Abstract
Background and Objectives
Hyaluronic acid (HA) is highly biocompatible with cells and the extracellular matrix. In contrast to degradation products of a synthetic polymer, degradation products of HA do not acidify the local environment. The aim of this study was to fabricate an HA-coated paclitaxel (PTX)-eluting stent via simple ionic interactions and to evaluate its effects in vitro and in vivo.
Materials and Methods
HA and catechol were conjugated by means of an activation agent, and then the stent was immersed in this solution (resulting in a HA-coated stent). After that, PTX was immobilized on the HA-coated stent (resulting in a hyaluronic acid-coated paclitaxel-eluting stent [H-PTX stent]). Study groups were divided into 4 groups: bare metal stent (BMS), HA, H-PTX, and poly (L-lactide)-coated paclitaxel-eluting stent (P-PTX). Stents were randomly implanted in a porcine coronary artery. After 4 weeks, vessels surrounding the stents were isolated and subjected to various analyses.
Results
Smoothness of the surface was maintained after expansion of the stent. In contrast to a previous study on a PTX-eluting stent, in this study, the PTX was effectively released up to 14 days (a half amount of PTX in 4 days). The proliferation of smooth muscle cells was successfully inhibited (by 80.5±12.11% at 7 days of culture as compared to the control) by PTX released from the stent. Animal experiments showed that the H-PTX stent does not induce an obvious inflammatory response. Nevertheless, restenosis was clearly decreased in the H-PTX stent group (9.8±3.25%) compared to the bare-metal stent group (29.7±8.11%).
Conclusion
A stent was stably coated with PTX via simple ionic interactions with HA. Restenosis was decreased in the H-PTX group. These results suggest that HA, a natural polymer, is suitable for fabrication of drug-eluting stents (without inflammation) as an alternative to a synthetic polymer.
Keywords: Stents, Hyaluronic acid, Paclitaxel, Preclinical drug evaluation, Coronary restenosis
Introduction
Drug-eluting stents (DESs) effectively reduce restenosis by addressing the biological mechanisms of neointimal proliferation and have become the mainstay of interventional treatment of coronary atherosclerotic disease.1) Paclitaxel (PTX) is a taxane derivative with antiproliferative properties, which makes it effective in the treatment of coronary restenosis.2) PTX inhibits microtubule disassembly and disrupts normal cellular processes including protein-mediated signaling, mitosis, and migration. PTX is generally cytostatic, though higher concentrations may cause tissue necrosis. PTX is highly lipophilic and poorly soluble in water. These properties promote rapid tissue uptake, which enables brief drug exposure of vessel walls, resulting in adequate tissue concentrations. Many researchers studied the efficacy of PTX-eluting stents using a porcine model of coronary restenosis. Neointimal formation decreases significantly in a dose-dependent manner in PTX-treated arteries.3),4),5) Nonetheless, it has been reported that severe limitations of PTX-coated stents are an uncontrollable drug release,6) poor safety,7) and thrombosis.8) Polymers have found widespread applications as coating matrices for DESs. Various biocompatible synthetic polymers such as poly ethylene, polyurethanes, polyglycolide, and polylactides (PLAs) have been materials of choice for fabrication of DESs. On the other hand, these synthetic-polymer-coated DESs are prone to cracking, peeling, strut bridging, and fragmentation during fabrication.9) Furthermore, blockage of peripheral vessels by bulk erosion may cause vessel occlusion.10) Therefore, despite the demonstrated efficacy of DESs, there is a small but unpredictable risk of thrombosis thought to be due to delayed vascular healing resulting from either the initial antiproliferative effect or an allergic reaction to the drug, a polymer coating, or their combination.11) For these reasons, alternative polymers of natural origin are needed. Hyaluronic acid (HA), a ubiquitous unsulfated glycosaminoglycan component of the extracellular matrix, is not only highly biocompatible with cells and the extracellular matrix but also enhances the proliferation and migration of endothelial cells at a later stage.12) HA can be efficiently degraded by specific enzymes, such as hyaluronidase. In contrast to degradation products of a synthetic polymer, degradation products of HA do not acidify the local environment. Both HA and immobilized sulfated HA have been shown to inhibit platelet aggregation and platelet adhesion and prolong bleeding-time measurements when administered systemically.13) The aim of this study was to fabricate an HA-coated PTX-eluting stent via simple ionic interactions and to evaluate its effects in vitro and in vivo.
Materials and methods
Preparation of bare-metal stents (BMSs)
In this study, a cobalt-chromium (Co-Cr, 3.0×18.0 mm, L605) alloy was used as the stent material because many researchers have reported that this alloy is the most suitable material in terms of biocompatibility.14) The stents were manufactured by Chonnam National University Hospital (CNUH).15) Fabrication of BMSs from the Co-Cr alloy was performed using a laser cutter (Rofin, Starcut, Hamburg, Germany). Thereafter, the stent was treated with an acidic atmosphere (50% H2SO4) for 1 h to remove particulates and burrs. Heat treatment and a polishing process were applied to restore the metal's mechanical properties. The cleaned BMSs were placed in a vacuum oven at 60℃ with incubation for 2 h to evaporate the residual water.
Preparation of PTX nanoparticles (NPs)
To prepare PTX NPs, human serum albumin (HSA) was dissolved in water (at 2 mg/mL) and mixed with β-mercaptoethanol. Then, PTX (10 mg/mL) was slowly added to the denatured-protein solution. The color of the solution changed to light blue and PTX-loaded HSA nanoparticles were formed.16) After that, the suspension was dialyzed against water for 24 h to remove β-mercaptoethanol. For preparation of an HA-coated stent, 3-hydroxytyramine hydrochloride (1400 mg), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (450 mg), and 5 m hydrochloric acid (100 µL) were added to a solution of HA (1000 mg) in 0.1 M 2-(N-morpholino) ethanesulfonic acid (MES) buffer (150 mL). The reaction solution was stirred at room temperature for 12 h. Additional 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (450 mg) was added to the solution at two time points (3 and 6 h). The resulting polymer was dialyzed via a molecular weight cutoff (MWCO) 3500 membrane against a 100 mM NaCl solution for 2 days and then against double-distilled water for 2 days.17)
Methods for coating of BMSs with HA and PTX
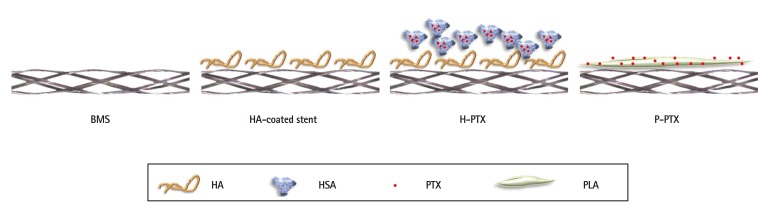
HA (10 mg/mL) and PLA (10 mg/mL) were dissolved in water and tetrahydrofuran (THF), respectively. Then, PTX NPs were resuspended in either HA or PLA solution and incubated for 24 h at room temperature. Next, the suspension was applied to the bare-metal stent (BMS) surface by means of an electrospinning coating device (NanoNC, Seoul, Korea). The flow rate of the reaction mixture was set to 50 L/min, and the flow was focused on the BMS assembled on a rotating mandrel. The samples were air dried by rotation for 24 h to evaporate the solvents. The stents were subdivided into 4 groups as follows: 1) BMSs, 2) HA-coated stents, 3) HA-and-PTX-coated stents (i.e., HA-coated PTX-eluting stents [H-PTX], and 4) PLA-coated PTX-eluting stents ([P-PTX]; Fig. 1).
Fig. 1. Schematic illustration of the coating approaches. To prepare and immobilize PTX NPs, HSA and HA were used. Ionic interactions between PTX NPs and HA were induced. BMS: bare-metal stent, HA: hyaluronic acid, H-PTX: hyaluronic-acid-coated paclitaxel-eluting stent, P-PTX: poly(L-lactide)-coated paclitaxel-eluting stent, HSA: human serum albumin, PTX: paclitaxel, PLA: polylactide, PTX NPs: paclitaxel nanoparticles.
In vitro kinetics of a PTX release
These kinetics were studied using an ultraviolet-visible (UV-Vis) spectrophotometer (Multiskan EX; Thermo Fisher Scientific, Waltham, MA, USA). In contrast to other studies where researchers used a simple shaking procedure, here, the equipment was designed to mimic the human body's circulation system. A peristaltic pump (JenieWell, Seoul, Korea) and silicon tubing of various thicknesses served as the heart and vasculature, respectively. Three stents were inserted and then expanded in the lumen of a silicon tube using a balloon (3.0×20.0 mm) under 10 mmHg pressure. Phosphate-buffered saline (PBS) was circulated through the lumen of the tubes by placing both open ends of a silicon tube into a temperature-controlled reservoir. The circulation was set to 150 rpm, and a one-directional flow was used to simulate the body's circulation system. PBS was sampled at some time points, and its absorbance at 276 nm was measured. The concentration of the released drug was calculated by comparing the measured values to the standard curve of the drug and was expressed as a cumulative value. The morphological features of the stent surfaces after the drug release experiment were examined by means of a scanning electron microscope (SEM; Hitachi, Tokyo, Japan).
In vitro cell proliferation
To study the effects of HA and PTX on smooth muscle cell (SMC) proliferation, a 2-dimensional version of the stents was immersed in the cell culture medium. After that, SMCs were seeded at 104/cm2 in a 24-well culture plate. The culture plate was incubated at 37 ℃ in a humidified atmosphere containing 5% of CO2. The proliferation of SMCs was evaluated by a XTT assay using an EZ-cytox Cell Viability Assay Kit (Daeil Lab Service Co., Seoul, Korea). Briefly, 40 mL of the EZ-cytox reagent was added to a 24-well culture plate. By the action of mitochondrial dehydrogenases, XTT is converted to a formazan dye that can be quantified by measuring absorbance at 450 nm using a spectrophotometric microplate reader (Bio-Tek Instruments, Winooski, VT, USA). The amount of formazan salt formed corresponds to the number of viable cells present in each well.
Animal preparation and stent implantation
The Ethics Committee of Chonnam National University Medical School and Chonnam National University Hospital approved the protocol of animal experiments for this study. Animal experiments were performed on castrated male pigs weighing 20-25 kg. Study groups were divided into 4 groups, which were BMS, HA, H-PTX, and P-PTX. Stents were implanted to the left anterior descending artery and left circumflex artery of 20 pigs (n=10). Mortality was zero in this study. The stents were deployed by inflating a balloon, and the resulting stent-to-artery ratio was 1.3:1. Four weeks after the implantation, the animals were euthanized by injection of 20 mL of potassium chloride into the left carotid artery. The vessels surrounding the stents were isolated and fixed in 10% neutral buffered formalin overnight. Samples were step-sectioned, processed routinely for light microscopy, and stained for histological analysis.
Histopathological analysis
This evaluation of the arteries was performed by an experienced cardiovascular pathologist. The specimens were embedded into paraffin, and sections of 50- to 100-µm thickness were prepared approximately 1-mm apart and subjected to hematoxylin-and-eosin (H&E) staining and Carstairs' fibrin staining for histological analysis. Analyses of the histopathological slices were performed using a calibrated microscope, a digital video imaging system, and special software (Visus 2000 Visual Image Analysis System; IMT Tech, San Diego, CA, USA). Borders were manually traced for the luminal area, the area circumscribed by the internal elastic lamina, and the innermost border of the external elastic lamina. The morphometric parameter of the neointimal area for a given vessel was calculated as the measured internal elastic lamina area minus the LA. The measurements were carried out on 5 cross-sections: at the proximal end, distal end, and 3 midpoints in each stent. The histopathological restenosis area was calculated as 100×(1−[lesion lumen area÷lesion internal elastic lamina area]). Immunofluorescence (IF) analysis was conducted by standard procedures as described elsewhere.18)
Calculation of inflammation and fibrin scores
Inflammation score for each individual strut was defined as follows: 0, no inflammatory cells surrounding the strut: 1, light, non-circumferential lymphohistiocytic infiltrate surrounding strut; 2, localized, moderate to dense cellular aggregate surrounding the strut non-circumferentially; and 3, circumferential dense lymphohistiocytic cell infiltration of the strut. The inflammation score for each cross section was calculated by dividing the sum of the individual inflammation scores by the total number of struts at the examined section.19) Arterial healing was assessed by fibrin deposition and ordinal data for fibrin were collected on each stent section using a scale of 0-3, as previously reported.20)
Morphological analysis of the stent surface
Longitudinally bisected stents 28 days after the implantation into a pig coronary artery were studied by means of the SEM (Hitachi, Tokyo, Japan).21) For imaging of the luminal area of the stents, samples were fixed with 2.5% glutaraldehyde for 2 h before serial dehydration with increasing concentrations of ethanol (40%, 50%, 60%, 70%, 80%, 90%, and 100%) for 10 min each. After dehydration, the samples were dried overnight and sputter-coated with gold prior to examination. The data were analyzed in special software (Visus 2000 Visual Image Analysis System; IMT Tech, San Diego, CA, USA).
Statistical analysis
This analysis was performed by means of commercially available software (SPSS version 15; SPSS Inc., Chicago, IL, USA). The data are presented as mean±standard. Unpaired Student's t test was used for the comparison of the stent groups. Differences with *p<0.05 were considered statistically significant.
Results
In vitro kinetics of the paclitaxel release
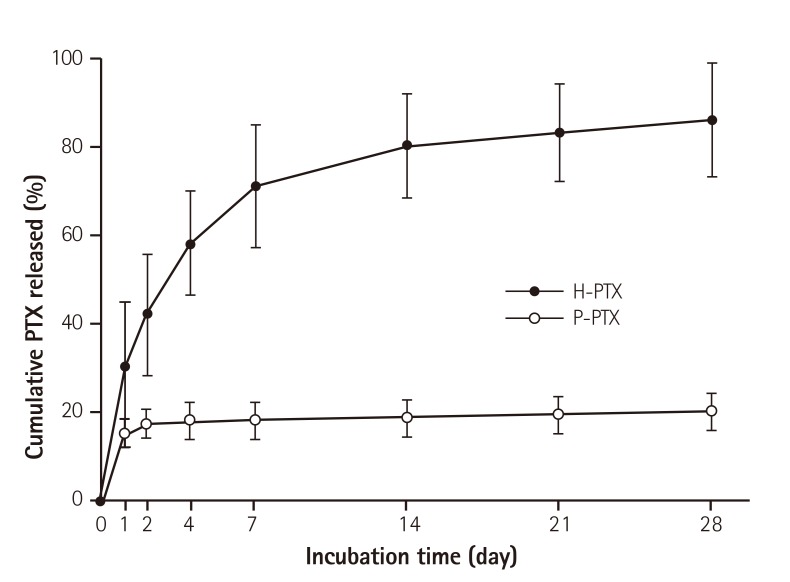
In order to make the total amount of PTX similar to that in a commercial PTX-eluting stent (180.0 mg/stent), PTX was dissolved in THF at 20 mg/mL as an initial concentration. To study the release kinetics of PTX, appropriate equipment mimicking the body's circulation system was designed for this study as described above. The amount of PTX released from a stent was measured as described above. In contrast to a previous study on a PTX-eluting stent,22) in this study, the PTX was effectively released up to 14 days (a half amount of PTX in 4 days). The additional release was allowed to continue up to 28 days, and showing an almost linear pattern (Fig. 2).
Fig. 2. In vitro cumulative kinetics of the PTX release from the stents. As described in the Materials and Methods, the stents were placed in tubing, and then PBS was circulated in the tubes. The amount of PTX released into the medium was measured at certain time points using a spectrophotometer. The data are shown as mean±standard (n=10). PTX: paclitaxel, H-PTX: hyaluronic-acid-coated paclitaxel-eluting stent, P-PTX: poly(L-lactide)-coated paclitaxel-eluting stent, PBS: phosphate-buffered saline.
Effects of released PTX on SMC proliferation
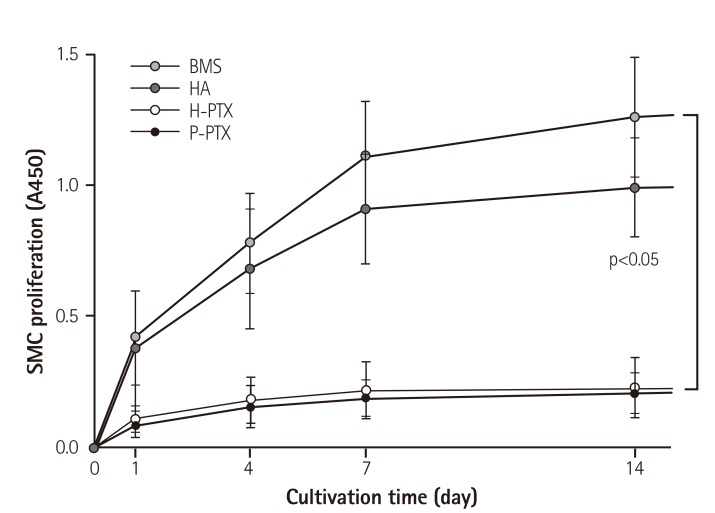
To study the inhibitory effect of PTX released from stents on the proliferation of SMCs, which are a component of the tunica media of the vascular wall,23) a XTT assay was performed. Absorbance in the control group, which was not treated with any additive, increased with the cultivation time. This means that the cells proliferated continuously. In contrast, the SMC proliferation was dramatically inhibited in both groups H-PTX and P-PTX (80.5± 12.11% at 7 days of culture compared to the control; Fig. 3).
Fig. 3. Inhibitory effects of PTX on SMC proliferation. To assess the effects of HA and PTX on cell proliferation, a 2-dimensional version of a stent was immersed in the cell culture medium. Cells were seeded in the stent-containing culture dish and incubated. Proliferation was assessed by an XTT assay. The results are shown as a mean±standard as compared to the control, n=10. SMC: smooth muscle cell, BMS: bare-metal stent, HA: hyaluronic acid, H-PTX: hyaluronic-acid-coated paclitaxel-eluting stent, P-PTX: poly(L-lactide)-coated paclitaxel-eluting stent, PTX: paclitaxel.
Histopathological analysis
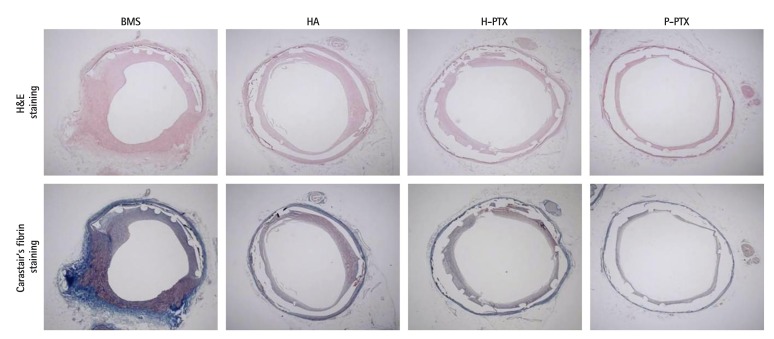
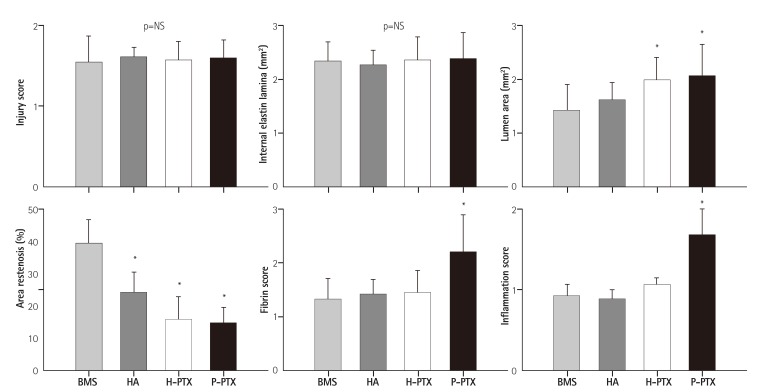
Four weeks after the implantation, the stents were isolated and subjected to quantitative histopathological analysis. Prior to euthanasia, angiographic images were obtained and showed the position of the properly expanded implanted stents and that the blood flow was passing through the lumen of the implanted stents (data not shown). After H&E and Carstairs' fibrin staining, the samples were examined using typical optical-microscope photographs. There were no significant differences in the injury score (BMSs: 1.5±0.33, HA-coated stents: 1.6±0.64, H-PTX: 1.6±0.23, and P-PTX: 1.6±0.22, p=NS), in the internal elastic lamina (BMSs: 4.6±0.71 mm2, HA; 4.5±0.55 mm2, H-PTX; 4.7±0.87 mm2, and P-PTX; 4.7±0.99, p=NS). However, the lumen areas were higher in PTX-containing groups (H-PTX; 4.0±0.83 mm2 and P-PTX: 4.1±1.20 mm2) compared to the other groups (BMSs: 2.8±0.96 mm2 and HA-coated stents: 3.2±0.66 mm2, p<0.05). The percent area of restenosis was slightly decreased in the HA group (24.1±6.25%, p<0.05) and greatly decreased in PTX-related groups (H-PTX: 15.8±7.22% and P-PTX: 14.8±4.90%, p<0.05) compared to the BMS group (39.3±8.12%). The fibrin score was higher in the P-PTX group (1.2±0.34, p<0.05) than in the other groups (BMSs: 0.7±0.19, HA-coated stents: 0.7±0.14, and H-PTX: 0.7±0.21). The inflammation score also higher in the P-PTX group (19±0.35, p<0.05) than in the other groups (BMSs: 0.9±0.15, HA-coated stents: 0.9±0.11, and H-PTX: 1.1±0.09; Fig. 4 and 5).
Fig. 4. Histopathological analysis of the porcine coronary model. Twenty-eight days postimplantation, the vessels containing the stents were isolated and subjected to a histopathological analysis. Typical optical photographs (25×) of the cross-sectional slices. BMS: bare-metal stent, HA: hyaluronic acid, H-PTX: hyaluronic-acid-coated paclitaxel-eluting stent, P-PTX: poly(L-lactide)-coated paclitaxel-eluting stent.
Fig. 5. Histomorphometric analysis of the stented arteries. The data are presented as mean±standard (n=10), *p<0.05. NS: not statistically significant, BMS: bare-metal stent, HA: hyaluronic acid, H-PTX: hyaluronic-acid-coated paclitaxel-eluting stent, P-PTX: poly(L-lactide)-coated paclitaxel-eluting stent.
Immunofluorescence analysis
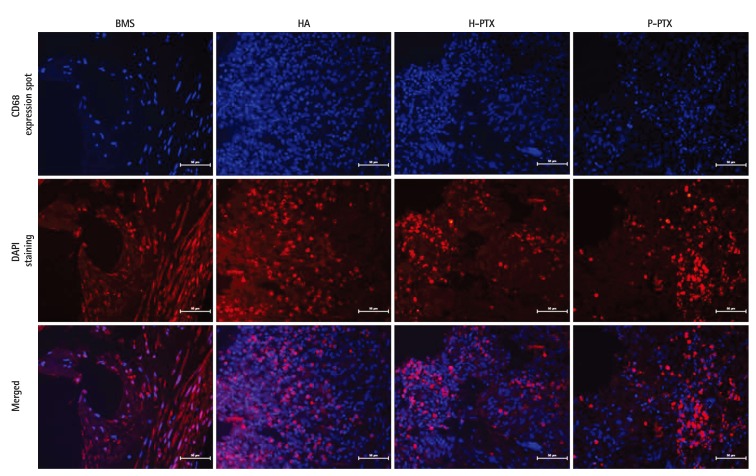
To investigate the effects of DES, immunofluorescence analysis was performed with antibodies against CD68, which is a classical marker for macrophage and inflammation cells. Their locations were prominently displayed in colors. There were more CD68 expression spots in the DSS P-PTX group than in the BMS, HA, and H-PTX groups (Fig. 6). This result was consistent to a histological analysis.
Fig. 6. Immunofluorescence analysis of the stents. Stent sections after coronary implantation were paraffin-embedded and stained for CD68. Red staining by secondary antibodies was Alexa fluor 568. DAPI staining was performed to visualize the cell nuclei (40×). BMS: bare-metal stent, HA: hyaluronic acid, H-PTX: hyaluronic-acid-coated paclitaxel-eluting stent, P-PTX: poly(L-lactide)-coated paclitaxel-eluting stent, DAPI: 4',6-diamidino-2-phenylindole.
Strut coverage rate
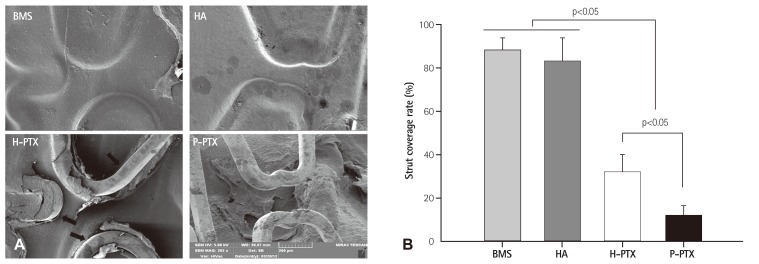
To evaluate the inhibitory effects of BMSs, HA-coated stents, H-PTXs, and P-PTXs on in-stent restenosis (ISR) in vivo, at 4 weeks post-implantation, the stents were collected and segmented by bisecting them longitudinally, with exposure of the luminal surface and examination under an SEM. The strut coverage rates dramatically decreased in PTX-related groups (H-PTX: 32.2 ±8.04% and P-PTX: 12.0±4.40%) compared to the other groups (BMS: 88.0 ±6.20% and HA: 83.2 ±7.24%, p<0.05; Fig. 7).
Fig. 7. The strut coverage rate. (A) Representative SEM images of the luminal area of the stents. The latter were harvested at 4 weeks postimplantation. They were bisected longitudinally and subjected to SEM examination. (B) Histomorphometric analysis of strut coverage rate ntified by ntified by special software (Visus 2000 Visual Image Analysis System; IMT Tech., San Diego, CA, USA). (Visus 2000 Visual Image Analysis System; IMT Tech., San Diego, CA, USA). special software. BMS: bare-metal stent, HA: hyaluronic acid, H-PTX: hyaluronic-acid-coated paclitaxel-eluting stent, P-PTX: poly(L-lactide)-coated paclitaxel-eluting stent, SEM: scanning electron microscope.
Discussion
The aim of this study was to fabricate an HA-coated PTX-eluting stent and to evaluate the differences between H-PTX and P-PTX in vitro and in vivo. We used the method of HSA nanoparticles for targeted immobilization of PTX on a stent. An HSA-PTX-coated stent was fabricated by unfolding (denaturing) HSA in an appropriate solution to expose more hydrophobic domains with consequent self-assembly into NPs with added PTX (Fig. 1).16) To study the release kinetics of PTX from the stents, special equipment was designed for this study, mimicking the body's circulation system. This was in contrast to other studies, which were based on a simple shaking procedure. The PTX release kinetics in the P-PTX group were consistent with data from other reports indicating no release caused by its hydrophobicity.24) In contrast to other studies on PTX-eluting stents, the release of PTX from H-PTX continued up to 14 days (Fig. 2). This extended release may be caused by the dissolution of ionic interactions between HSA-PTX and HA rather than by PTX self-release. The BMS surface was coated with PTX via HA rather than a durable polymer, which is a conventional material for PTX-eluting stents. In this study, we focused on how to coat the stents on materials not meant for coating. Nevertheless, the PTX was applied to the BMS surface via HA, and the pattern of release kinetics was similar to that of other drugs that are used for fabrication of second-generation DESs.
Restenosis, which is the in-growth of vascular SMCs into the lumen of the stent after a percutaneous coronary intervention, remains a major problem.25) In the response to vessel injury, SMCs are an integral part of neointimal hyperplasia (related to ISR).26) Drug-releasing coronary stents are known to effectively prevent restenosis.27) To investigate the inhibitory effects of HA and PTX on the proliferation of SMCs, which are a component of the tunica media of the vascular wall,22) an XTT assay was performed. As shown in Fig. 3, absorbance in the control groups, which were not treated with PTX, increased with the cultivation time. This means that the cells proliferated continuously. The SMC proliferation was not affected significantly in the HA-coated stent group, though it decreased slightly. By contrast, SMC proliferation in the H-PTX group dramatically decreased (by 80.5±12.11% at 7 days of culture as compared to control; Fig. 3). It is well known that PTX can inhibit cell proliferation. The results of this study showed that PTX released from a stent retains an inhibitory effect on cell proliferation. It was reported that HA shows excellent biocompatibility and inhibitory effects on SMC growth.28)
A porcine model was utilized here for a histological evaluation. The stents were implanted into coronary arteries, and the vessels containing the stent were isolated and subjected to a histological analysis. Prior to euthanasia, angiographic images were obtained. These images showed the positions of properly expanded implanted stents and that blood flow was passing freely through the lumen of the implanted stents (data not shown). Mortality among pigs in this study was zero. There were no significant differences in the stent-to-artery ratio among the stent groups. There were no significant differences in the injury score and internal elastic lamina among the groups. Nevertheless, the luminal area and % area of stenosis decreased in PTX-related groups (H-PTX and P-PTX; Fig. 5). This finding may be the result of the anti-proliferative effect of PTX released from stents. The fibrin score and inflammation score were higher in the P-PTX group than in the other groups. Notably, the fibrin score and inflammation score in the H-PTX group were similar to that in the BMS and HA groups. It has been reported that synthetic-polymer-coated DESs have limitations such as induction of local inflammation and thrombosis. Moreover, degrading polymers may generate fragments potentially leading to emboli.29),30) The risks of in-stent thrombosis and restenosis have been attributed to characteristics of a polymer that induces inflammation.31) To understand the inhibitory effect of PTX on ISR, morphological characteristics of the stent strut in the luminal area were studied. At 4 weeks post-implantation, the vessels containing the stents were isolated and segmented by bisecting them longitudinally to expose the luminal surface. After that, they were examined under an SEM. As shown in Fig. 7, the struts were fully covered by the attached and in-grown cells in both BMS and HA-coated stent groups. In contrast, the stent struts were free in the PTX-related groups (H-PTX and P-PTX groups). It was difficult to identify the species of cells around the stent struts as either SMCs or endothelial cells. According to one report, endothelial cells have a shuttlelike shape.32) Nevertheless, it is difficult to see the shuttlelike shape of the cells in all the images. Even if the shape were well visible, the identification process for the cells surrounding the stent struts should include other methods such as immunohistochemistry. Assuming that we see SMCs in the images, the images show a lower degree of strut coverage in the groups with a PTX coating compared to groups BMS and HA-coated stents. This result provides evidence that PTX coating of BMSs may prevent ISR. In conclusion, in the present study, BMSs were coated with PTX to inhibit SMC proliferation and prevent ISR. The effect was evaluated both in vitro and in vivo. To compare the natural-polymer-coated and synthetic-polymer-coated PTX-eluting stents, HA and PLA were used for attachment of PTX to the stents. The release kinetics of PTX were delayed in the H-PTX group compared to the P-PTX group. Furthermore, SMC proliferation was strongly suppressed in the groups with PTX coating. The animal experiments confirmed the decrease in ISR and in the strut coverage rate under the influence of PTX. Especially, the fibrin score and inflammation score significantly decreased in the H-PTX group compared to the P-PTX group. Thus, an HA-coated PTX-eluting stent may be especially beneficial to patients who are at an increased risk of stent-related inflammation. Such stents have a great potential for cardiovascular applications.
Acknowledgments
This study was supported by a research grant from the Korean Society of Cardiology (201203-04).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Kirtane AJ, Gupta A, Iyengar S, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119:3198–3206. doi: 10.1161/CIRCULATIONAHA.108.826479. [DOI] [PubMed] [Google Scholar]
- 2.Kamath KR, Barry JJ, Miller KM. The Taxus drug-eluting stent: a new paradigm in controlled drug delivery. Adv Drug Deliv Rev. 2006;58:412–436. doi: 10.1016/j.addr.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 3.Kee WJ, Jeong MH, Jang SY, et al. Very late stent thrombosis due to neointimal rupture after paclitaxel-eluting stent implantation. Korean Circ J. 2011;41:754–758. doi: 10.4070/kcj.2011.41.12.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herdeg C, Oberhoff M, Baumbach A, et al. Local paclitaxel delivery for the prevention of restenosis: biological effects and efficacy in vivo. J Am Coll Cardiol. 2000;35:1969–1976. doi: 10.1016/s0735-1097(00)00614-8. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht T, Speck U, Baier C, Wolf KJ, Böhm M, Scheller B. Reduction of stenosis due to intimal hyperplasia after stent supported angioplasty of peripheral arteries by local administration of paclitaxel in swine. Invest Radiol. 2007;42:579–585. doi: 10.1097/RLI.0b013e31804f5a60. [DOI] [PubMed] [Google Scholar]
- 6.Regar E, Sianos G, Serruys PW. Stent development and local drug delivery. Br Med Bull. 2001;59:227–248. doi: 10.1093/bmb/59.1.227. [DOI] [PubMed] [Google Scholar]
- 7.McFadden EP, Stabile E, Regar E, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- 8.Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 9.Nebeker JR, Virmani R, Bennett CL, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol. 2006;47:175–181. doi: 10.1016/j.jacc.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 10.Otsuka Y, Chronos NA, Apkarian RP, Robinson KA. Scanning electron microscopic analysis of defects in polymer coatings of three commercially available stents: comparison of BiodivYsio, Taxus and Cypher stents. J Invasive Cardiol. 2007;19:71–76. [PubMed] [Google Scholar]
- 11.Nakazawa G, Vorpahl M, Finn AV, Narula J, Virmani R. One step forward and two steps back with drug-eluting-stents: from preventing restenosis to causing late thrombosis and nouveau atherosclerosis. JACC Cardiovasc Imaging. 2009;2:625–628. doi: 10.1016/j.jcmg.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Gouëffic Y, Guilluy C, Guérin P, Patra P, Pacaud P, Loirand G. Hyaluronan induces vascular smooth muscle cell migration through RHAMM-mediated PI3K-dependent Rac activation. Cardiovasc Res. 2006;72:339–348. doi: 10.1016/j.cardiores.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Barbucci R, Lamponi S, Magnani A, et al. Influence of Sulfation on Platelet Aggregation and Activation with Differentially Sulfated Hyaluronic Acids. J Thromb Thrombolysis. 1998;6:109–115. doi: 10.1023/A:1008841303634. [DOI] [PubMed] [Google Scholar]
- 14.Buszman P, Trznadel S, Zurakowski A, et al. Prospective registry evaluating safety and efficacy of cobalt-chromium stent implantation in patients with de novo coronary lesions. Kardiol Pol. 2007;65:1041–1046. discussion 1047-8. [PubMed] [Google Scholar]
- 15.Bae IH, Lim KS, Park JK, et al. Mechanical behavior and in vivo properties of newly designed bare metal stent for enhanced flexibility. J Indst Eng Chem. 2015;21:1295–1300. [Google Scholar]
- 16.Ding D, Tang X, Cao X, et al. Novel self-assembly endows human serum albumin nanoparticles with an enhanced antitumor efficacy. AAPS PharmSciTech. 2014;15:213–222. doi: 10.1208/s12249-013-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Che HL, Bae IH, Lim KS, et al. Novel fabrication of microRNA nanoparticle-coated coronary stent for prevention of post-angioplasty restenosis. Korean Circ J. 2016;46:23–32. doi: 10.4070/kcj.2016.46.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian Z, Haessler M, Lemos JA, et al. Targeting vascular injury using Hantavirus-pseudotyped lentiviral vectors. Mol Ther. 2006;13:694–704. doi: 10.1016/j.ymthe.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz RS, Edelman E, Virmani R, et al. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv. 2008;1:143–153. doi: 10.1161/CIRCINTERVENTIONS.108.789974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolodgie FD, John M, Khurana C, et al. Sustained reduction of in-stent neointimal growth with the use of a novel systemic nanoparticle paclitaxel. Circulation. 2002;106:1195–1198. doi: 10.1161/01.cir.0000032141.31476.15. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues SN, Gonçalves IC, Martins MC, Barbosa MA, Ratner BD. Fibrinogen adsorption, platelet adhesion and activation on mixed hydroxyl-/methyl-terminated self-assembled monolayers. Biomaterials. 2006;27:5357–5367. doi: 10.1016/j.biomaterials.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Jabara R, Chronos N, Conway D, Molema W, Robinson K. Evaluation of a novel slow-release paclitaxel-eluting stent with a bioabsorbable polymeric surface coating. JACC Cardiovasc Interv. 2008;1:81–87. doi: 10.1016/j.jcin.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Seidel CL. Cellular heterogeneity of the vascular tunica media. Implications for vessel wall repair. Arterioscler Thromb Vasc Biol. 1997;17:1868–1871. doi: 10.1161/01.atv.17.10.1868. [DOI] [PubMed] [Google Scholar]
- 24.Waugh J, Wagstaff AJ. The paclitaxel (TAXUS)-eluting stent: a review of its use in the management of de novo coronary artery lesions. Am J Cardiovasc Drugs. 2004;4:257–268. doi: 10.2165/00129784-200404040-00006. [DOI] [PubMed] [Google Scholar]
- 25.Popma JJ, Califf RM, Topol EJ. Clinical trials of restenosis after coronary angioplasty. Circulation. 1991;84:1426–1436. doi: 10.1161/01.cir.84.3.1426. [DOI] [PubMed] [Google Scholar]
- 26.Clowes AW, Clowes MM. Kinetics of cellular proliferation after arterial injury. II. Inhibition of smooth muscle growth by heparin. Lab Invest. 1985;52:611–616. [PubMed] [Google Scholar]
- 27.Baek S, March KL. Gene therapy for restenosis: getting nearer the heart of the matter. Circ Res. 1998;82:295–305. doi: 10.1161/01.res.82.3.295. [DOI] [PubMed] [Google Scholar]
- 28.Park S, Bhang SH, La WG, Seo J, Kim BS, Char K. Dual roles of hyaluronic acids in multilayer films capturing nanocarriers for drug-eluting coatings. Biomaterials. 2012;33:5468–5477. doi: 10.1016/j.biomaterials.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Ceonzo K, Gaynor A, Shaffer L, Kojima K, Vacanti CA, Stahl GL. Polyglycolic acid-induced inflammation: role of hydrolysis and resulting complement activation. Tissue Eng. 2006;12:301–308. doi: 10.1089/ten.2006.12.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Commandeur S, van Beusekom HM, van der Giessen WJ. Polymers, drug release, and drug-eluting stents. J Interv Cardiol. 2006;19:500–506. doi: 10.1111/j.1540-8183.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 31.Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ. 2013;347:f6625. doi: 10.1136/bmj.f6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng S, Liu Z, Shen L, et al. The effect of a layer-by-layer chitosan-heparin coating on the endothelialization and coagulation properties of a coronary stent system. Biomaterials. 2009;30:2276–2283. doi: 10.1016/j.biomaterials.2008.12.075. [DOI] [PubMed] [Google Scholar]