL of 5 mg/mL) was added into each well and PC-12 cells were incubated for another 4 hr. The upper layer of the medium was then removed. Dark blue formazan crystals precipitated in the cell culture plate were dissolved in dimethyl sulfoxide (200 µL) and the absorbance was measured at wavelength of 570 nm on a microplate reader.
Inflammation-inhibiting activity
Inhibitory effect on NO production
The inhibitory effect of the ME on NO production was determined using published method [30] with minor modifications. Briefly, RAW 264.7 murine macrophages were seeded into 96-well culture plate (5 × 105 cells/well) and incubated for 12 hr at 37℃ with humidified 5% CO2 atmosphere. The medium was then changed to fresh medium (0.2 mL) and incubated for an additional 60 min. The RAW 264.7 cells were then stimulated with lipopolysaccharide (LPS, 1 µg/mL) for 24 hr without or with ME (0.5, 1.0, and 2.0 µg/mL). For the analysis of NO production, 50 µL of the supernatant was used. NO production in the media of RAW 264.7 cells was determined with Griess assay. Briefly, the cell culture medium (120 µL) was mixed with an equal volume of Griess reagent. The reaction mixture was then incubated at 25℃ for 30min. The absorbance of each well was measured at 540 nm on a micro-titer plate reader. The amount of NO produced in the medium was calculated using standard curve of sodium nitrite.
Western blot analysis of iNOS level
Western blot analysis for iNOS level was performed using published method [31] with slight modifications. Briefly, RAW 264.7 cells were treated as described in the section of inhibitory effect on NO production. Cell extracts were prepared and used for western blot analysis using anti-iNOS antibody (Santa Cruz Biotechnology). Equivalent amounts of proteins were verified by re-probing the blot with anti-β-actin antibody (Santa Cruz Biotechnology). β-Actin expression was used as loading control. The expression of each protein and β-actin was detected using ECL western blotting detection system (Amersham Biosciences).
Carrageenan-induced acute inflammatory model
Carrageenan-induced hind paw edema assay [32] was performed to determine the in vivo anti-inflammation effect of ME. Rats were divided into five groups (five rats per group). For the edema assay, 50 µL of saline containing different concentrations of ME (5, 15, and 50 mg/kg per body weight), and indomethacin (5 mg/mL), the positive control, was injected into sub-plantar of rat hind-paw. After 30 min of administration of ME or indomethacin, 1% of 0.1 mL carrageenan (carrageenan, type IV; Sigma) was injected into the right hind paw of rats. Paw volumes were determined using a plethysmometer (MK-101P; Muromachi Kikai, Tokyo, Japan) at times just before injection of carrageenan and at 2, 4, and 6 hr after the administration of carrageenan. Paw volume was measured by the difference in paw volume at pre-injection volume and paw volume at different time points. The inhibitory effect of ME on inflammation was calculated using the following formula:
| Increase of paw volume (%) = [(Vt − Vo)/Vo] × 100, |
where Vt was the final paw volume of each rat at different time point after the injection and Vo was the paw volume of each rat at pre-injection time.
Statistical analysis
Data are presented as means ± standard deviations (SD). Statistical differences between control and treatment groups were tested by one-way analysis of variance (ANOVA) followed by Tukey's post-hoc test using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA). A p-value of less than 0.05 was considered as statistically significant.
RESULTS AND DISCUSSION
Antioxidant activity of Pleurotus pulminarius extracts
DPPH free radical scavenging activity
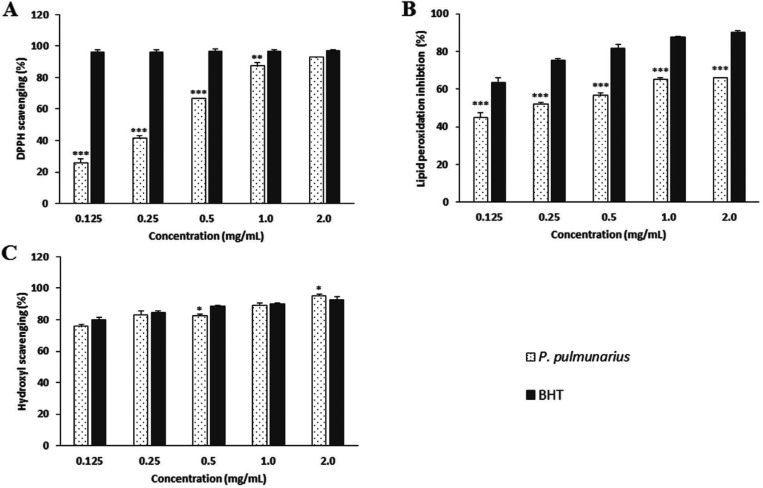
The DPPH free radical scavenging activity of the ME from fruiting bodies from P. pulmonarius was increased as the extract concentration was increased. The DPPH radical scavenging activity of ME at concentration range of 0.125~2.0 mg/mL was 25.67~92.73%. However, BHT, the positive control, showed excellent scavenging ability (from 96.19% to 96.97%) at the same concentration range (Fig. 1A). The scavenging activities of ME at all concentrations tested were significantly lower than those of BHT at similar concentrations. It has been reported that the DPPH radical scavenging activities of ME and hot water extract of fruiting bodies of mushroom Lentinula edodes are 3.39~29.4% and 38.3~40.04%, respectively, at concentration range of 1.5~9.0 mg/mL [33]. The DPPH radical scavenging activities of ME and hot water extract of fruiting bodies of mushroom Volvariella volvacea have been reported to be 17.8~57.8% and 20.2~37.9%, respectively, at similar concentration range (1.5~9.0 mg/mL) [33]. The DPPH scavenging activities of ethanol extracts of wild mushrooms such as Termitomyces robustus, Termitomyces clypeatus, Lenzites species, and Lentinus subnidus have been reported to be ranging from 29.8~75.2% at 2.0 mg/mL [34]. The DPPH scavenging activities of the ME of P. pulmonarius fruiting bodies obtained in this study were relatively higher than those of mushrooms mentioned above. Therefore, it has the potential of being used for promoting good health.
Fig. 1. Antioxidant activities of methanol extract from fruiting bodies of Pleurotus pulmunarius. A, DPPH radical scavenging activity; B, Lipid peroxidation inhibition activity; C, Hydroxyl radical (OH−) scavenging activity. Values are means ± SD (n = 3). BHT, butylated hydroxytoluene; DPPH, 1,1-Diphenyl-2-picryl-hydrazy. ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05 vs. BHT group.
Inhibition activity against lipid peroxidation
The inhibitory activity of ME of P. pulmonarius fruiting bodies against lipid peroxidation was investigated at five different concentrations. Its inhibitory activities against lipid peroxidation ranged from 44.99~65.87% at concentration of 0.125 to 2.0 mg/mL (Fig. 1B), demonstrating increased inhibition activity against lipid peroxidation with increasing concentration of the ME. However, the inhibitory activity of BHT against lipid peroxidation was 90.30%, which was significantly (p < 0.001) higher than that of the ME. The IC50 values of ME of wild and cultivated mushrooms such as Pleurotustuber-regium, Termitomycesrobustus, Lentinus squarrosulus, Pleurotus ostreatus, Pleurotus sajor-caju, and Auricularia auricula against lipid peroxidation have been reported to be 1.04, 0.43, 1.00, 0.15, 0.75, and 1.51 mg/mL, respectively [35]. The inhibitory activities of hot water extracts of 14 different mushrooms collected from Malaysia at 10 mg/mL against lipid peroxidation have been reported to be from 33.33% to 58.18% [36]. In this study, the inhibitory ability of ME of P. pulmonarius against lipid peroxidation was found to be 65.87% at concentration of 2.0mg/mL and its IC50 value was 0.21 mg/mL. These results indicate that the ME of P. pulmonarius fruiting bodies has relatively good inhibitory activity against lipid peroxidation compared to all mushrooms mentioned above. Therefore, P. pulmonarius fruiting bodies could be used as natural antioxidant agents.
Hydroxyl radical (OH−) scavenging activities
The hydroxyl radical scavenging activity of ME of P. pulmonarius at various concentrations (0.125~2.0 mg/mL) ranged from 75.60~95.13%, whereas those of BHT ranged from 79.87~92.66% (Fig. 1C). Therefore, the ME of P. pulmonarius had potent hydroxyl radical scavenging activities at the concentration tested. In this study, it was found that various ME concentrations of P. pulmonarius prevented the degradation of 2-deoxyribose by eliminating hydroxyl radicals in the solution. The ME exhibited a concentration-dependent scavenging activity against hydroxyl radicals. The observed IC50 value of ME of P. pulmonarius was 0.116 mg/mL, whereas the IC50 value of BHT was 0.111 mg/mL, indicating that the hydroxyl radical scavenging activity of the ME of P. pulmonarius was comparable to BHT. It has been reported that a ME of fruiting bodies of Pleurotus florida has 59% of hydroxyl radical scavenging activity at concentration of 1.0 mg/mL [37], which is lower than that (89.04%) of ME of P. pulmonarius tested in this study at the same concentration. It has been documented that the hydroxyl scavenging ability of hot water extract of mushroom Vovariella vovacea fruiting bodies is 69.34%, whereas its ME has activity of 62.45% at concentration of 0.25 mg/mL [33]. Taken together, these results suggest that the scavenging effect of ME of P. pulmonarius is better than the extracts of the above-mentioned mushrooms. Therefore, the ME of P. pulmonarius is a powerful OH− radical scavenger that can be used to prevent OH− radical related disorders.
Identification and quantification of phenolic compounds in Pleurotus pulmonarius extracts
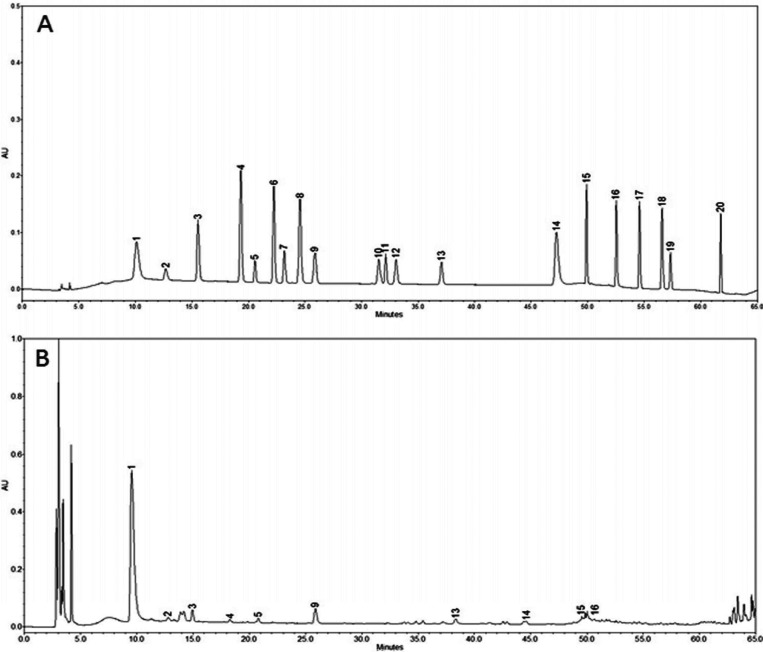
To identify and quantify the phenolic compounds present in the ME of P. pulmonarius fruiting bodies, HPLC analysis was performed. Ten phenolic compounds were identified from the ME of P. pulmonarius fruiting bodies (Fig. 2). Their total concentration was 135.89 µg/g. Phenolic compounds detected in the ME of P. pulmonarius fruiting bodies included gallic acid (84.85 µg/g), homogentisic acid (10.82 µg/g), procatechuic acid (4.99 µg/g), (+)-catechin (1.15 µg/g), chlorogenic acid (8.12 µg/g), vanillin (12.19), naringin (4.71 µg/g), myricetin (1.29 µg/g), resveratrol (2.29 µg/g), and quercetin (5.48 µg/g) (Fig. 2B). The phenolic compounds with the lowest and highest concentrations in the ME of P. pulmonarius fruiting bodies were (+)-catechin and gallic acid, respectively. The ME of P. pulmonarius fruiting bodies possessed different numbers of phenolic compounds, ranging from 3 to 15, with gallic acid and protocatechuic acid being the most common compounds present in this mushroom. The profiles of phenolic compounds and their concentrations are good indicators of the antioxidant potential of mushrooms [38]. Phenolic compounds are regarded as the most important antioxidant components in mushrooms. The correlation between the concentration of phenolic compounds and the total antioxidant capability of mushroom has been reported previously [39,40]. Consistent with earlier reports, our results also showed that phenolic compounds present in the ME of P. pulmonarius fruiting bodies corresponded to its DPPH radical scavenging, lipid peroxidation inhibition, and hydroxyl radical scavenging of activities.
Fig. 2. High-performance liquid chromatography analysis of phenolic compounds. A, Standard coumpounds; B, Pleurotus pulmunarius. 1, gallic acid; 2, homonogentisic acid; 3, procatechuic acid; 4, (+)-catechin; 5, chlorogenic acid; 6, (−)-epicatechin; 7, (−)-epigallocatechin gallate; 8, caffeic acid; 9, vanillin; 10, rutin hydrate; 11, p-coumaric acid; 12, ferullic acid; 13, naringin; 14, myricetin; 15, resveratrol; 16, quercetin; 17, naringenin; 18, kaempferol; 19, formonoentin; 20, biochanin-A.
Anti-cholinesterase activity of Pleurotus pulmonarius extracts
AChE inhibitory activity
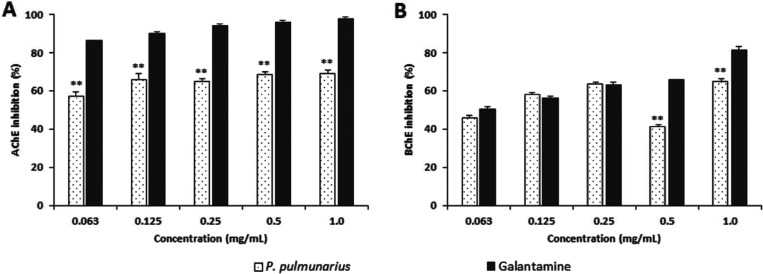
The AChE inhibitory effects of the ME of P. pulmonarius fruiting bodies ranged from 57.24~69.05% at concentrations of 0.063~1.0 mg/mL (Fig. 3A). It also exhibited inhibitory activities toward AChE in a concentration-dependent manner. However, the inhibitory activity of the ME of P. pulmonarius fruiting bodies toward AChE was significantly (p < 0.001) lower than that of galanthamine, the positive control, at all concentration tested. A previous study has reported that the AChE inhibitory activities of 7 wild mushroom species belonging to genus Polyporus and 3 other mushroom species (Cantharellus cibarius, Lactarius deliciosus, and Trametes versicolor) are ranged from 6.81~37.61% at concentration of 0.5 mg/mL [41], which are significantly lower than the AChE inhibitory activity (68.60%) of the ME of P. pulmonarius fruiting bodies in this study. Phenolic acids and flavonoid derivatives have been reported to be potent inhibitors of AChE [42]. The HPLC results of this study showed that the ME of P. pulmonarius fruiting bodies possessed 10 phenolic compounds, including gallic acid (84.85 µg/g), chlorogenic acid (8.12 µg/g), resveratrol (2.29 µg/g), and quercetin (5.48 µg/g) (Fig. 2B). These compounds have been reported to possess strong AChE inhibitory potential [43]. Therefore, that AChE inhibitory activity found in the ME of P. pulmonarius fruiting bodies might be due to its contents of phenolic compounds.
Fig. 3. Cholinesterase inhibitory activities of methanol extract from fruiting bodies of Pleurotus pulmunarius. A, Acetylcholinesterase inhibitory actibity; B, Butylrylcholinesterase inhibitory activity. AChE, acetylcholinesterase; BChE, butyrylcholinesterase. Values are means ± SD (n = 4). **p ≤ 0.01 vs. galantamine group.
Butyrylcholinesterase inhibitory activity
The inhibitory effect of the ME of P. pulmonarius fruiting bodies on BChE was analyzed in this study. The inhibitory activities of the ME of P. pulmonarius fruiting bodies toward BChE were 45.67~64.56% at concentrations of 0.063~1.0 mg/mL. These activities were significantly (p < 0.001) lower than that of positive control galanthamine (50.55~81.12%). The BChE inhibitory activity of the ME of P. pulmonarius fruiting bodies was increased with increasing concentration of the extract (Fig. 3B). It has been reported that phenolic acids (such as chlorogenic and gallic acids) and flavonoid derivatives (such as quercetin, genistein, lucolin-7-O-galactoside, naringin, silibinin, and silymarin) possess BChE inhibitory activities [44]. In this experiment, two of these phenolic acids (gallic acid and chlorogenic acid) and one flavonoid compound (quercetin) were detected in the ME of P. pulmonarius fruiting bodies. These compounds might have contributed to the moderate inhibitory effect of the ME of P. pulmonarius fruiting bodies toward BChE.
Glutamate-Induced cytotoxicity of Pleurotus pulmonarius extracts
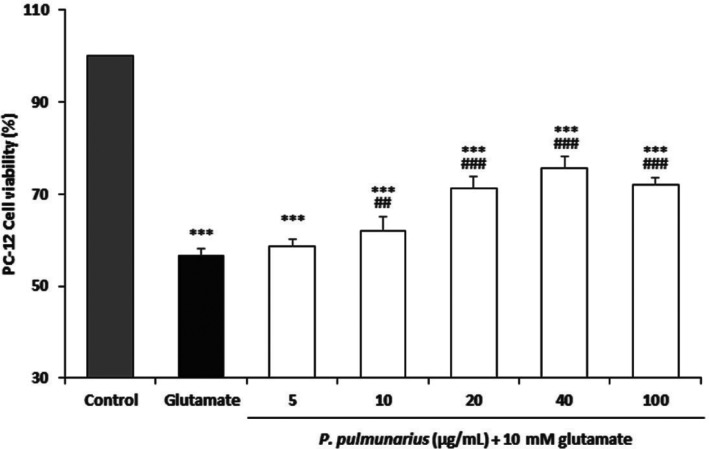
Glutamate-induced cytotoxicity was evaluated by using PC-12 cells. The viability of PC-12 cells in the medium supplemented with glutamate (10mM) was found to be 56.65% compared to that of control PC-12 cells without glutamate treatment. The viabilities of PC-12 cells after treatment with 5, 10, 20, 40, and 100 µg/mL of the ME of P. pulmonarius fruiting bodies were 58.67%, 62.08%, 71.19%, 75.59%, and 71.94%, respectively (Fig. 4). These results showed that the cytotoxicity of PC-12 cells caused by glutamate treatment was significantly lessened by supplementation of the ME at concentrations of 10~100 µg/mL. PC-12 cells treated with (−)-epigallocatechingallate, a polyphenolic compound isolated from green tea leaves, have mitigated glutamate-induced [Ca2+]i increase, while the viability of PC-12 cells caused by glutamate-induced toxicity is increased [44]. Varying concentrations of biochanin A also reduced the cytotoxic effect of glutamate treatment on PC-12 cells [45]. Our experimental results suggest that the cytotoxicity caused by glutamate treatment on PC-12 cells can be attenuated by increasing the concentrations of ME of P. pulmonarius fruiting bodies.
Fig. 4. Glutamate-induced cytotoxicity activity of methanol extracts from fruiting body of Pleurotus pulmunarius against PC-12 cells. Values are means ± SD (n = 3). ***p ≤ 0.001 vs. control group; ###p ≤ 0.001, ##p ≤ 0.01 vs. glutamate group.
Anti-inflammatory activity of Pleurotus pulmonarius extracts
Inhibition on NO production
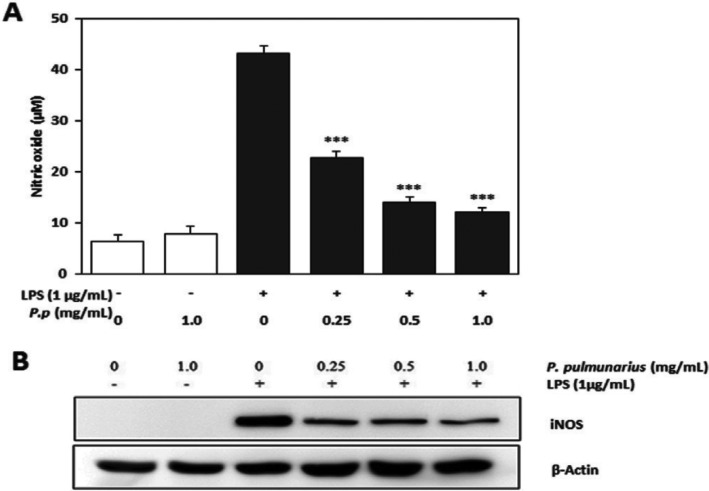
To determine the NO concentration in the media of LPS-stimulated RAW 264-7 cells, Griess assay [46] was employed. NO concentration in the media of RAW 264.7 cells after 24 hr of LPS treatment was increased ~6.80-fold from 6.35 to 43.17 µM, whereas the NO concentration produced in the media of RAW 264.7 cells treated with various concentrations of ME of P. pulmonarius fruiting bodies was decreased significantly in a concentration-dependent manner. RAW 264.7 cells treated with 1 mg/mL of ME of P. pulmonarius fruiting bodies produced 7.83 µM of NO, which was 1.23-fold higher compared to that by control RAW 264.7 cells with LPS treatment (Fig. 5A). Therefore, the ME of P. pulmonarius fruiting bodies showed significantly higher inhibition activity against NO production in LPS-stimulated RAW 264.7 cells (p ≤ 0.001). No cytotoxic effect of the ME was observed based on MTT test (data not shown). The reduced production of NO in ME treated RAW 264.7 macrophages might be due to the inhibition of ME on iNOS protein expression. It has been reported that ethanol extracts of mycelia and fruiting bodies of mushroom Cordyceps militaris can inhibit the production of NO in a concentration-dependent manner upon stimulation by LPS in RAW 264.7 macrophages [47]. Furthermore, MEs from fruiting bodies of edible wild mushrooms such as Agaricus bisporus, Cantherellus cibarius, Lactarius deliciosus, and Craterellus cornucopioides can also suppress the NO production in LPS-induced RAW 264.7 macrophages. The strong inhibition effect on NO production by these mushrooms are related to their phenolic compounds such as caffeic acid, gallic acid, homogentisic acid, protocathechuic acid, myricetin, and pyrogallol [48]. Therefore, the inhibitory effect of the ME on LPS-stimulated production of NO in RAW 264.7 macrophages might be partly due to its phenolics and other compounds.
Fig. 5. Inhibitory effect of Pleurotus pulmunarius methanol extract on lipopolysaccharide (LPS)-induced nitric oxide production and expression of inducible nitric oxide synthase (iNOS) in RAW 264.7 cells. A, Nitric oxide production; B, Expression of iNOS. β-Actin was used as an internal control. Accumulated nitric oxide in the culture medium was determined by the Griess method. The values are means ± SD (n = 3). ***p ≤ 0.001 vs. LPS treated group.
Western blot analysis
NO production of LPS-stimulated RAW264.7 cells was decreased with a dose-dependently after treatment with ME of P. pulmonarius fruiting bodies at different concentrations. Western blot analysis was performed under the assumption that the previous experimental results on iNOS expression might be responsible for the effect of ME in reducing NO production. The amount of iNOS protein was reduced by ME of P. pulmonarius fruiting bodies in a dose-dependent manner. However, β-actin protein expression was not changed by ME treatment, indicating that the ME of P. pulmonarius fruiting bodies only inhibited the expression of iNOS protein (Fig. 5B). It has been reported water extract of Ganoderma lucidum fruiting bodies can inhibit NO production and the expression of iNOS in LPS-induced RAW 264.7 cells in a dose-dependent manner [49]. Ethyl acetate extract of fruiting bodies of P. baumii can also inhibit the production of NO and iNOS protein expression in LPS-induced RAW 264.7 cells [31]. These results revealed that the ME of P. pulmonarius fruiting bodies could decrease the production of NO and the expression of iNOS protein.
Carrageenan-induced paw edema of rats
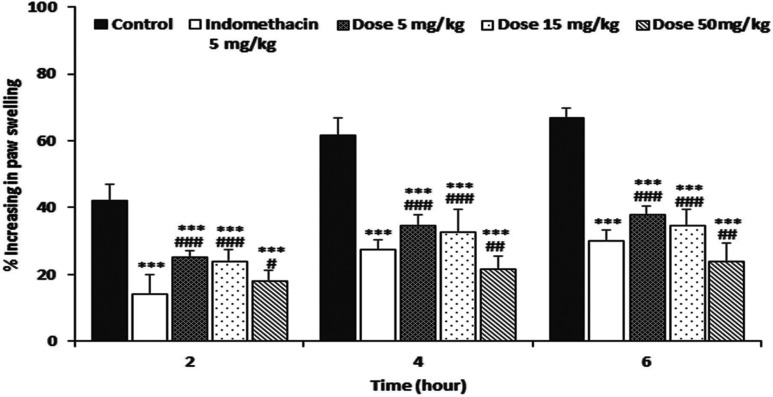
The carrageenan-stimulated hind-paw edema in the control group was developed progressively as time went by, whereas the positive control administered with indomethacin (5 mg/kg) reduced the edema volume of rats significantly at 2, 4, and 6 hr after the administration by 68.02%, 55.26%, and 55.09%, respectively, compared to the control group. The administration of various concentrations of ME of P. pulmonarius fruiting bodies (5, 15, and 50 mg/kg body weight) also significantly (p < 0.001) inhibited edema development at 2~6 hr after the injection. The highest inhibition (66.16%) was observed at 4 hr after injection with 50mg/kg of ME (Fig. 6). The edema inhibitory activity of the ME was dose-dependent. Although the ant-edema effect of the ME at 50 mg/kg was significantly higher (p < 0.01) than that of indomethacin at 5 mg/kg, the edema inhibitory effects of the ME at 5 mg/kg was significantly lower (p < 0.01) than that of indomethacin at the same concentration of 5 mg/kg. The results of this study showed that the ME of P. pulmonarius fruiting bodies could significantly inhibit rat paw edema development from the first phase to the second phase (2~6 hr), suggesting that ME of P. pulmonarius fruiting bodies might play a crucial role in inhibiting the release and action of histamine, serotonin, and prostaglandin. Since the ME of P. pulmonarius fruiting bodies possessed high anti-inflammatory potential, it might be useful for treating oxidative stress-induced inflammatory disorders.
Fig. 6. Effect of methanol extract from fruiting bodies of Pleurotus pulmunarius on carrageenan-induced paw edema. The values are means ± SD (n = 5). ***p ≤ 0.001 vs. control group; ###p ≤ 0.001, ##p ≤ 0.01, #p ≤ 0.05 vs. indomethacin group.
In conclusion, the ME of P. pulmonarius fruiting bodies possessed moderate DPPH scavenging and lipid peroxidation inhibiting activities, while its hydroxyl radical scavenging activity was higher than that of BHT. The ME of P. pulmonarius fruiting bodies also showed moderate cholinesterase inhibitory effect. It also exhibited protective effect against glutamate-induced cytotoxicity to PC-12 cells at 10~100 µg/mL. The ME of P. pulmonarius fruiting bodies also showed high inflammation inhibitory activities by inhibiting NO production, iNOS expression, and carrageenan-induced hind paw edema in rats. Its high phenolic contents might have contributed to its good antioxidant, anti-cholinesterase, and anti-inflammatory activities. Our results suggest that P. pulmonarius might be used as a natural product to promote human health through its antioxidant, anti-cholinesterase, and anti-inflammation activities.
ACKNOWLEDGEMENTS
This research was supported by the Golden Seed Project (Center for Horticultural Seed Development; No. 213003-04-4-CGI00), Ministry of Agriculture, Food and Rural Affairs (MAFRA), Ministry of Oceans and Fisheries (MOF), Rural Development Administration (RDA), and Korea Forest Service (KFS).
References
- 1.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 2.Fukumoto LR, Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem. 2000;48:3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- 3.Mylonas C, Kouretas D. Lipid peroxidation and tissue damage. In Vivo. 1999;13:295–309. [PubMed] [Google Scholar]
- 4.Gates KS. An overview of chemical processes that damage cellular DNA: spontaneous hydrolysis, alkylation, and reactions with radicals. Chem Res Toxicol. 2009;22:1747–1760. doi: 10.1021/tx900242k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson PA, Wright DE, Counsell CE, Zajicek J. Statistical analysis, trial design and duration in Alzheimer's disease clinical trials: a review. Int Psychogeriatr. 2012;24:689–697. doi: 10.1017/S1041610211001116. [DOI] [PubMed] [Google Scholar]
- 6.Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8:2003–2014. doi: 10.3969/j.issn.1673-5374.2013.21.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Čolović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan JW, Tham CL, Israf DA, Lee SH, Kim MK. Neuroprotective effects of biochanin A against glutamate-induced cytotoxicity in PC12 cells via apoptosis inhibition. Neurochem Res. 2013;38:512–518. doi: 10.1007/s11064-012-0943-6. [DOI] [PubMed] [Google Scholar]
- 9.Connelly L, Palacios-Callender M, Ameixa C, Moncada S, Hobbs AJ. Biphasic regulation of NF-κB activity underlies the pro- and anti-inflammatory actions of nitric oxide. J Immunol. 2001;166:3873–3881. doi: 10.4049/jimmunol.166.6.3873. [DOI] [PubMed] [Google Scholar]
- 10.Posadas I, Bucci M, Roviezzo F, Rossi A, Parente L, Sautebin L, Cirino G. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br J Pharmacol. 2004;142:331–338. doi: 10.1038/sj.bjp.0705650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang ST, Buswell JA. Mushroom nutriceuticals. World J Microbiol Biotechnol. 1996;12:473–476. doi: 10.1007/BF00419460. [DOI] [PubMed] [Google Scholar]
- 12.Kalač P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J Sci Food Agric. 2013;93:209–218. doi: 10.1002/jsfa.5960. [DOI] [PubMed] [Google Scholar]
- 13.Ying JZ, Mao XL, Ma QM, Zong YC, Wen HA. Icons of medicinal fungi from China. Beijing: Science Press; 1987. [Google Scholar]
- 14.Pak WH, Lee HD. Illustrated book of Korean medicinal mushrooms. 2nd ed. Seoul: Kyo-Hak Publishing Co. Ltd.; 2003. [Google Scholar]
- 15.Wasser SP, Weis AL. Medicinal properties of substances occurring in higher basidiomycetes mushrooms: current perspectives (review) Int J Med Mushrooms. 1999;1:31–62. [PubMed] [Google Scholar]
- 16.Lindequist U. The merit of medicinal mushrooms from a pharmaceutical point of view. Int J Med Mushrooms. 2013;15:517–523. doi: 10.1615/intjmedmushr.v15.i6.10. [DOI] [PubMed] [Google Scholar]
- 17.Patel Y, Naraian R, Singh VK. Medicinal properties of Pleurotus species (oyster mushroom): a review. World J Fungal Plant Biol. 2012;3:1–12. [Google Scholar]
- 18.Chang ST, Miles PG. Mushrooms cultivation, nutritional value, medicinal effect, and environmental impact. 2nd ed. Boca Raton (FL): CRC Press; 2004. [Google Scholar]
- 19.Oliveira Silva S, Gomes da Costa SM, Clemente E. Chemical composition of Pleurotus pulmonarius (Fr.) Quél., substrates and residues after cultivation. Braz Arch Biol Technol. 2002;45:531–535. [Google Scholar]
- 20.Smiderle FR, Olsen LM, Carbonero ER, Baggio CH, Freitas CS, Marcon R, Santos AR, Gorin PA, Iacomini M. Anti-inflammatory and analgesic properties in a rodent model of a (1→3),(1→6)-linked β-glucan isolated from Pleurotus pulmonarius. Eur J Pharmacol. 2008;597:86–91. doi: 10.1016/j.ejphar.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Alam N, Yoon KN, Lee KR, Shin PG, Cheong JC, Yoo YB, Shim JM, Lee MW, Lee UY, Lee TS. Antioxidant activities and tyrosinase inhibitory effects of different extracts from Pleurotus ostreatus fruiting bodies. Mycobiology. 2010;38:295–301. doi: 10.4489/MYCO.2010.38.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuendet M, Hostettmann K, Potterat O, Dyatmiko W. Iridoid glucosides with free radical scavenging properties from Fagraea blumei. Helv Chim Acta. 1997;80:1144–1152. [Google Scholar]
- 23.Ruberto G, Baratta MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69:167–174. [Google Scholar]
- 24.Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: a simple “test-tube” assay for the determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 25.Kim MY, Seguin P, Ahn JK, Kim JJ, Chun SC, Kim EH, Seo SH, Kang EY, Kim SL, Park YJ, et al. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J Agric Food Chem. 2008;56:7265–7270. doi: 10.1021/jf8008553. [DOI] [PubMed] [Google Scholar]
- 26.Im KH, Nguyen TK, Shin DB, Lee KR, Lee TS. Appraisal of antioxidant and anti-inflammatory activities of various extracts from the fruiting bodies of Pleurotus florida. Molecules. 2014;19:3310–3326. doi: 10.3390/molecules19033310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 28.Orhan I, Aslan S, Kartal M, Şener B, Hüsnü Can Başer K. Inhibitory effect of Turkish Rosmarinus officinalis L. on acetylcholinesterase and butyrylcholinesterase enzymes. Food Chem. 2008;108:663–668. doi: 10.1016/j.foodchem.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Ma S, Liu H, Jiao H, Wang L, Chen L, Liang J, Zhao M, Zhang X. Neuroprotective effect of ginkgolide K on glutamate-induced cytotoxicity in PC 12 cells via inhibition of ROS generation and Ca2+ influx. Neurotoxicology. 2012;33:59–69. doi: 10.1016/j.neuro.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Abas F, Lajis NH, Israf DA, Khozirah S, Kalsom YU. Antioxidant and nitric oxide inhibition activities of selected Malay traditional vegetables. Food Chem. 2006;95:566–573. [Google Scholar]
- 31.Yayeh T, Oh WJ, Park SC, Kim TH, Cho JY, Park HJ, Lee IK, Kim SK, Hong SB, Yun BS, et al. Phellinus baumii ethyl acetate extract inhibits lipopolysaccharide-induced iNOS, COX-2, and proinflammatory cytokine expression in RAW264.7 cells. J Nat Med. 2012;66:49–54. doi: 10.1007/s11418-011-0552-8. [DOI] [PubMed] [Google Scholar]
- 32.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 33.Punitha SC, Rajasekaran M. Free radical scavenging activity of fruiting body extracts of an edible mushroom, Volvariella volvacea (Bull. ex Fr.) Singer: an in vitro study. Asian J Biomed Pharmaceut Sci. 2014;4:6–11. [Google Scholar]
- 34.Oyetayo VO. Free radical scavenging and antimicrobial properties of extracts of wild mushrooms. Braz J Microbiol. 2009;40:380–386. doi: 10.1590/S1517-838220090002000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obodai M, Ferreira IC, Fernandes A, Barros L, Mensah DL, Dzomeku M, Urben AF, Prempeh J, Takli RK. Evaluation of the chemical and antioxidant properties of wild and cultivated mushrooms of Ghana. Molecules. 2014;19:19532–19548. doi: 10.3390/molecules191219532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdullah N, Ismail SM, Aminudin N, Shuib AS, Lau BF. Evaluation of selected culinary-medicinal mushrooms for antioxidant and ACE inhibitory activities. Evid Based Complement Alternat Med. 2012;2012:464238. doi: 10.1155/2012/464238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menaga D, Rajakumar S, Ayyasamy PM. Free radical scavenging activity of methanolic extract of Pleurotus florida mushroom. Int J Pharm Pharm Sci. 2013;5(Suppl 4):601–606. [Google Scholar]
- 38.Yoon KN, Alam N, Lee KR, Shin PG, Cheong JC, Yoo YB, Lee TS. Antioxidant and antityrosinase activities of various extracts from the fruiting bodies of Lentinus lepideus. Molecules. 2011;16:2334–2347. doi: 10.3390/molecules16032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walter M, Marchesan E. Phenolic compounds and antioxidant activity of rice. Braz Arch Biol Technol. 2011;54:371–377. [Google Scholar]
- 40.Zhao M, Yang B, Wang J, Li B, Jiang Y. Identification of the major flavonoids from pericarp tissues of lychee fruit in relation to their antioxidant activities. Food Chem. 2006;98:539–544. [Google Scholar]
- 41.Orhan I, Üstün O. Determination of total phenol content, antioxidant activity and acetylcholinesterase inhibition in selected mushrooms from Turkey. J Food Compost Anal. 2011;24:386–390. [Google Scholar]
- 42.Roseiro LB, Rauter AP, Serralheiro ML. Polyphenols as acetylcholinesterase inhibitors: structural specificity and impact on human disease. Nutr Aging. 2012;1:99–111. [Google Scholar]
- 43.Dundar A, Okumus V, Ozdemir S, Celik KS, Boga M, Ozcagli E, Ozhan G, Yildiz A. Antioxidant, antimicrobial, cytotoxic and anticholinesterase activities of seven mushroom species with their phenolic acid composition. J Hortic. 2015;2:161. [Google Scholar]
- 44.Orhan I, Kartal M, Tosun F, Sener B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z Naturforsch C. 2007;62:829–832. doi: 10.1515/znc-2007-11-1210. [DOI] [PubMed] [Google Scholar]
- 45.Lee JH, Song DK, Jung CH, Shin DH, Park J, Kwon TK, Jang BC, Mun KC, Kim SP, Suh SI, et al. (−)-Epigallocatechin gallate attenuates glutamate-induced cytotoxicity via intracellular Ca2+ modulation in PC12 cells. Clin Exp Pharmacol Physiol. 2004;31:530–536. doi: 10.1111/j.1440-1681.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- 46.Ong CK, Lirk P, Tan CH, Seymour RA. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin Med Res. 2007;5:19–34. doi: 10.3121/cmr.2007.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jo WS, Choi YJ, Kim HJ, Lee JY, Nam BH, Lee JD, Lee SW, Seo SY, Jeong MH. The anti-inflammatory effects of water extract from Cordyceps militaris in murine macrophage. Mycobiology. 2010;38:46–51. doi: 10.4489/MYCO.2010.38.1.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elsayed EA, El Enshasy H, Wadaan MA, Aziz R. Mushrooms: a potential natural source of anti-inflammatory compounds for medical applications. Mediators Inflamm. 2014;2014:805841. doi: 10.1155/2014/805841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song YS, Kim SH, Sa JH, Jin C, Lim CJ, Park EH. Anti-angiogenic and inhibitory activity on inducible nitric oxide production of the mushroom Ganoderma lucidum. J Ethnopharmacol. 2004;90:17–20. doi: 10.1016/j.jep.2003.09.006. [DOI] [PubMed] [Google Scholar]