ABSTRACT
Background: An acellular Pertussis (aP) vaccine containing recombinant genetically detoxified Pertussis Toxin (PTgen), Filamentous Hemagglutinin (FHA) and Pertactin (PRN) has been developed by BioNet-Asia (BioNet). We present here the results of the first clinical study of this recombinant aP vaccine formulated alone or in combination with tetanus and diphtheria toxoids (TdaP). Methods: A phase I/II, observer-blind, randomized controlled trial was conducted at Mahidol University in Bangkok, Thailand in healthy adult volunteers aged 18–35 y. The eligible volunteers were randomized to receive one dose of either BioNet's aP or Tetanus toxoid-reduced Diphtheria toxoid-acellular Pertussis (TdaP) vaccine, or the Tdap Adacel® vaccine in a 1:1:1 ratio. Safety follow-up was performed for one month. Immunogenicity was assessed at baseline, at 7 and 28 d after vaccination. Anti-PT, anti-FHA, anti-PRN, anti-tetanus and anti-diphtheria IgG antibodies were assessed by ELISA. Anti-PT neutralizing antibodies were assessed also by CHO cell assay. Results: A total of 60 subjects (20 per each vaccine group) were enrolled and included in the safety analysis. Safety laboratory parameters, incidence of local and systemic post-immunization reactions during 7 d after vaccination and incidence of adverse events during one month after vaccination were similar in the 3 vaccine groups. One month after vaccination, seroresponse rates of anti-PT, anti-FHA and anti-PRN IgG antibodies exceeded 78% in all vaccine groups. The anti-PT IgG, anti-FHA IgG, and anti-PT neutralizing antibody geometric mean titers (GMTs) were significantly higher following immunization with BioNet's aP and BioNet's TdaP than Adacel® (P< 0.05). The anti-PRN IgG, anti-tetanus and anti-diphtheria GMTs at one month after immunization were comparable in all vaccine groups. All subjects had seroprotective titers of anti-tetanus and anti-diphtheria antibodies at baseline. Conclusion: In this first clinical study, PTgen-based BioNet's aP and TdaP vaccines showed a similar tolerability and safety profile to Adacel® and elicited significantly higher immune responses to PT and FHA.
KEYWORDS: clinical trial, phase I/II, Thailand, recombinant acellular pertussis vaccine, TdaP
Introduction
Although vaccines against pertussis have been used for the past 45 years, pertussis remains a public health concern.1 In the 1990s, acellular Pertussis vaccines (aP) were developed with the intent to improve the safety of existing whole-cell Pertussis (wP) vaccines1,2 as well as to address the great variability in the production of wP vaccines.1 Large safety and efficacy studies showed that overall wP vaccines were more reactogenic but had a similar safety profile as aP vaccines whereas the efficacy of aP vaccines was similar or inferior to that of wP vaccines.1,2
Despite high vaccine coverage with wP or aP vaccines, pertussis remains one of the major causes of childhood morbidity and mortality worldwide, responsible for 60,000 deaths in 2013,3,4 mostly in infants less than 1 y old who are unvaccinated or incompletely vaccinated.5 Resurgence of pertussis in the adolescent and adult population has been documented in countries that use aP-based vaccines, despite high infant vaccine coverage and children/adolescent boosters.1 The failure of aP vaccines to confer long term protection may result from the induction of short-lived immunity,6,7 loss of B-cell binding neutralizing epitopes,8,9 insufficient T-cell type 1 (Th1) and type 17 (Th17) responses to promote mucosal responses10,11 and/or genetic changes in circulating B. pertussis strains.12,13 Potential strategies for better pertussis control include the improvement of pertussis vaccines with recombinant genetically detoxified Pertussis Toxin (rPT) and/or increase of antigenic content, adjustment in formulations to include novel adjuvants, additional booster doses, cocooning and/or maternal immunization.14
In infants, aP vaccines containing rPT were shown as safe, highly immunogenic, efficacious and able to elicit antibodies and protection which persisted up to 6 y.15,16 The superior immune response of rPT-containing vaccines15 was associated with the conservation of 75–80% of native PT, enabling efficient B-cell epitope binding.8,17 The production and supply of this first generation of pediatric rPT-containing vaccines was interrupted by patent issues. BioNet-Asia (BioNet, Bangkok, Thailand) has developed a new recombinant B. pertussis strain expressing a genetically-detoxified PT (PTgen)18 which retains the functional antigenic properties of native PT but without its toxicity.17,18,19
Here, we report the results of the first clinical trial designed to evaluate the safety and immunogenicity of BioNet's aP vaccine alone or combined with tetanus and diphtheria toxoids (TdaP) in healthy adults.
Results
Study subjects and demographic characteristics
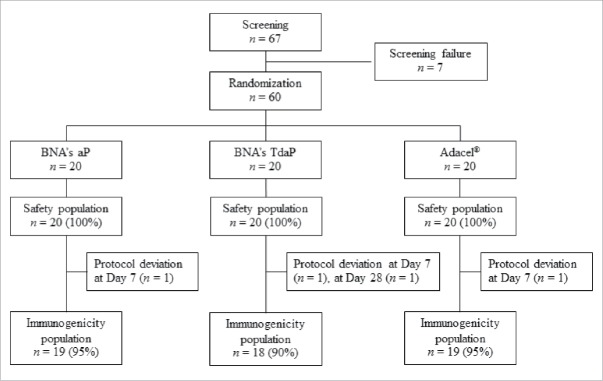
A total of 67 subjects were screened, of whom 60 were enrolled, vaccinated and included in the safety analysis (Fig. 1). Four subjects were excluded from the immunogenicity analysis: 3 subjects at Day 7 after vaccination (2 for incorrect labeling of the serum samples, one for missed visit) and one subject at Day 28 after vaccination for having received tetanus vaccine due to a squirrel bite during the study. Demographic and baseline characteristics of study subjects were similar among the 3 vaccine groups (Table 1).
Figure 1.
Subjects Disposition.
Table 1.
Summary of demographics at baseline by vaccine group.
| Demographics at baseline (Screening day) | BNA's aP | BNA's TdaP | Adacel® | Total | P-value |
|---|---|---|---|---|---|
| Gender: n (%) | 0.81 [1] | ||||
| -Male | 10 (50.00) | 9 (45.00) | 11 (55.00) | 30 (50.00) | |
| -Female | 10 (50.00) | 11 (55.00) | 9 (45.00) | 30 (50.00) | |
| Age | 0.60 [2] | ||||
| -N | 20 | 20 | 20 | 60 | |
| -Mean (SD) | 28.23 (4.67) | 27.00 (4.63) | 28.30 (4.31) | 27.85 (4.50) | |
| -Min/Max | 18.87 – 34.93 | 18.29 – 35.86 | 20.85 – 34.34 | 18.29 – 35.86 | |
| Height (m) | |||||
| -N | 20 | 20 | 20 | 60 | 0.65 [2] |
| -Mean (SD) | 1.62 (0.09) | 1.64 (0.08) | 1.62 (0.06) | 1.63 (0.08) | |
| -Min/Max | 1.52 – 1.84 | 1.52 – 1.75 | 1.52 – 1.74 | 1.52 – 1.84 | |
| Weight (kg) | |||||
| -N | 20 | 20 | 20 | 60 | 0.18 [2] |
| -Mean (SD) | 59.11 (9.89) | 63.74 (13.78) | 66.38 (13.14) | 63.07 (12.55) | |
| -Min/Max | 41.80 – 79.50 | 44.50 – 90.30 | 44.40 – 89.40 | 41.80 – 90.30 |
Note. [1] P-value based on Chi-square test. [2] P-value based on One-way ANOVA.
Safety
No immediate reactions or adverse events were reported during the 4-hour observation period after vaccination. At Day 7 and Day 28 after vaccination, none of the subjects had clinically significant deviation of hemato-chemical and urinalysis tests compared to baseline values (not shown).
Pain at injection site was the most frequently reported (75–85%) local post-immunization reaction (Table 2). A significantly higher incidence of induration (20%), mostly mild in severity, was observed in BioNet's TdaP vaccine group (P < 0.05). One subject reported severe induration which resolved in a few days without sequelae. The systemic post-immunization reactions were similar in all vaccine groups, most of which were mild in severity. The most frequently reported systemic post-immunization reaction was myalgia (10–35%), followed by fatigue (10–25%) and malaise (5–25%) ( Table 2). Mild fever was reported by one subject in the Adacel® group. All post-immunization reactions were transient and resolved without sequelae.
Table 2.
Local and systemic reactions during 7 d after vaccination by vaccine group.
| Visit 2 (Vaccination) | Visit 3 (Day 1) | Visit 4 (Day 7) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BNA's aP | BNA's TdaP | Adacel® | BNA's aP | BNA's TdaP | Adacel® | BNA's aP | BNA's TdaP | Adacel® | ||||
| Local andSystemicReactions |
n (%) |
n (%) |
n (%) |
P-value |
n (%) |
n (%) |
n (%) |
P- value |
n (%) |
n (%) |
n (%) |
P-value |
| Local | ||||||||||||
| Pain | 12 (60.00) | 13 (65.00) | 13 (65.00) | — | 16 (80.00) | 15 (75.00) | 16 (80.00) | — | 16 (80.00) | 15 (75.00) | 17 (85.00) | 0.73 |
| (38.53–81.47) | (44.10–85.90) | (44.10–85.90) | (62.47–97.53) | (60.62–97.28) | (67.81–100.00) | (62.47–97.53) | (56.02–93.98) | (69.35–100.00) | ||||
| Redness | 0 (0.00) | 0 (0.00) | 1 (5.00) | — | 0 (0.00) | 3 (15.00) | 1 (5.00) | 0.12 | 0 (0.00) | 3 (15.00) | 1 (5.00) | 0.15 |
| (0.00–0.00) | (0.00–0.00) | (0.00–14.55) | (0.00–0.00) | (0.00–32.19) | (0.00–15.30) | (0.00–0.00) | (0.00–30.65) | (0.00–14.55) | ||||
| Induration | 0 (0.00) | 1 (5.00) | 0 (0.00) | — | 0 (0.00) | 4 (20.00) | 1 (5.00) | 0.04* | 0 (0.00) | 4 (20.00) | 1 (5.00) | 0.06 |
| (0.00–0.00) | (0.00–14.55) | (0.00–0.00) | (0.00–0.00) | (2.72–39.38) | (0.00–15.30) | (0.00–0.00) | (2.47–37.53) | (0.00–14.55) | ||||
| Systemic | ||||||||||||
| Fever | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (5.00) | 0.66 | 0 (0.00) | 0 (0.00) | 1 (5.00) | — | |
| (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–15.30) | (0.00–0.00) | (0.00–0.00) | (0.00–14.55) | ||||
| Headache | 0 (0.00) | 0 (0.00) | 2 (10.00) | 0.32 | 1 (5.00) | 0 (0.00) | 2 (10.00) | 0.53 | 2 (10.00) | 1 (5.00) | 3 (15.00) | 0.86 |
| (0.00–0.00) | (0.00–0.00) | (0.00–23.15) | (0.00–14.55) | (0.00–0.00) | (0.00–24.33) | (0.00–23.15) | (0.00–14.55) | (0.00–30.65) | ||||
| Fatigue | 2 (10.00) | 0 (0.00) | 2 (10.00) | 0.53 | 2 (10.00) | 2 (10.00) | 5 (25.00) | 0.28 | 3 (15.00) | 2 (10.00) | 5 (25.00) | 0.43 |
| (0.00–23.15) | (0.00–0.00) | (0.00–23.15) | (0.00–23.15) | (0.00–24.33) | (6.52–46.12) | (0.00–30.65) | (0.00–23.15) | (6.02–43.98) | ||||
| Arthralgia | 1 (5.00) | 0 (0.00) | 1 (5.00) | — | 1 (5.00) | 1 (5.00) | 2 (10.00) | 0.84 | 1 (5.00) | 2 (10.00) | 3 (15.00) | 0.86 |
| (0.00–14.55) | (0.00–0.00) | (0.00–14.55) | (0.00–14.55) | (0.00–15.30) | (0.00–24.33) | (0.00–14.55) | (0.00–23.15) | (0.00–30.65) | ||||
| Chills | 1 (5.00) | 0 (0.00) | 0 (0.00) | — | 1 (5.00) | 0 (0.00) | 1 (5.00) | — | 1 (5.00) | 0 (0.00) | 1 (5.00) | — |
| (0.00–14.55) | (0.00–0.00) | (0.00–0.00) | (0.00–14.55) | (0.00–0.00) | (0.00–15.30) | (0.00–14.55) | (0.00–0.00) | (0.00–14.55) | ||||
| Malaise | 1 (5.00) | 0 (0.00) | 1 (5.00) | — | 1 (5.00) | 1 (5.00) | 4 (20.00) | 0.30 | 1 (5.00) | 1 (5.00) | 5 (25.00) | 0.08 |
| (0.00–14.55) | (0.00–0.00) | (0.00–14.55) | (0.00–14.55) | (0.00–15.30) | (2.72–39.38) | (0.00–14.55) | (0.00–14.55) | (6.02–43.98) | ||||
| Myalgia | 1 (5.00) | 1 (5.00) | 2 (10.00) | — | 4 (20.00) | 2 (10.00) | 7 (35.00) | 0.14 | 5 (25.00) | 2 (10.00) | 7 (35.00) | 0.21 |
| (0.00–14.55) | (0.00–14.55) | (0.00–23.15) | (2.47–37.53) | (0.00–24.33) | (15.15–58.53) | (6.02–43.98) | (0.00–23.15) | (14.10–55.90) | ||||
| Vomiting | 0 (0.00) | 0 (0.00) | 0 (0.00) | — | 0 (0.00) | 0 (0.00) | 0 (0.00) | — | 0 (0.00) | 0 (0.00) | 0 (0.00) | — |
| (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | ||||
P-value based on Fisher's exact,
P < 0.05, statistically significant.
During the 28-day study period, unsolicited AEs were reported by 20–25% of subjects in each vaccine group, with similar frequencies. In each group, one subject reported a vaccine-related AE (injection site pain or induration that lasted for more than 7 days). All AEs were transient and resolved without sequelae. One unrelated SAE (dysfunctional uterine bleeding 8 d after vaccination) was reported in one subject in BioNet's aP vaccine group. This female subject, whose pregnancy test was negative at screening, was admitted to the hospital for curettage, after which the SAE resolved without sequelae.
Immunogenicity
ELISA anti-PT, anti-FHA, anti-PRN, anti-tetanus and anti-diphtheria IgG antibodies
Seven days after vaccination, seroresponse rates to PT and PRN were similar in all vaccine groups. Anti-FHA seroresponse rates were significantly higher in BioNet's aP and TdaP than in the Adacel® group (P = 0.001, Table 3A). One month after vaccination, seroresponse rates to PT, FHA and PRN ranged from 78% to 100% in all vaccine groups (Table 3A), with no statistically significant difference.
Table 3A.
Seroresponse rates as defined by the percentage of subjects with ≥ 4-fold increase as compared to baseline values of anti-PT IgG, anti-FHA IgG, anti-PRN IgG and anti-PT neutralizing antibody titers at 7 and 28 d after vaccination.
| Day 7 | Day 28 | |||||||
|---|---|---|---|---|---|---|---|---|
| BNA's aP | BNA's TdaP | Adacel® | BNA's aP | BNA's TdaP | Adacel® | |||
| N = 19 | 18 | 19 | 19 | 18 | 19 | |||
| |
n (%) (95% CI) |
n (%) (95% CI) |
n (%) (95% CI) |
P-value |
n (%) (95% CI) |
n (%) (95% CI) |
n (%) (95% CI) |
P-value |
| Anti-PT ELISA | 9 (47.37) (25.0–70.0) | 8 (44.44) (21.0–67.0) | 11 (57.89) (36.0–80.0) | 0.688 [1] | 17 (89.47) (76.0–100.0) | 17 (94.44) (84.0–100.0) | 16 (84.21) (68.0–100.0) | 0.862 [2] |
| Anti- FHA ELISA | 16 (84.21) (68.0–100.0) | 14 (77.78) (59.0–97.0) | 6 (31.58) (11.0–52.0) | 0.001* [1] | 19 (100.00) (100.0–100.0) | 17 (94.44) (84.0–100.0) | 16 (84.21) (68.0–100.0) | 0.209 [2] |
| Anti-PRN ELISA | 9 (47.37) (25.0–70.0) | 8 (44.44) (21.0–67.0) | 11 (57.89) (36.0–80.0) | 0.688 [1] | 15 (78.95) (61.0–97.0) | 15 (83.33) (66.0–100.0) | 16 (84.21) (68.0–100.0) | 1.000 [2] |
| Anti-PT Nab | 10 (52.63) (30.0–75.0) | 12 (66.67) (45.0–88.0) | 16 (84.21) (68.0–100.0) | 0.110 [1] | 17 (89.47) (76.0–100.0) | 16 (88.89) (74.0–100.0) | 16 (84.21) (68.0–100.0) | 1.000 [1] |
[1] Based on chi-square test, [2] Based on Fisher's exact,
P < 0.05, statistically significant.
Baseline anti-PT, anti-FHA and anti-PRN IgG GMTs were similar across vaccine groups (Table 3B). At 7 d after vaccination, the GMTs for the 3 antigens had only slightly increased in all vaccine groups, except anti-FHA IgG GMTs which were significantly higher (P < 0.01) in BioNet's aP and TdaP than in the Adacel® group (Table 3B). At Day 28, anti-PT and anti-FHA IgG GMTs were significantly higher in BioNet's aP and TdaP vaccine groups [anti-PT antibody: 264.0 IU/mL (95% CI, 113.70–612.92) and 268.5 IU/mL (95% CI, 162.20–444.39), respectively; anti-FHA: 728.0 IU/mL (95% CI, 545.94–970.66) and 666.1 IU/mL (95% CI, 498.61–889.79), respectively] compared to Adacel® group [anti-PT: 50.79 IU/mL (95% CI, 36.98–69.75); anti-FHA: 159.6 IU/mL (95% CI, 114.49–222.49)] (Table 3B).
Day 28 anti-PRN IgG GMTs were higher in Adacel® than BioNet's aP and BioNet's TdaP vaccinees (Table 3B), although the difference was not statistically significant.
At baseline, all subjects in BioNet's TdaP vaccine and Adacel® groups had seroprotective level (≥0.1 IU/mL) of anti-tetanus and anti-diphtheria IgG antibodies. At 7 d after Adacel® immunization, subjects had significantly higher (P < 0.05) anti-diphtheria antibody titers [0.72 IU/mL (95% CI, 0.46–1.12)] than those in BioNet's TdaP vaccine group [0.39 IU/mL (95% CI, 0.24–0.62)]. There was no statistically significant difference in the anti-tetanus and anti-diphtheria GMTs at 28 d after vaccination in both BioNet's TdaP vaccine and Adacel® groups [7.22 IU/mL (95% CI, 5.35–9.76) and 7.66 IU/mL (95% CI, 6.53–8.98), respectively for anti-tetanus antibody and 0.53 IU/mL (95% CI, 0.31–0.90) and 0.88 IU/mL (95% CI, 0.59–1.32), respectively for anti-diphtheria antibody].
PT neutralizing assay
At 7 and 28 d after vaccination, the seroresponse rates to anti-PT neutralizing titers were similar in all 3 vaccines groups with no statistically significant difference (Table 3A).
At 28 d post-immunization, the GMTs of anti-PT neutralizing antibody (Nab) in BioNet's aP [151.5 IU/mL (95% CI, 54.48–421.33)] and TdaP [149.5 IU/mL (95% CI, 81.62–273.74)] vaccinees were significantly higher than in Adacel® group [33.40 IU/mL (95% CI, 21.22–52.58)] (P < 0.01).
Proportion of subjects with ELISA anti-PT IgG and anti-PT Nab above cut-off antibody levels
The proportion of subjects with ELISA anti-PT IgG antibody cut-off titers between 20 and >120 IU/mL is shown in Fig. 2. At 28 d post-immunization, more than 80% of subjects in BioNet's aP and BioNet's TdaP vaccine groups had anti-PT IgG titer above 80 IU/mL compared to approximately 20% of subjects in the Adacel® group (Fig. 2).
Figure 2.
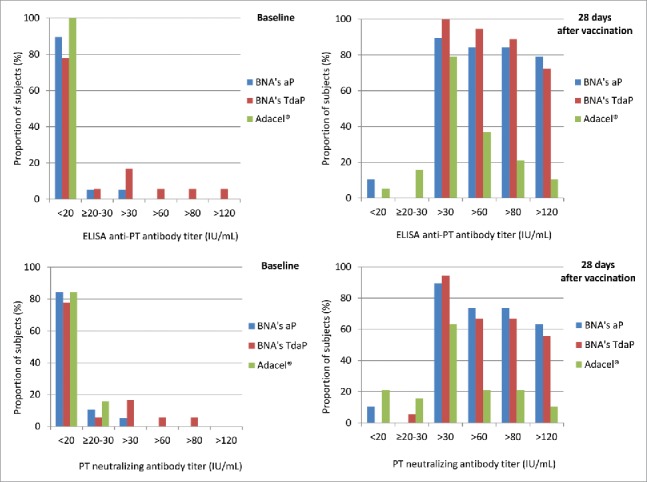
Percentages of subjects with cut-off value titers of ELISA and Nab anti-PT at baseline and 28 d after vaccination.
The distribution of anti-PT Nab titers was similar, with approximately 70% of subjects in BioNet's aP and TdaP vaccine groups with anti-PT Nab titer >80 IU/mL vs approximately 20% of subjects in the Adacel® group (Fig. 2).
Discussion
This first-in-human study indicated a similar reactogenicity and safety profile of BioNet's PTgen-containing vaccines and Adacel®, except for a few more transient local reactions following BioNet's TdaP immunization. It is unlikely that these reactions were due to recent exposure to Tetanus or Diphtheria vaccines as potential volunteers were excluded during screening visit if they had received Tetanus or Diphtheria vaccines within 5 y from study start. However, BioNet's TdaP reactogenicity and safety will be further studied in a larger phase II/III clinical trial.
Adacel® induced significantly higher anti-diphtheria antibody titers at Day 7. However, there was no statistically significant difference at Day 28 in antibody response for diphtheria, tetanus and pertactin antigens among the 3 vaccine groups. On the other hand, antibody titers elicited by PTgen were significantly higher in subjects vaccinated with BioNet's vaccines than Adacel®, whether assessed by ELISA or the CHO cell assay for neutralizing antibodies, confirming the good correlation between ELISA anti-PT and CHO cell assays when PTgen is used as vaccine antigen.20 Anti-FHA GMTs were also significantly higher following immunization with BioNet's vaccines, for reasons which are yet unclear. This suggests that the immunogenicity to PT and FHA of BioNet's formulations may be higher than that of current aP containing vaccines, a hypothesis to be confirmed in Phase II trials. Importantly, these trials may include aP alone or with Td given the similar antibody response to PT, FHA or PRN elicited by BioNet's aP and TdaP vaccines. The use of stand-alone aP vaccines could be of interest for repeat immunization at close intervals, for example at each pregnancy.21
An interesting observation is that on Day 7, no booster effect was observed for anti-PT nor anti–PRN antibodies. This suggests that in Thai adults previously primed with whole-cell vaccines in infancy, baseline PT- and PRN-specific memory B cells were insufficient to enable their prompt reactivation. The Day 7 booster effect was observed for FHA likely reflects the fact that it is a common antigen to other Bordetella spp22 which circulate widely in the population and periodically reactivate FHA immunity upon exposure.
Although immuneresponse to study vaccines was evaluated against BioNet's antigens, it is unlikely that this would have favored BioNet's vaccines. In fact, inter-laboratories collaborative studies have not identified any significant difference in seroresponse to pertussis vaccination or disease when different sources of PT antigens are used.23,24
There is still a search for serological correlates of protection against whooping cough and several candidate pertussis antigens were suggested in some studies.1,2,25-29 But so far correlates of protection induced by vaccination against pertussis have not been clearly established. However, it is well accepted that PT plays a major role in the protection and PT is the only pertussis component included in all types of acellular pertussis vaccines. In addition, countries that have been using monocomponent PT vaccines have effectively controlled pertussis as reported from Denmark on a 15 y nationwide pertussis surveillance study.30 Thus, even though extrapolations should be careful, one may expect enhanced protective efficacy to result from the use of more immunogenic vaccines. A 4-fold increase to baseline titers has been recommended by WHO for the evaluation of responses to new acellular pertussis infant vaccines25 but is unlikely to apply to adults nor correlate with protection. As a rule, pertussis antibody titers of a new vaccine should be compared to a licensed vaccine and the persistence of antibody titers will have to be assessed.25 Adacel® was selected as comparator vaccine as it is the TdaP vaccine most used in Thailand in adolescents with a well-established record of safety and immunogenicity.31
In conclusion, the high immunogenicity of PTgen demonstrated here for the first time in adults is consistent with previous studies that demonstrated high and sustained efficacy of rPT-containing aP vaccines in infants.14,15 It paves the way to the further development of BioNet's aP vaccines.
Methods
Study design and subjects
This study was designed as a phase I/II single-center, observer-blind, randomized, controlled ICH-GCP compliant study to evaluate the safety and immunogenicity of BioNet's TdaP and aP vaccines in healthy subjects aged 18–35 y. Adacel®, a licensed Tdap vaccine (Sanofi Pasteur, Canada) was used as a control. The study was conducted as single center study at the Faculty of Tropical Medicine, Mahidol University, Thailand between June and December 2014.
A pamphlet, previously approved by EC, containing brief information on the study was distributed to hospital visitors. Potential volunteers who were interested in participating into the study contacted the study site. After written informed consent was obtained, the subjects were screened according to predefined inclusion (be between 18 to 35 y of age; be free of obvious health problems as established by medical history and screening evaluations including physical examination and laboratory test; capable to comply with the study protocol; for women, willing to take reliable birth control measures for entire study duration) and exclusion criteria (history of significant medical illness such as immune deficiency, uncontrolled diabetes or hypertension, heart or renal or hepatic diseases; pregnant or breast-feeding women; history of allergy to any vaccine component; history of serious adverse event or neurological adverse event after injection with DTP vaccine; having received any Diphtheria or Tetanus or Pertussis vaccine within 5 y prior enrollment in the present study; individuals with any progressive or severe neurological disorder, seizure disorder or Guillain-Barré syndrome; history of alcoholism and/or intravenous drug abuse; history of any illness that, in the opinion of the investigator, might interfere with the results of the study or pose additional risk to the subjects due to participation in the study; for subjects screened at Day -14 to Day -1, presence of any clinically or laboratory significant abnormality on physical examination or laboratory tests upon investigator judgment) and hemato-chemical laboratory tests (WBC,RBC,HG,HT, Platelets, Differential WBC, ESR, liver and renal functionality tests. For the complete list please refer to http://www.clinicaltrials.in.th/ as TCTR20140703001). Those eligible to enter into the study were randomly assigned in a 1:1:1 ratio to receive one intramuscular injection of one of the 3 study vaccines in the non-dominant deltoid region. PROC PLAN (SAS version 9.2) was used to generate the randomization list with blocks of three. As the syringe containing Adacel® had different appearance than BioNet's vaccines syringes, at study site, the unblind personnel was responsible for subjects randomization, vaccine preparation, administration and accountability. All other site study staff was blind to vaccine assignment groups as well as data management personnel, statistician and laboratory staff. The study was approved by the Institutional Ethics Committee (MUTM 2014-018-01) at the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand and registered at http://www.clinicaltrials.in.th/ as TCTR20140703001. The study was conducted according to ICH-GCP, Helsinki Declaration and local ethical guidelines.
Study vaccines
BioNet's recombinant acellular Pertussis vaccine contains PTgen, FHA (Filamenteous Hemagglutinin) and PRN (Pertactin), in proportions similar to the one included in the first generation recombinant pertussis vaccines commercialized in the 90s.
A single dose (0.5 mL) of each study vaccine composition is listed in Table 4.
Table 3B.
ELISA anti-PT, anti-FHA and anti-PRN IgG Geometric Mean Titers at baseline and 7 and 28 d after vaccination.
| PT |
FHA |
PRN |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Day 7 | Day 28 | Baseline | Day 7 | Day 28 | Baseline | Day 7 | Day 28 | |
| |
GMT (IU/mL)(95% CI) |
GMT (IU/mL)(95% CI) |
GMT (IU/mL)(95% CI) |
GMT (IU/mL)(95% CI) |
GMT (IU/mL)(95% CI) |
GMT (IU/mL)(95% CI) |
GMT (IU/mL)(95% CI) |
GMT (IU/mL)(95% CI) |
GMT (IU/mL)(95% CI) |
| Vaccine | |||||||||
| BNA's aP | 6.98 (5.37–9.07) | 31.38 (12.92–76.24) | 264.0b (113.70–612.92) | 12.57 (8.27–19.11) | 130.2b (78.59–215.62) | 728.0b (545.94–970.66) | 9.20 (5.84–14.47) | 28.77 (11.95–69.31) | 120.4 (51.57–280.90) |
| BNA's TdaP | 10.00 (5.78–17.31) | 41.46 (17.67–97.29) | 268.5b (162.20–444.39) | 15.50 (8.95–26.84) | 154.0b (102.66–230.90) | 666.1b (498.61–889.79) | 7.91 (5.63–11.13) | 29.73 (14.31–61.79) | 118.3 (59.02–237.11) |
| Adacel® | 6.77 (5.43–8.44) | 25.98 (16.54–40.80) | 50.79a (36.98–69.75) | 16.64 (11.05–25.05) | 60.38a (43.22–84.36) | 159.6a (114.49–222.49) | 8.48 (6.33–11.37) | 49.46 (21.32–114.75) | 175.0 (77.51–395.11) |
| P-value | 0.226 | 0.655 | <0.001* | 0.640 | 0.004* | <0.001* | 0.835 | 0.548 | 0.708 |
P-value based on One-way ANOVA,
P < 0.05, statistically significant.
Table 4.
Study vaccines composition.
| Vaccine composition per 0.5-mL of individual dose | BNA's aP* | BNA's TdaP** | Adacel®*** |
|---|---|---|---|
| Active Ingredients | |||
| Tetanus Toxoid (TT) | — | 7.5 Lf | 5 Lf |
| Diphtheria Toxoid (DT) | — | 2.0 Lf | 2 Lf |
| Pertussis Toxoid (PT) | 5 µg**** | 5 µg**** | 2.5 µg |
| Filamentous hemagglutinin (FHA) | 5 µg | 5 µg | 5 µg |
| Pertactin (PRN) | 2.5 µg | 2.5 µg | 3 µg |
| Fimbriae type 2/3 | — | — | 5 µg |
| Excipients | |||
| Aluminum content | 0.3 mg | 0.3 mg | 0.33 mg |
Lot No. PER4001-1.
Lot No. TDA4001-1.
Lot U4700BA.
containing PTgen.
All study vaccines were presented as monodose prefilled syringe for intramuscular administration.
Safety assessment
After vaccination subjects were observed at study site for 4 hours for immediate or any adverse reactions. Subjects were asked to return for a visit at 1, 7 and 28 d after vaccination. Blood samples were taken at screening visit (considered as baseline), 7 and 28 d after vaccination for routine hematology, blood chemistry and urinalysis tests and for evaluation of vaccine immunogenicity. The day of vaccination diary cards were distributed to study subjects to record solicited local (pain, redness and induration) and systemic (fever, headache, fatigue, arthralgia, chills, malaise, myalgia and vomiting) reactions for 7 d after vaccination, as well as any other adverse events (AEs) including serious adverse events (SAEs) for the entire study period. AEs and SAEs relationship to study vaccines was determined by the investigator according to ICH guidelines and specified in the protocol.
Immunogenicity assessment
Anti-PT, anti-FHA and anti-PRN IgG antibodies were measured by standardized enzyme-linked immunosorbent assay (ELISA) in the Center for Vaccinology (Geneva). Plates were coated with either BioNet's purified PTgen, FHA or PRN at 4°C overnight, then blocked with bovine serum albumin (BSA). Serial dilutions of the World Health Organization (WHO) International Standard Pertussis Antiserum (Human) 06/140, WHO Reference Reagent Pertussis Antiserum (Human) 06/142 (NIBSC, UK) and serum samples were incubated at room temperature for 30 minutes. The limit of detection was 1 IU/mL. Seroresponse was defined as 4-fold increase of antibody titers compared to baseline titers (seroconversion). Anti-tetanus and anti-tetanus IgG were determined similarly, using SERION ELISA classic (Virion/Serion, Germany) with protective cut-off at 0.1 IU/mL. Samples with titers below the assay cut-off were arbitrarily attributed a titer of 50% of the cut-off to allow for statistical analyses. Anti-Fimbriae antibodies were not evaluated in this study.
Functional anti-PT antibody was assessed by measuring the PT neutralizing titer in Chinese Hamster Ovary (CHO) cells. Two-fold serial dilutions of the clinical sera were added to a 96- well reaction plate, before incubating with the standard PT, JNIH-5 at room temperature for PT-toxin neutralization reaction. The reacting sera were then incubated in CHO cells at 37°C for 48 hours. The highest dilution of complete neutralization observed as the absence of cell clustering of crystal violet stained CHO cells was considered the end point dilution. The PT neutralizing titer was reported as IU/mL based on the relative activity of the WHO International Standard Pertussis Antiserum (Human) 06/140. Samples with titers below the assay cut-off were arbitrarily attributed a titer of 50% of the cut-off to allow for statistical analyses.
Data management and statistical analysis
Data management and statistical analyses were performed by the Center of Excellence for Biomedical and Public Health Informatics (BIOPHICS), Bangkok, Thailand using Statistical Analysis System (SAS) version 9.2. The seroresponse rate and geometric mean titer (GMT) were calculated with exact 95% Confidence Interval (CI). The difference between each vaccine group was assessed by either Chi-square or Fisher's exact test for categorical variables and Mann-Whitney U test for continuous variables. P < 0.05 was considered to be statistically significant.
Disclosure of potential conflicts of interest
PHT, JP, SV, WW, MC and PChi are employed by BioNet-Asia Co., Ltd. CAS was appointed as member of the BioNet-Asia Scientific Advisory Board at its creation in March 2016, after completion of this study.
Acknowledgments
The authors thank all study investigators, clinical study site staff, and participating subjects at the Faculty of Tropical Medicine, Mahidol University for their contribution to the study. The authors would also like to thank BioNet-Asia's team for their contribution; Aruna Poredi, Aucha Sachair and Walaya Amornratanayut for clinical monitoring; Indrajeet Poredi for vaccine testing, Greanggrai Hommalai for CHO serological assay and Porntip Wudtisun for regulatory submissions. Special thanks to Vitoon Vonghangool for his continuous support and to Drs Nicole Guiso and Stanley Plotkin, members of BioNet-Asia Scientific Advisory Board, for reviewing the manuscript.
Funding
This study was funded by BioNet-Asia Co., Ltd., Thailand.
Author contributions
SV, WW, JP, PHT, MC and PChi contributed to the design of the study. CS, PC and KL conducted the clinical study. CAS performed ELISA assays and provided input to the study design. All authors had access to the statistical analysis report and contributed to interpretation of the results. All authors contributed to drafting the manuscript, reviewing and approving the final version.
References
- [1].World Health Organization (WHO) Pertussis vaccines: WHO position paper, August 2015. Recommendations. Vaccine. 2016; 34(12):1423-5; http://dx.doi.org/ 10.1016/j.vaccine.2015.10.136. Epub 2015 Nov 10. [DOI] [PubMed] [Google Scholar]
- [2].Zhang L, Prietsch SO, Axelsson I, Halperin SA. Acellular vaccines for preventing whooping cough in children. Cochrane Database Syst Rev 2014; (9):CD001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Libster R, Edwards KM. Re-emergence of pertussis: what are the solutions? Expert Rev Vaccines 2012; 11:1331-46; PMID:23249233; http://dx.doi.org/ 10.1586/erv.12.118 [DOI] [PubMed] [Google Scholar]
- [4].Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn J, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385:430-40; PMID:25280870; http://dx.doi.org/ 10.1016/S0140-6736(14)61698-6 [DOI] [PubMed] [Google Scholar]
- [5].Hiappini E, Stival A, Galli L, de Martino M. Pertussis re-emergence in the post-vaccination era. BMC Infect Dis. 2013;13:151 . [DOI] [PMC free article] [PubMed]
- [6].Quinn HE, Snelling TL, Macartney KK, McIntyre PB. Duration of protection after first dose of acellular pertussis vaccine in infants. Pediatrics 2014; 133:e513-9; PMID:24515514; http://dx.doi.org/ 10.1542/peds.2013-3181 [DOI] [PubMed] [Google Scholar]
- [7].Acosta AM, Debolt C, Tasslimi A, Lewis M, Stewart LK. Tdap vaccine effectiveness in adolescents during the 2012 Washington State pertussis epidemic. Pediatrics 2015; 135:981-9; PMID:25941309; http://dx.doi.org/ 10.1542/peds.2014-3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ibsen PH. The effect of formaldehyde, hydrogen peroxide and genetic detoxification of pertussis toxin on epitope recognition by murine monoclonal antibodies. Vaccine 1996; 14:359-68; PMID:8735545; http://dx.doi.org/ 10.1016/0264-410X(95)00230-X [DOI] [PubMed] [Google Scholar]
- [9].Sutherland JN, Chang C, Yoder SM, Rock MT, Maynard JA. Antibodies recognizing protective pertussis toxin epitopes are preferentially elicited by natural infection versus acellular immunization. Clin Vaccine Immunol 2011; 18:954-62; PMID:21508166; http://dx.doi.org/ 10.1128/CVI.00561-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ausiello CM, Urbani F, Sala A, Lande R, Cassone A. Vaccine- and Antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect Immun 1997; 65(6):2168-74; PMID:9169747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Higgs R, Higgins SC, Ross PJ, Mills KHG. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol 2012; 5:485-500; PMID:22718262 [DOI] [PubMed] [Google Scholar]
- [12].Mooi FR, Van Der Maas NA, De Melker HE. Pertussis resurgence: waning immunity and pathogen adaptation - two sides of the same coin. Epidemiology and infection 2014; 142:685-94; PMID:23406868; http://dx.doi.org/ 10.1017/S0950268813000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hegerle N, Guiso N. Bordetella pertussis and pertactin-deficient clinical isolates: lessons for pertussis vaccines. Expert Rev Vaccines 2014; 13:1135-46; PMID:24953157; http://dx.doi.org/ 10.1586/14760584.2014.932254 [DOI] [PubMed] [Google Scholar]
- [14].Plotkin S. The pertussis problem. Clin Infect Dis 2014; 58:830-3; PMID:24363332; http://dx.doi.org/ 10.1093/cid/cit934 [DOI] [PubMed] [Google Scholar]
- [15].Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi AE, Anemona A, Ciofi degli Atti ML, Giammanco A, Panei P, Blackwelder WC, et al.. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N Engl J Med 1996; 334:341-8; PMID:8538704; http://dx.doi.org/ 10.1056/NEJM199602083340601 [DOI] [PubMed] [Google Scholar]
- [16].Salmaso S, Mastrantonio P, Tozzi AE, Stefanelli P, Anemona A, Ciofi degli Atti ML, Giammanco A. Sustained efficacy during the first 6 years of life of 3-component acellular pertussis vaccines administered in infancy: the Italian experience. Pediatrics 2001; 108:E81; PMID:11694665; http://dx.doi.org/ 10.1542/peds.108.5.e81 [DOI] [PubMed] [Google Scholar]
- [17].Di Tommaso A, De Magistris MT, Bugnoli M, Marsili I, Rappuoli R, Abrignani S. Formaldehyde treatment of proteins can constrain presentation to T cells by limiting antigen processing. Infect Immun 1994; 62:1830-4; PMID:7513307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Buasri W, Impoolsup A, Boonchird C, Luengchaichawange A, Prompiboon P, Petre J, Panbangred W. Construction of Bordetella pertussis strains with enhanced production of genetically-inactivated Pertussis Toxin and Pertactin by unmarked allelic exchange. BMC Microbiol 2012; 12:61; PMID:22524455; http://dx.doi.org/ 10.1186/1471-2180-12-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Seubert A, D'Oro U, Scarselli M, Pizza M. Genetically detoxified pertussis toxin (PT-9K/129G): implications for immunization and vaccines. Expert Rev Vaccines 2014; 13:1191-204; PMID:25183193; http://dx.doi.org/ 10.1586/14760584.2014.942641 [DOI] [PubMed] [Google Scholar]
- [20].Meade BD, Lynn F, Reed GF, Mink CM, Romani T, Deforest A, Deloria MA. Relationships between functional assays and enzyme immunoassays as measurements of responses to acellular and whole-cell pertussis vaccines. Pediatrics 1995; 96:595-600; PMID:7659484 [PubMed] [Google Scholar]
- [21].Broder KR, Cortese MM, Iskander JK, Kretsinger K, Slade BA, Brown KH, Mijalski CM, Tiwari T, Weston EJ, Cohn AC, et al.. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1-34 [PubMed] [Google Scholar]
- [22].Watanabe M, Nagai M. Reciprocal protective immunity against Bordetella pertussis and Bordetella parapertussis in a murine model of respiratory infection. Infect Immun 2001; 69:6981-6; http://dx.doi.org/ 10.1128/IAI.69.11.6981-6986.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xing D, Markey K, Newland P, Rigsby P, Hockley J, He Q. EUVAC NET collaborative study: Evaluation and standardisation of serology for diagnosis of pertussis. J Immunol Methods 2011; 372:137-45 [DOI] [PubMed] [Google Scholar]
- [24].Kapasi A, Meade BD, Plikaytis B, Pawloski L, Martin MD, Yoder S, Rock MT, Coddens S, Haezebroeck V, et al.. Comparative study of different sources of pertussis toxin (PT) as coating antigens in IgG anti-PT enzyme-linked immunosorbent assays. Clin Vaccine Immunol 2012; 19(1):64-72; http://dx.doi.org/ 10.1128/CVI.05460-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Recommendations to assure the quality, safety and efficacy of acellular pertussis vaccines. Replacement of Annex 2 of WHO Technical Report Series, No. 878. In: WHO Expert Committee on Biological Standardization. Sixty-second report. Geneva, World Health Organization, 2013 (WHO Technical Report Series, No. 927), Annex 4. [Google Scholar]
- [26].Taranger J, Trollfors B, Lagergård T, Sundh V, Bryla DA, Schneerson R, Robbins JB. Correlation between pertussis toxin IgG antibodies in postvaccination sera and subsequent protection against pertussis. J Infect Dis 2000; 181:1010-3; PMID:10720524; http://dx.doi.org/ 10.1086/315318 [DOI] [PubMed] [Google Scholar]
- [27].Gorringe AR, Vaughan TE. Bordetella pertussis fimbriae (Fim): relevance for vaccines. Expert Rev Vaccines 2014; 13:1205-14; PMID:25102891; http://dx.doi.org/ 10.1586/14760584.2014.930667 [DOI] [PubMed] [Google Scholar]
- [28].Olin P, Hallander HO, Gustafsson L, Reizenstein E, Storsaeter J. How to make sense of pertussis immunogenicity data. Clin Infect Dis 2001; 33 Suppl 4:S288-91; PMID:11709761; http://dx.doi.org/ 10.1086/322564 [DOI] [PubMed] [Google Scholar]
- [29].Cherry JD, Gornbein J, Heininger U, Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 1998; 16:1901-6; PMID:9796041; http://dx.doi.org/ 10.1016/S0264-410X(98)00226-6 [DOI] [PubMed] [Google Scholar]
- [30].Thierry-Carstensen B, Dalby T, Stevner MA, Robbins JB, Schneerson R, Trollfors B. Experience with monocomponent acellular pertussis combination vaccines for infants, children, adolescents and adults–a review of safety, immunogenicity, efficacy and effectiveness studies and 15 years of field experience. Vaccine 2013; 31(45):5178-91; http://dx.doi.org/ 10.1016/j.vaccine.2013.08.034 [DOI] [PubMed] [Google Scholar]
- [31].Sanofi Pasteur 306 - Adacel® Full Prescribing Information. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM142764.pdf [Google Scholar]