Leukocyte telomere length (TL) provides an index of cellular age that predicts the incidence of age-related diseases and early mortality in older adults.1 Telomerase adds telomeric repeats to terminal DNA, a critical process that helps stall genomic instability and apoptotic events that are triggered when telomeres shorten to a critical length. Evidence from a telomerase-deficient mouse model demonstrated the widespread consequences of telomere attrition on neurodegeneration including reduced proliferation of neural progenitor cells, restricted neurogenesis, and atrophy of white matter tracts. Remarkably, these age-related degenerative phenotypes were reversed following reactivation of endogenous telomerase activity.2 Clinical evidence also suggests a correlation between chromosomal and neural aging but direct evidence in humans is limited. In this study, we characterized the relationship between telomerase activity, TL, and hippocampal volume in humans to assess whether cellular markers of aging reflect early age-related structural brain changes.
Methods
Participants included 47 midlife women (mean [SD] age, 57.89 [4.71] years; range, 49–66 years) who were at risk for early cognitive decline based on apolipoprotein E(APOE)–ε4 carrier status (n = 19) or were noncarrier control individuals (n = 28). Groups were comparable across demographic and clinical variables (Table). Participants were enrolled in a parallel longitudinal study and the data reported here reflect baseline measurements to avoid confounding effects of randomization with and without hormone replacement therapy, the focus of the parent study. Participants gave written informed consent to participate in the study, which was approved by the institutional review board for human research at Stanford University. Exclusion criteria included a history of neuropsychiatric disorder, history of drug or alcohol abuse, significant cognitive impairment (defined as any impairment in daily functions and/or Mini-Mental State Examination score <24), current depression, and history of major medical illness. The final sample consisted of currently healthy, high-functioning adults (Mini-Mental State Examination score ≥27; Full Scale Intelligence Quotient score >122; education >16 years). Clinical evaluations and assessments of peripheral blood mononuclear cell TL and telomerase activity were performed as previously described.3–5 Magnetic resonance imaging acquisition parameters were previously reported.3 Structural magnetic resonance imaging data were analyzed using FreeSurfer version 5.0.0 software (http://surfer.nmr.mgh.harvard.edu), which provides automated algorithms for the volumetric segmentation of subcortical structures and calculation of intracranial volume. The accuracy of structural estimates derived by FreeSurfer has been validated against histological analysis and manual measurements. The values in the Figure display the ratio of raw (left or right) hippocampal volume to total intracranial volume (cubic centimeters). Block linear regression models were conducted to determine the relationship between leukocyte TL and the telomerase to TL ratio (a summary measure of telomere maintenance) with hippocampal volume. Regression models were conducted separately for both the left and right hippocampus and were adjusted for standard covariates known to be associated with TL including age, education, and body mass index (BMI, calculated as weight in kilograms divided by height in meters squared). Furthermore, because APOE-ε4 genotype is a strong risk factor for accelerated cell aging4 and was a selection variable for the parent longitudinal study, analyses distinguished between ε4 carriers and noncarriers. All regression models that were significant in the full model (which partialed out variance associated with age, education, and BMI) were highly robust and remained significant when only age or age and education were entered as predictor variables. Details of the full model are reported. To compare characteristics of the sample by APOE status, t tests were conducted on continuous variables where equal variance was assumed (Table). A Mann-Whitney U test was conducted for Mini-Mental State Examination because the assumption of equal variance was violated. A statistical significance of P < .05 was used for all analyses. Analyses were performed using SPSS version 20.0.
Table.
Characteristics of the Study Sample by Apolipoprotein E–ε4 Carrier Status
| Characteristic | Mean (SD) | P Valuea | |
|---|---|---|---|
| Noncarrier (n = 28) | Carrier (n = 19) | ||
| Age, y | 57.96 (4.73) | 57.79 (4.80) | .90 |
| Education, y | 16.13 (1.98) | 16.32 (2.00) | .75 |
| BMI | 25.78 (4.04) | 24.65 (3.44) | .32 |
| Age at menopause, y | 47.87 (5.70) | 48.44 (4.91) | .73 |
| Baseline cognitive function | |||
| MMSE | 29.46 (0.64) | 29.28 (1.01) | .72 |
| FSIQ | 122.36 (7.19) | 122.78 (8.23) | .86 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FSIQ, Full Scale Intelligence Quotient; MMSE, Mini-Mental State Examination.
P values were determined using t tests (for continuous variables with equal variance) and a Mann-Whitney U test (MMSE).
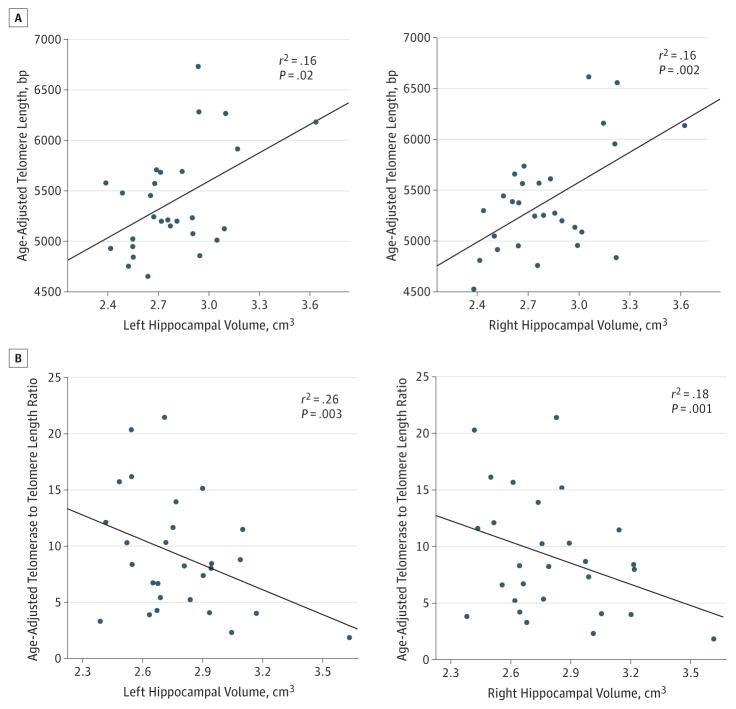
Figure. Leukocyte Telomere Length, Telomerase, and Hippocampal Volume.
A, Scatterplots display the correlation between telomere length and hippocampal volume in women with no known genetic risk for early cognitive decline or dementia. Age-adjusted telomere length is depicted in base pairs (bp). Hippocampal values represent the ratio of raw (left or right) hippocampal volume to total intracranial volume. B, Higher telomerase to telomere length ratio was negatively associated with hippocampal volume. R2 values reflect the correlation strength after partialing out variance associated with age, education, and body mass index (for R2 values of the full model, see the Results section).
Results
For healthy, high-functioning adults with no known genetic risk for AD (ε4 negative), longer leukocyte TL was associated with greater left (F4,27 = 3.67; model P = .02; TL P = .02; overall R2 = 0.39; adjusted R2 = 0.28) and right (F4,27 = 5.79; model P = .002; TL P = .01; overall R2 = 0.50; adjusted R2 = 0.42; Figure) hippocampal volume, adjusting for age, education, and BMI. Considered independently, telomerase activity did not show a significant relationship with hippocampal volume (all P > .05) (left: F4,27 = 1.97; model P = .13; telomerase P = .13 and right: F4,27 = 2.67; model P = .06; telomerase P = .26). However, we examined the relationship between hippocampal volume and the ratio between telomerase activity and TL, which is thought to provide a more comprehensive marker of telomere maintenance. After partialing out variance attributable to age, education, and BMI, the telomerase to TL ratio was a strong predictor of left hippocampal volume (F4,27 = 5.66; model P = .003; telomerase to TL ratio P = .002; overall R2 = 0.50; adjusted R2 = 0.41). This effect was mirrored in the right hippocampus (F4,27 = 6.43; model P = .001; telomerase to TL ratio P = .007; overall R2 = 0.53; adjusted R2 = 0.45; Figure). For individuals at an increased risk for developing Alzheimer disease (APOE-ε4 positive), the relationship between TL, telomerase activity, and hippocampal volume was not evident (all P > .50).
Discussion
This study showed that leukocyte TL and, more strongly, the ratio between telomerase activity and TL are associated with age-related structural brain changes in currently healthy, high-functioning adults, a relationship that was robust in APOE-ε4 noncarriers and obscured in ε4 carriers. Other studies have found that short leukocyte TL predicts pre-clinical cognitive decline in function. We extended these findings by demonstrating a relationship between chromosomal and neural markers of aging. Furthermore, we found that a profile of short telomeres and high telomerase is most characteristic of neurodegeneration. It is thought that when telomeres shorten to a critical length, telomerase increases as a compensatory response to cellular damage. In contrast, longer TL in the face of lower telomerase activity is thought to be a marker of stable maintenance, with no need for upregulated telomerase to maintain short telomeres. Thus, a higher telomerase to TL ratio likely reflects a state of stress-related telomere repair.6
Several limitations of the current study should be considered. First, the sample size was limited (N = 47) and there were fewer carriers than noncarriers, which could contribute to the absence of an association in APOE-ε4–positive individuals. Second, linear regression models were conducted for several predictor variables (TL, telomerase, and telomerase to TL ratio) after partialing out variance attributable to several potential confounders (ie, age, BMI, and years of education), thus constraining the degrees of freedom for each model. Moving forward, it will be critical to extend these findings to a larger cohort to determine the robustness of the model and generalizability. Chronic exposure to inflammatory cytokines, oxidative stress, and glucocorticoids can accelerate telomere shortening and lead to hippocampal atrophy,7 suggesting common pathways through which markers of telomere maintenance could reflect hippocampal integrity. Examining these pathways in greater depth will advance our understanding of the extent to which alterations in telomerase activity and TL reflect, or perhaps drive, age-related neural deficits.
Acknowledgments
Funding/Support: This study was funded by grant R01 AG22008 from the National Institute on Aging (Dr Rasgon) and grant M01 RR-00070 from the National Center for Research Resources, National Institutes of Health. This study also received support from the Robert Wood Johnson Foundation Health and Society Scholars program (Dr Jacobs).
Footnotes
Conflict of Interest Disclosures: Drs Epel, Lin, and Blackburn were cofounders of Telome Health Inc, a telomere length measurement company. No other disclosures were reported.
Author Contributions: Dr Rasgon had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Jacobs, Epel, Rasgon.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Jacobs.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Jacobs.
Obtained funding: Jacobs, Rasgon.
Administrative, technical, or material support: Epel, Lin, Rasgon.
Study supervision: Epel, Blackburn, Rasgon.
Role of the Sponsor: The funding agencies had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript, but not the decision to submit the manuscript for publication.
Additional Contributions: We thank Allan Reiss, MD (Stanford University), for overseeing brain imaging acquisition and volumetric analyses; Ryan Kelley, BS (Stanford University), for help with volumetric analyses; Joachim Hallmayer, MD (Stanford University), for overseeing apolipoprotein E genotyping; and Heather Kenna, MA (Stanford University), and Candyce Kroenke, MPH, ScD (Kaiser Permanente), for managerial and analytic support. They did not receive compensation from a funding sponsor.
References
- 1.Honig LS, Kang MS, Schupf N, Lee JH, Mayeux R. Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch Neurol. 2012;69(10):1332–1339. doi: 10.1001/archneurol.2012.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaskelioff M, Muller FL, Paik JH, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469(7328):102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasgon NL, Kenna HA, Wroolie TE, et al. Insulin resistance and hippocampal volume in women at risk for Alzheimer’s disease. Neurobiol Aging. 2011;32(11):1942–1948. doi: 10.1016/j.neurobiolaging.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs EG, Kroenke C, Lin J, et al. Accelerated cell aging in female APOE-ε4 carriers: implications for hormone therapy use. PLoS One. 2013;8(2):e54713. doi: 10.1371/journal.pone.0054713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin J, Kroenke CH, Epel E, et al. Greater endogenous estrogen exposure is associated with longer telomeres in postmenopausal women at risk for cognitive decline. Brain Res. 2011;1379:224–231. doi: 10.1016/j.brainres.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beery AK, Lin J, Biddle JS, Francis DD, Blackburn EH, Epel ES. Chronic stress elevates telomerase activity in rats. Biol Lett. 2012;8(6):1063–1066. doi: 10.1098/rsbl.2012.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010;27(4):327–338. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]