ABSTRACT
Ebola virus disease (EVD) has become a great threat to humans across the world in recent years. The 2014 Ebola epidemic in West Africa caused numerous deaths and attracted worldwide attentions. Since no specific drugs and treatments against EVD was available, vaccination was considered as the most promising and effective method of controlling this epidemic. So far, 7 vaccine candidates had been developed and evaluated through clinical trials. Among them, the recombinant vesicular stomatitis virus-based vaccine (rVSV-EBOV) is the most promising candidate, which demonstrated a significant protection against EVD in phase III clinical trial. However, several concerns were still associated with the Ebola vaccine candidates, including the safety profile in some particular populations, the immunization schedule for emergency vaccination, and the persistence of the protection. We retrospectively reviewed the current development of Ebola vaccines and discussed issues and challenges remaining to be investigated in the future.
KEYWORDS: clinical trial, Ebola virus, efficacy, immunogenicity, safety, vaccine
Introduction
Ebola virus was first identified in Central Africa with 2 simultaneous outbreaks in Sudan and Zaire (now Democratic Republic of the Congo) separately in 1976, and then be named after the Ebola River in Zaire.1 Ebola virus is an enveloped, single stranded, negative sense RNA virus, which comprises 5 distinct subspecies: Zaire Ebola virus (EBOV), Sudan Ebola virus (SUDV), Bundibugyo Ebola virus (BDBV), Tai Forest Ebola virus (TAFV) and Reston Ebola virus (RESTV).2 Among them, EBOV is the most dangerous subspecies which cause a high case fatality among human and non-human primates (NHPs).3-5 Ebola virus could lead to EVD, formerly known as Ebola hemorrhagic fever,6 which is a severe acute viral illness and often characterized by the sudden onset of fever, weakness, headache, muscle pain, sore throat, hiccups conjunctivitis, red eyes, rash, diarrhea, vomiting, internal and external bleeding.6 Ebola virus can transmit from animals to humans and then spread among human beings quickly through closely contacts with the infected blood, bodily fluids or tissues. Moreover, the release of Ebola virus by small-particle aerosol dispersion would probably result in mucosal infection.6 Due to the high level of infectivity and severity, Ebola virus has been listed as Biosafety Level-4 Virus by World Health Organization (WHO).
The 2014 West Africa outbreak is the largest and the most serious Ebola virus outbreak in history, with an approximate total reported cases count of 28646 and 11323 deaths till 27 March, 2016.7 This EVD outbreak had spread from Guinea into Liberia, Nigeria, Senegal, Sierra Leone and Mali, with individual case exportations or transport of patients to France, Germany, Norway, Spain, United Kingdom (UK), and United States (US), causing a worldwide alarm.6 Since there were no specific drugs or treatments for EVD, vaccination was considered as a most efficient method to control the spreading of Ebola virus. Several Ebola vaccine development campaigns were swiftly launched for clinical trials in order to cope with the Ebola epidemics.8 In this review, we aim to provide an overview of the current research and development of vaccine candidates against Ebola virus, including the efficacy, immunogenicity, and safety profiles of the vaccines, and discuss further research topics and directions in the future.
Structural and functional characteristics of Ebola
The mature Ebola virion is comprised of 2 main components that nucleocapsid and envelope. The glycoprotein (GP) spikes form on the surface of envelope and the matrix consisted of virion protein (VP) 24 and VP40 locates in the middle of nucleocapsid and envelope (Fig. 1A). The Ebola virus genome is 19kb in size and comprised of 7 non-segmented genes, which encodes the nucleoprotein (NP), VP35, VP40, GP, VP30, VP24 and RNA-dependent RNA polymerase (L), respectively9 (Fig. 1B). The viral RNA genome is encapsidated by the NP and together with L, the L cofactor VP35 and the transcriptional activator VP30, as well as VP24, which form a central nucleocapsid in the virus particle.10 The structure of Ebola virus NP, VP35, VP30 and L are responsible for replication and transcription of viral RNA, while VP40 and VP24 are responsible for assembly, budding and release of virion particles. Furthermore, VP35 and VP24 are implicated in immune evasion by blocking interferon (IFN) production and signaling. The former blocks detection of the dsRNA stage of viral replication/transcription, while the latter blocks a number of IFN signaling pathways.11 The surface GP is a multimer of a single structural GP, which is responsible for cell attachment, fusion and cell entry, helps in immune evasion and plays a role in pathogenesis of disease.8 The role of the viral GP makes it a key antigenic target for designing new Ebola vaccine candidates and immunotherapies.
Figure 1.
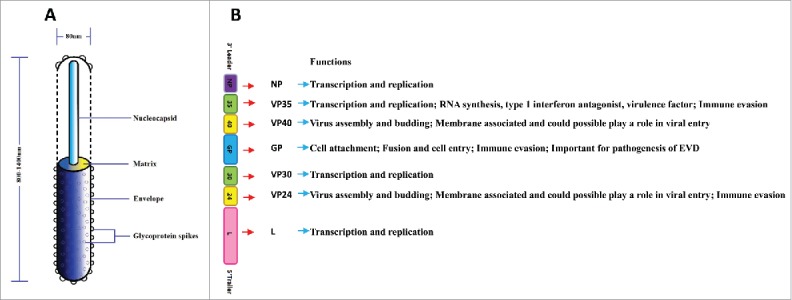
Structure and functions of Ebola virus and Ebola virus genes. (A) Shown are the structure of Ebola virus which comprised of two main factors that nucleocapsid and envelope. In the middle of nucleocapsid and envelope is matrix which is comprised of VP40 and VP24. Glycoprotein spikes are located on the surface of envelope. (B) Shown are schematic representations of Ebola virus genome which is comprised of seven non-segmented genes. These seven genes encode the nucleoprotein, virion protein35, VP40, glycoprotein, VP30, VP24 and RNA-dependent RNA polymerase.
Overview of the development of Ebola vaccines
The research on Ebola vaccines had already started in 1980 after the first discovery of Ebola virus. However, most of the researches were still in the animal study stages before 2014, and no evidence of protection in human beings had been obtained. Results from pre-clinical trial studies indicated that both humoral and cellular immunity play an essential role in controlling and eliminating virus due to the spiking of Ebola virus envelope GP forms on the surface of mature virions.12-14
The 2014 Ebola epidemic has significantly accelerated the development of Ebola vaccines, 46 clinical trials with Ebola vaccines were launched according to the registration on Clinicaltrial.gov and Pan African Clinical Trials Registry since then (Tables 1 and 2).15 Different kinds of Ebola vaccines had been developed and evaluated, which can be roughly divided into 3 categories: non-replicative vector-based Ebola vaccines, replicative vector-based Ebola vaccines and others (Fig. 2). Non-replicative vector-based Ebola vaccines are those vaccines based on adapted vectors encoding the GP or other antigens of Ebola with deletions of genes essential for the life cycle of the vector virus to restrict the transcription and replication, including modified vaccinia strain Ankara (MVA)-vectored vaccines, venezuelan equine encephalitis virus (VEEV)-like replicon particles vaccine, human adenovirus vector-based Ebola vaccines, replication-defective recombinant chimpanzee adenovirus type 3-vectored vaccine (ChAd3 vaccine) and Kunjin replicon virus-like particle vaccine (KUN VLPs). Though the non-replicative vector-based Ebola vaccines were considered to have a better tolerability profile without causing a viremia after vaccination, high dosage of viral particles (vp) were needed to elicit a significant response. While the replicative vector-based Ebola vaccines could encode Ebola antigens with replicative vectors which were highly efficient with relatively low dosage, including rVSV-EBOV, human parainfluenza virus type 3-based vaccine (HPIV3), recombinant cytomegalovirus (rCMV)-based vaccine and recombinant rabies virus (RABV)-based vaccine. However, there are still some safety concerns associated with the replicative vector-based vaccines. Other Ebola vaccines included inactivated Ebola vaccine, DNA vaccine encoding the GP from EBOV and SUDV, virus-like particles (VLPs) and recombinant EBOVΔVP30 (rEBOVΔVP30). The timeline of the development of various Ebola vaccines were showed in Fig. 3. The rVSV-EBOV, ChAd3 vaccine, Ad26-EBOV, Ad5-EBOV, HPIV3, DNA vaccine and MVA-vectored vaccine have been evaluated in clinical trials, and the rVSV-EBOV has successfully showed a high protection against EVD from the preliminary results of a phase III trial conducted in West Africa.
Table 1.
List of clinical trials which are single use of promising candidates.
| Single Use Candidate Vaccines | Current Status | Sponser | Start Date | Phase | Estimated Enrollments | Ages | Locations | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|---|---|
| Ad5-EBOV | Completed | NIAID | Sept.2010 | 1 | 48 | 18-50 | USA | NCT00374309 |
| Ad5-EBOV | Completed | JSCDC | Dec.2014 | 1 | 120 | 18-60 | China | NCT02326194 |
| Completed | JSCDC | Jul.2015 | 1 | 110 | 18-60 | China | NCT02533791 | |
| Completed | FAHZU | May.2015 | 1 | 61 | 18-60 | China | NCT02401373 | |
| Ongoing | JSCDC | Oct.2015 | 2 | 500 | 18-50 | Sierra Leone | NCT02575456 | |
| DNA | Completed | NIAID | Oct.2003 | 1 | 27 | 18-44 | USA | NCT00072605 |
| Completed | NIAID | Jan.2008 | 1 | 20 | 18-60 | USA | NCT00605514 | |
| Completed | NIAID | Fre.2010 | 1 | 108 | 18-50 | Uganda | NCT00997607 | |
| rVSV-EBOV | Completed | Merck Sharp & Dohme Corp | Oct.2014 | 1 | 120 | 18-65 | USA | NCT02280408 |
| Completed | Merck Sharp & Dohme Corp | Oct.2014 | 1 | 39 | 18-50 | USA | NCT02269423 | |
| Completed | Dalhousie University | Nov.2014 | 1 | 40 | 18-65 | Not Provided | NCT02374385 | |
| Completed | UHE | Nov.2014 | 1 | 30 | 18-65 | Germany | NCT02283099 | |
| Completed | Merck Sharp & Dohme Corp | Dec.2014 | 1 | 512 | 18-60 | USA | NCT02314923 | |
| Completed | University Hospital, Geneva | Dec.2014 | 1 & 2 | 115 | 18-65 | Switzerland | NCT02287480 | |
| Ongoing | University of Oxford | Dec.2014 | 1 | 40 | 18-55 | Kenya | NCT02296983 | |
| Ongoing | Novavax | Feb.2015 | 1 | 230 | 18-50 | Australia | NCT02370589 | |
| Ongoing | CDC | Apr.2015 | 2 & 3 | 8000 | 18+ | Sierra Leone | NCT02378753 | |
| Ongoing | Merck Sharp & Dohme Corp | Aug.2015 | 3 | 1198 | 18-65 | USA, Canada, Spain | NCT02503202 | |
| Ongoing | Profectus BioSciences, Inc. | Jan.2016 | 1 | 38 | 18-60 | USA | NCT02718469 | |
| Not recruit | NIAID | Jan.2016 | 2 | 300 | 18+ | USA | NCT02788227 | |
| cAd3-EBO | Completed | CHUV | Oct.2014 | 1 & 2 | 120 | 18-65 | Switzerland | NCT02289027 |
| Ongoing | GSK | Jul.2015 | 2 | 2796 | 18+ | Senegal | NCT02485301 | |
| cAd3-EBO/ChAd3-EBO-Z | Ongoing | NIAID | Aug.2014 | 1 | 50 | 18-65 | USA | NCT02231866 |
| HPIV3-EBO-Z | Ongoing | NIAID | Aug.2015 | 1 | 30 | 18-50 | USA | NCT02564575 |
Abbreviations: NIAID = National Institute of Allergy and Infectious Diseases; JSCDC = Jiangsu Province Centers for Disease Control and Prevention FAHZU = First Affiliated Hospital of Zhejiang University; NLGC = NewLink Genetics Corporation; UHE = Universitätsklinikum Hamburg-Eppendorf; CDC = Centers for Disease Control and Prevention; CHUV = Center Hospitalier Universitaire Vaudois; GSK = GlaxoSmithKline; USA = United States.
Table 2.
List of clinical trials which are combined use of promising candidates.
| Combine Use Candidate Vaccines | Current Status | Sponser | Start Date | Phase | Estimated Enrollments | Ages | Locations | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|---|---|
| Ad26-ZEBOV+MVA-BN Filo | Completed | Crucell Holland BV | Dec.2014 | 1 | 88 | 18-50 | UK | NCT02313077 |
| Ongoing | Crucell Holland BV | Jan.2015 | 1 | 164 | 18-50 | USA | NCT02325050 | |
| Ongoing | Crucell Holland BV | Apr.2015 | 1 | 78 | 18-50 | Uganda;Tanzania | NCT02376400 | |
| Ongoing | Crucell Holland BV | Mar.2015 | 1 | 72 | 18-50 | Ghana; Kenya | NCT02376426 | |
| Recruiting | Crucell Holland BV | Jun.2015 | 2 | 612 | 18-65 | France, UK | NCT02416453 | |
| Recruiting | Crucell Holland BV | Sept.2015 | 3 | 525 | 18-50 | USA | NCT02543567 | |
| Recruiting | Crucell Holland BV | Sept.2015 | 3 | 728 | 1-65 | Sierra Leone | NCT02509494 | |
| Recruiting | Crucell Holland BV | Oct.2015 | 2 | 1188 | 1-70 | Africa countries | NCT02564523 | |
| Ongoing | Crucell Holland BV | Sept.2015 | 3 | 329 | 18-50 | USA | NCT02543268 | |
| Recruiting | Crucell Holland BV | Jan.2016 | 2 | 575 | 18-70 | USA,Kenya,Nigeria | NCT02598388 | |
| Recruiting | Crucell Holland BV | Jan.2016 | 4 | 5500 | 1-71 | Not Provided | NCT02661464 | |
| Ad26.Filo+MVA-BN Filo | Not recruit | Janssen Vaccines & Prevention B.V. | Aug.2016 | 1 | 72 | 18-50 | USA | NCT02860650 |
| ChAd3-EBO-Z+MVA-BN Filo | Recruiting | University of Oxford | Dec.2014 | 1 | 92 | 18-50 | USA | NCT02240875 |
| Ongoing | University of Maryland | Nov.2014 | 1 | 91 | 18-50 | Mali | NCT02267109 | |
| ChAd3-EBO-Z+MVA-ZEBOV | Completed | University of Oxford | Jul.2015 | 1 | 40 | 18-50 | Senegal | NCT02485912 |
| Ongoing | University of Oxford | Apr.2015 | 1 | 38 | 18-50 | UK | NCT02451891 | |
| cAd3-EBO+MVA-ZEBOV | Ongoing | University of Maryland | May.2015 | 1 | 60 | 18-65 | Mali | NCT02368119 |
| Ongoing | NIAID | Mar.2015 | 1 | 64 | 18-66 | USA | NCT02408913 | |
| cAd3-EBO/ChAd3-EBO-Z+MVA-ZEBOV | Ongoing | NIAID | Jan.2015 | 1 | 90 | 18-65 | Uganda | NCT02354404 |
| ChAd3-EBO-Z+Ad26-ZEBOV | Ongoing | University of Oxford | Sept.2015 | 1 | 32 | 18-50 | UK | NCT02495246 |
| ChAd3-EBO-Z+rVSV-EBOV | Ongoing | NIAID | Jan.2015 | 2 | 28170 | 18+ | Liberia | NCT02344407 |
| ChAd3-EBO-Z+Nimenrix | Ongoing | GSK | Nov.2015 | 2 | 600 | 1-17 | Not Provided | NCT02548078 |
Abbreviations: NIAID = National Institute of Allergy and Infectious Diseases; GSK = GlaxoSmithKline; USA = United States; UK = United Kingdom.
Figure 2.
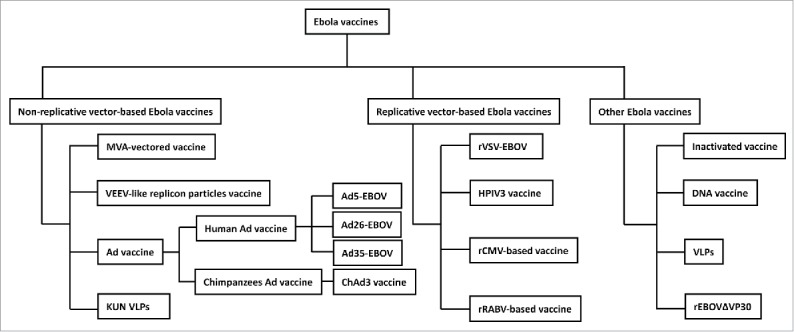
Classification of Ebola vaccines. Ebola vaccines can be roughly divided into three classifies that non-replicative vector-based Ebola vaccines, replicative vector-based Ebola virus vaccines and other Ebola vaccines.
Figure 3.
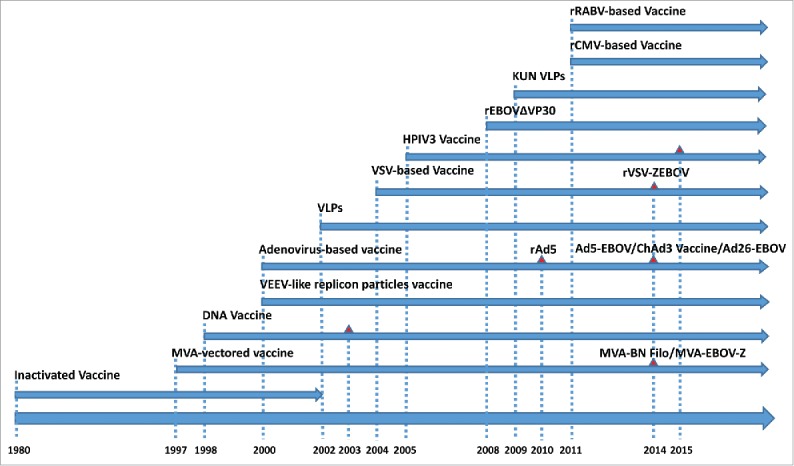
Development history of Ebola virus vaccines. Shown are schematic representations of the sequence of development of Ebola virus vaccines and the start time of clinical trials. Each line represents the development of a kind of vaccine with its name ahead. The red triangle represents the start point of clinical trials of promising candidate. Almost every vaccine continues research up to date except inactivated vaccine which was stopped in 2002.
Non-replicative vector-based Ebola vaccines
VEEV-like replicon particle vaccines
VEEV is a positive-sense RNA virus.16 By replacement of VEEV structural protein genes, the Ebola NP or GP gene was packaged into a recombinant propagation-deficient VEEV replicon particles (VRP) expressed from an RNA expression vector and defined as NP-VRP or GP-VRP, respectively.17 Guinea pigs were inoculated subcutaneously with a total of 0.5 ml containing 107 infectious units (IU)/ml of VRP then challenged subcutaneously with 1000 LD50 (104 plaque-forming units (PFU)) of guinea pig-adapted Ebola virus, while BALB/c mice were inoculated subcutaneously with 0.2 ml containing 106 IU of VRP then challenged intraperitoneally with 30-300 LD50 (1-10 PFU) of mouse-adapted Ebola virus.17 Complete protection was noticed in both mice and guinea pigs after the vaccination of GP-VRP alone, or in combination with NP-VRP. (Table 3) By contrast, immunization with NP-VRP alone protected mice, but not guinea pigs. Besides, another study proved that 75-80% C57BL/6 mice vaccinated with 2 × 106 focus-forming units (FFU) of NP-VRP survived lethal challenge with 10 PFU of mouse-adapted Ebola virus intraperitoneally.18 On the basis of the protection observed in rodents challenge model, another study demonstrated that cynomolgus macaques can also be completely protected from the lethal challenge with 1000 PFU of Ebola virus after administrating with one single dose 1010 FFU of GP-VRP intramuscularly, which lighted the potential of VEEV Ebola vaccines.19
Table 3.
Comparison of Ebola vaccine candidates in animal studies.
| Vaccine | Animal Model | Doses | Origin of antigen | Challenge Dose/Manner | Protection | ref |
|---|---|---|---|---|---|---|
| VEEV | Guinea pig | 107 IU | EBOV (1976 Mayinga) | 1000LD50 (104PFU) of guinea pig-adapted Ebola virus/S.C. | 100% | 17 |
| Mice | 106 IU | EBOV (1976 Mayinga) | 30-300 LD50 (1-10 PFU) of mouse-adapted Ebola virus/I.P. | 100% | 17 | |
| Mice | 2 × 106 FFU | EBOV (1995 Kikwit) | 300 LD50 (10 PFU) of mouse-adapted Ebola virus/I.P. | 75-80% | 18 | |
| NHPs | 1010 FFU | EBOV (1995 Kikwit)SUDV (Boniface) | 887-1050 PFU of SUDV; 943-1012 PFU of EBOV/I.M.16-132 PFU of SUDV/Aerosol | 100% | 19 | |
| DNA+Ad5 | NHPs | 1010 FFU | EBOV (1976 Mayinga)SUDV ; Ivory Coast | 6 PFUs of EBOV/I.P | 100% | 20 |
| Ad5 | NHPs | 2 × 1012 vp | EBOV (1995 Kikwit) | 10/1000 PFU of EBOV/I.M. | 100% | 21 |
| NHPs | 1010vp | EBOV (1995 Kikwit) | 1000 PFU of EBOV/I.M. | 100% | 24 | |
| Ad26+Ad35 | NHPs | 1010vp | EBOV (1995 Kikwit) | 1000 PFU of EBOV/I.M. | 100% | 28 |
| ChAd3 | NHPs | 1010vp | EBOV (1995 Kikwit)SUDV | 1000 PFU of EBOV/I.M. | 0% | 34 |
| ChAd3 | 1011vp | 50% | ||||
| ChAd3+ChAd3 | 1010vp/1010vp | 33% | ||||
| ChAd3+ChAd63 | 1010vp/1010vp | 25% | ||||
| ChAd3+MVA | 1010vp/108vp | 100% | ||||
| MVA | NHPs | — | EBOV (1995 Kikwit) | 1000 PFU of EBOV/I.M. | 0% | 41 |
| KUN VLPs | Guinea pig | 106/5 × 106 vp | EBOV (1976 Mayinga) | 200 LD50 of guinea pig-adapted EBOV/I.P. | 25–75% | 43 |
| NHPs | 109vp | EBOV (1976 Mayinga) | 600 PFU of EBOV/I.M. | 75% | 44 | |
| VSV | Mice | 2 × 104 PFU | EBOV (1976 Mayinga)MARV (1980 Musoke);Lassa virus(Josiah) | 1000 LD50 of mouse-adapted Ebola virus/I.P. | 100% | 45 |
| NHPs | 107PFU | EBOV (1976 Mayinga)MARV (1980 Musoke); | 1000 PFU of EBOV/I.M. | 100% | 46 | |
| Mice | 2 × 105 PFU | EBOV (1976 Mayinga)MARV (1980 Musoke);Lassa virus(Josiah) | 1000 PFU of mouse-adapted EBOV/I.P. | 100% | 47 | |
| Guinea pig | 2 × 105 PFU | 1000 PFU of guinea pig-adapted EBOV/I.P. | 50% | |||
| NHPs | 2 × 107 PFU | 1000 PFU of EBOV/I.M. | 50% | |||
| HPIV3 | Guinea pig | 105.3PFU | EBOV (1976 Mayinga) | 1000 PFU of guinea pig-adapted EBOV/I.P. | 100% | 60 |
| NHPs | 4 × 106 /2 × 107 PFU | EBOV (1976 Mayinga) | 1000 PFU of EBOV/I.M. | 100% | 9 | |
| Guinea pig | 4 × 105 /4 × 106 PFU | EBOV (1976 Mayinga) | 1000 PFU of guinea pig-adapted EBOV/I.P. | 100% | 62 | |
| rCMV | Mice | 105/5 × 105 PFU | EBOV (1976 Mayinga) | 1000 PFU of mouse-adapted EBOV/I.P. | 100% | 63 |
| rRABV | Mice | 105PFU | EBOV (1976 Mayinga) | 1000 PFU of mouse-adapted EBOV/I.P. | 100% | 68 |
| Mice | 5 × 105 FFU | EBOV (1976 Mayinga) | 1000 PFU of mouse-adapted EBOV/I.P. | 100% | 69 | |
| NHPs | 5 × 107 FFU | EBOV (1976 Mayinga) | 1000 PFU of EBOV/I.M. | 100% | 70 | |
| Inactivated vaccine | Guinea pig | 1.7 × 105 PFU | EBOV (1976 Mayinga) | 10000 PFU of guinea pig-adapted EBOV/I.P. | 100% | 73 |
| Mice | 1.4ug | EBOV (1995 Kikwit) | 300 LD50 (10 PFU) of mouse-adapted Ebola virus/I.P. | 100% | 74 | |
| NHPs | 108 PFU | EBOV (1995 Kikwit) | 1000 PFU of EBOV/I.M. | 0% | ||
| NHPs | 8.0 log10 PFU | EBOV (1995 Kikwit) | 1000 PFU of EBOV/I.M. | 0% | 41 | |
| DNA | Mice | 0.5ug+1.5ug | EBOV (1995 Kikwit) | 30 LD50 of mouse-adapted EBOV/I.P. | 100% | 76 |
| Mice | 5ug/20ug | EBOV (1995 Kikwit) | 1000 PFU of mouse-adapted EBOV/I.P. | 100% | 77 | |
| VLPs | Mice | 0.1/1/10ug | EBOV (1995 Kikwit)MARV (1980 Musoke) | 10/300 PFU of mouse-adapted EBOV/I.P. | 100% | 83 |
| NHPs | 250ug | 1000 PFU of EBOV/I.M. | 100% | |||
| rEBOVΔVP30 | Mice | 2 × 106 FFU | EBOV (1976 Mayinga) | 1000 PFU of mouse-adapted EBOV/I.P. | 100% | 89 |
| Guinea pig | 107FFU | EBOV (1976 Mayinga) | 1000 PFU of guinea pig-adapted EBOV/I.P. | 100% | ||
| NHPs | 107 FFU | EBOV (1976 Mayinga) | 1000 PFU of EBOV/I.M. | 100% | 90 |
Abbreviations: FFU, focus-forming units; PFU, plaque-forming units; vp, viral particles; EBOV, Zaire Ebola virus; SUDV, Sudan Ebola virus; MARV, Marburg virus; GMT, Geometric mean titer; GMC, Geometric mean concentration; NHPs, nonhuman primates; I.M., intramuscular; I.P., intraperitoneally; S.C., subcutaneously; infectious units, IU.
Adenovirus vector-based Ebola vaccines
In 2000, Sullivan et al.20 first used a recombinant replication defective human adenovirus 5 (rAd5)-vectored Ebola vaccine, which was generated by introducing gene of EBOV-GP into the rAd5 full-length plasmid. NHPs was prime immunized with DNA vaccine at week 0, 4 and 8, then boosted with the rAd5-based Ebola vaccine at week 32, resulting in a 100% protection against the lethal challenge with 6 PFU of Ebola virus intraperitoneally. Considering the long immunization schedule could not meet the requirement of emergency immunization in Ebola outbreak, they further vaccinated NHPs with a single dose of an improved rAd5-EBOV containing both GP and NP antigens, which also achieved a complete protection in NHPs (Table 3).21 However, the prior prime-boost strategy may provide a greater durability and efficacy than a single injection of rAd5-EBOV. Besides, CD8+ cells were found to play a major role in rAd5-GP-induced immune protection against EBOV infection in NHPs.22
In 2010, Ledgerwood et al.23 conducted the very first human clinical trial with a rAd5-based Ebola vaccine developed by Crucell Holland BV in healthy adults at National Institutes of Health (NIH) Clinical Center. This was a randomized, double-blinded, placebo-controlled, and dose-escalating phase I trial (ClinicalTrials.gov NCT00374309) (Table 1). The rAd5-based Ebola vaccine encoding both the GPs of the EBOV (Kikwit 1995) and SUDV subspecies, which demonstrated a 100% protection in previously assessed NHPs challenging models.24 31 healthy adults were allocated randomly to receive intramuscular injection of either rAd5-based Ebola vaccine at 2 × 109 vp, or 2 × 1010 vp or placebo. The results showed that this rAd5-based Ebola vaccine was able to elicit both specific humoral and cellular immune responses, and the most common adverse reaction is mild and short-lived headache. Both the low dose and high dose vaccines were well tolerated, while the Ebola GP-specific antibody titers and the T-cell responses were significant greater in the high-dose groups. However, a critical concern of the human Ad5 vector-based vaccines was the commonly existed pre-existing immunity (PEI) to human Ad5 with a baseline positive rate of 60-90% in the populations, which may compromise the effectiveness of the Ad5 vectored vaccine in inducing humoral and cellular immunogenicity.25 Thus some scientists had tried to replace the human Ad5 vector with other less common human adenovirus such as Ad26 and Ad35, which exhibited a low pre-existing antibody in humans.26-28 In 2011, a NHPs challenging study with a prime-boost regimen using heterologous Ad26-vectored and Ad35-vectored Ebola vaccines demonstrated a significant protection after challenging with 1000 PFU of EBOV28 (Table 3). In addition, Ad35 and Ad26-vectored vaccines could induce potent antibody and T-cell responses to multiple filovirus species.29 Currently, the Ad26-EBOV vaccine has been evaluated in clinical trials (Table 2). A single-center, randomized, placebo-controlled, observer-blind, phase I trial adopting prime-boost regimen (prime with Ad26-EBOV or MVA-BN Filo and boost with the alternative vaccine 28 or 56 d later) enrolled 87 participants to evaluate the safety and immunogenicity of the Ad26-EBOV (Table 4).30 Mild to moderate injection-site pain was the most commonly reported adverse event. Though 4 serioues adverse events occurred, none of these were considered related to the experimental vaccines. According to the report, more than 90% of vaccinees generated Ebola GP-specific IgG 4 weeks after a priming dose of Ad26-EBOV, and 55% developed specific T cells. Furthermore, responses were enhanced by administration of an MVA-BN Filo booster dose and were sustained at 8 months after the prime vaccination. The immunogenicity and safety of these vaccines are being further assessed in phase II and III studies. These results hinted that choosing a vector with low pre-existing antibody in human may become an approach to solve PEI.
Table 4.
Comparison of Ebola vaccine candidates in clinical trials.
| Vaccine | Dosages | Origin antigens | Immune responses (EBOV GP) | Most common AE | Ref |
|---|---|---|---|---|---|
| Ad5 | Low (2 × 109 vp) High (2 × 1010 vp) |
EBOV(Kikwit,1995) SUDV(Gulu) |
GMT: Low:85 (Day28) High:155 (Day28) |
Headache | 23 |
| Ad5 | Low (4 × 1010 vp) High (1.6 × 1011 vp) |
EBOV(Guinea,2014) | GMT: Low:682.7 (Day28) High: 1305.7 (Day28) |
Injection-site pain | 31 |
| Ad26+MVA | Group1Prime:Ad26 (5 × 1010 vp) Boost:MVA (108TCID50) Group2 Prime: MVA (108TCID50) Boost:Ad26 (5 × 1010 vp) |
EBOV(Mayinga,1967)SUDV;MARV;TAFV | GMC:Group1:7553 (Day21) Group2:18474 (Day21) | Injection-site pain | 30 |
| ChAd3 | Low (2 × 1010 vp)High (2 × 1011 vp) | EBOV(Mayinga,1967)SUDV(Gulu) | GMT: Low:331 (Day28)High: 2037 (Day28) | Fever | 35 |
| ChAd3+MVA | Prime: ChAd3 Group1 (1 × 1010 vp) Group2 (2.5 × 1010 vp) Group3 (5 × 1010 vp) Boost: MVA1.5 × 108 PFU 3 × 108 PFU |
EBOV(Mayingaq,1967) SUDV;MARV;TAFV |
GMT: Prime:758 (6 Month)Boost:1750 (6Month) | Injection-site pain | 33/,37 |
| ChAd3 | Group1 (2.5 × 1010 vp) Group2(5 × 1010 vp) | EBOV(Mayinga,1967) | GMC:Group1:51μg/mL (Day28)Group2:44·9μg/mL (Day28) | Fatigue/Malaise | 38 |
| ChAd3+MVA | Prime:ChAd3Group1(1 × 1010 vp) Group2 (2.5 × 1010 vp)Group3 (5 × 1010 vp)Group4(1 × 1011 vp)Boost: MVA (2 × 108 vp) | EBOV(Mayinga,1967)SUDV;MARV;TAFV | GMT: PrimeGroup1:295.0 (Day28)Group2:204.6 Day28)Group3:555.8 (Day28)Group4:1493.6 (Day28)Boost:9279.6 (Day28) | Injection-site pain | 39 |
| rVSV | Site1and2:Group1 (3 × 106 PFU) Group2 (2 × 107 PFU) Site3: Group1 (3 × 105 PFU) Group2 (3 × 106 PFU) Site4: Group1(1 × 107 PFU) Group2 (5 × 107 PFU) |
EBOV(Kikwit,1995) | GMT:Site1:Group1:1392.9 (Day28)Group2:1969.8 (Day28)Site2:Group1:1492.9 (Day28)Group2: (−) (Day28)Site3:Group1:1055.6 (Day28)Group2:2570.9 (Day28)Site4:Group1:1064.2 (Day28)Group2:1780.1 (Day28) | Injection-site pain | 53 |
| rVSV | Group1 (3 × 105 PFU) | EBOV(Kikwit,1995) | GMT:Group1:344.5 (Day28) | Injection-site pain | 54 |
| rVSV | Group1 (3 × 106 PFU)Group2(2 × 107 PFU) | EBOV(Kikwit,1995) | GMT:Group1:1300 (Day28)Group2:4079 (Day28) | Injection-site pain | 55 |
| rVSV | Group (2 × 107 PFU) | EBOV(Kikwit,1995) | — | Injection-site pain | 56 |
| DNA | Group1(2.0mg)Group2(4.0mg)Group3 (8.0mg) | EBOV(Mayinga,1967)SUDV | — | Local reactions | 78 |
| DNA | Prime: Group(4.0mg)Boost: Group (4.0mg) | EBOV(Mayinga,1967)SUDV;MARV | GMT:Group:31.8 (Day28) after 3rdGroup:34.0 (Day28) after 4th | Injection-site pain | 79 |
| DNA | Group (4.0mg) | EBOV(Mayinga,1967)SUDV;MARV | GMT:Group:31.0 (Day28) after 3rd | Injection-site pain | 32 |
Abbreviations: TCID50, median tissue culture infective dose; PFU, plaque-forming units; vp, viral particles; EBOV, Zaire Ebola virus; SUDV, Sudan Ebola virus; MARV, Marburg virus; GMT, Geometric mean titer; GMC, Geometric mean concentration.
Even though there were several questions or doubts about the Ad5-based vaccine, the development of the Ebola vaccine based on the Ad5 vector did not stop. After the 2014 Ebola outbreak, a novel recombinant human Ad5 vector based Ebola vaccine (Ad5-EBOV) expressing the GP of the 2014 epidemic Ebola strain (Guinea, 2014) was jointly developed by Beijing Institute of Biotechnology and Tianjin CanSino Biotechnology Inc.31 Two outstanding advantages of this Ad5-EBOV was noticed: first, the Ad5-EBOV is the first Ebola vaccine developed according to the 2014 epidemic strain, which was considered to be a new epidemic strain, with 96.7% homology of the nucleotide sequence and 97.6% homology of amino acid sequence31 compared to the GP gene of the strain in 1976 which was based on by other vaccines;32,33 Second, the Ad5-EBOV is lyophilized white powder (can be stored at 2-8°C), which may be more suitable for the areas where the cold chain system is incomplete than those liquid formulations.
After a preliminary efficacy was observed in the pre-clinical animal studies, the Ad5-EBOV was quickly put into the clinical trials at the end of 2014 (Table 1). The safety, tolerability and immunogenicity of the Ad5-EBOV was evaluated in 120 healthy adults in China (ClinicalTrials.gov NCT02326194), receiving either one shot of the Ad5-EBOV at 4 × 1010 vp, 1.6 × 1011 vp, or placebo.31 The safety observation for adverse reactions post-vaccination indicated a good safety profile of the Ad5-EBOV, which was in line with the previous reports of other Ad5-based Ebola vaccines. Though higher incidence of injection-site reactions was associated with the higher dosage of Ad5-EBOV, most of the reactions were mild or moderate. Ebola GP-specific antibody titers were significantly increased in participants in both the 4 × 1010 vp and 1.6 × 1011 vp Ad5-EBOV groups with a geometric mean titer (GMT) of 421.4 and 820.5 at day 14, and 682.7 and 1305.7 at day 28, respectively. Moreover, T-cell responses peaked at day 14. The magnitude of both humoral and cell responses were greater in participants with low or negative pre-existing Ad5 neutralizing antibody than in those with high pre-existing Ad5 antibody (Table 4). The results indicated that the high-dose 1.6 × 1011 vp Ad5-EBOV could overcome the negative effects of PEI and still induced robust Ebola GP-specific antibody and T-cell responses.
In May 2015, another single-center, open-label phase I clinical trial was conducted, recruiting 60 healthy African adults in China (ClinicalTrials.gov NCT02401373), to further assess the safety profile of the experimental Ad5-EBOV in Africans. Participants were allocated into 2 groups to receive either low dose (4 × 1010 vp) or high dose (1.6 × 1011 vp) vaccine, while the particular data is unpublished (Table 1).
In October 2015, a phase II clinical trial of the experimental Ad5-EBOV was launched and ongoing in Sierra Leone, West Africa (ClinicalTrials.gov NCT02575456). A total of 500 healthy local adults were recruited and randomly allocated to receive one dose of 1.6 × 1011 vp, 8 × 1010 vp, or placebo at a ratio of 2:1:1 to further evaluate the safety and immunogenicity of Ad5-EBOV (Table 1).
ChAd3 vector was considered as a replacement of the human Ad5 vector for Ebola vaccine in order to cope with the PEI against Ad5. ChAd3 vectored Ebola vaccines can be divided into 2 kinds, one is the monovalent recombinant chimpanzee adenovirus type 3-vectored vaccine expressing wild-type GP from EBOV (ChAd3-EBO-Z), the other one is the bivalent recombinant chimpanzee adenovirus type 3-vectored vaccine expressing wild-type GP from EBOV or/and SUDV (cAd3-EBO). Stanley et al.34 immunized cynomologous macaques with a single inoculation of 1 × 1011 or 1 × 1010 vp of ChAd3-EBO-Z and a protection against EBOV was noticed 5 weeks after challenging with 1000 PFU of EBOV (Table 3). Then they added SUDV GP into vaccine to advance the diversity of protection in a natural outbreak setting. The team immunized 4 macaques with cAd3-EBO and challenged them with a lethal dose of EBOV 5 weeks after vaccination. As seen with the monovalent vaccine, the bivalent vaccine also protected the macaques from infection demonstrating that inclusion of an additional GP species did not interfere with the protection from EBOV observed with the monovalent vaccine. However, the humoral and cellular immunity elicited by ChAd3 vaccine waned gradually over time and lost protection for NHPs from challenging 10 months after vaccination. Even in the NHPs inoculated of 1 × 1011 vp of ChAd3 vaccine, only a 50% protection was observed 10 months later. Thus, the research team attempted to adopt a prime-boost immune strategy: macaques was immunized with a prime dose of 1 × 1010 vp ChAd3 vaccine, and then a booster dose of ChAd3 vaccine, chimpanzee adenovirus type 63-vectored Ebola vaccine (ChAd63-EBO), or multivalent MVA-vectored vaccine (MVA-BN Filo) 8 weeks later.34 The prime-boost regimen with ChAd3 vaccine and MVA-BN Filo achieved a full protection and durable immunity at month 10 post-immunization after challenging with 1000PFU of EBOV. On the contrary, the other 2 regimens (ChAd3 vaccine+ChAd3 vaccine and ChAd3 vaccine+ChAd63-EBO) provided a compromised protection to one third of NHPs or one fourth of NHPs (Table 3). These results from animal studies advanced development of the ChAd3 vaccine into clinical trials.
In September 2014, a phaseI, dose-escalation, open-label trial was conducted to evaluate safety, and immunogenicity of cAd3-EBO by enrolling 20 healthy adults into 2 sequentially groups of 10 each at dosage of 2 × 1010 vp or 2 × 1011 vp (ClinicalTrials.gov NCT02231866).35 This trial found that both the reactogenicity and immunogenicity of the experimental cAd3-EBO were dose-dependent. The GP Zaire-specific antibody titer at week 4 in 2 × 1011 vp dose group was significant higher than that in the 2 × 1010 vp dose group (2037 in 2 × 1011 vp dose group vs 331 in the 2 × 1010 vp dose) (Table 4). The incidences and severity of local and systemic adverse reactions were similar to those observed in previous studies of other adenovirus vectored vaccines.23,36
Beside, another phaseI, dose-escalation, open-label study was conducted to assess the safety and immunogenicity of a single dose of the ChAd3-EBO-Z at 3 different dosages 1 × 1010, 2.5 × 1010 and 5 × 1010 vp, with 20 participants per group, respectively33 (ClinicalTrials.gov NCT02240875) (Table 1). The highest dosage of the experimental vaccines in this trial only a quarter of that in the previous trial. No safety concerns were identified at any of the dosage levels studied, majority of the recorded local and systemic adverse events was mild and short-lived. The experimental vaccine had successfully induced both specific antibody and T-cell responses, but the immune response levels were lower than those induced in previous trial at a dosage of 2 × 1011 vp (Table 4). All these results indicated that the immune responses and antibody titers are highly dose-dependent.
Later, the team added a booster dose of MVA to access the effect in 30 of the 60 participants and evaluated a reduced prime–boost interval in another 16 participants.37 At 3 to 10 weeks after the priming immunization, the team further administered 18 participants with 1.5 × 108 PFU of MVA vaccine, while at a dose of 3 × 108 PFU to 12 participants. Significant increases in neutralizing antibodies were seen after boosting in all 30 participants (GMT139). ChAd3-EBO-Z boosted with MVA elicited B-cell and T-cell immune responses to EBOV that were superior to those induced by the ChAd3-EBO-Z alone. Besides, the prime–boost intervals as short as 1 week, which may facilitate vaccine deployment in outbreak regions.
Between October 24, 2014, and June 22, 2015, a phase I/II study included 120 health participants randomly assigned into deployed and non-deployed groups to receive a single intramuscular dose of ChAd3-EBO-Z at 5 × 1010 vp, 2.5 × 1010 vp or placebo.38 (ClinicalTrials.gov NCT02289027) Fatigue or malaise was the most common systemic adverse event and no serious adverse events were reported. GMC of IgG antibodies against Ebola GP peaked at 51 μg/mL in the high-dose group, 44·9 μg/mL in the low-dose group on day 28. A single dose was immunogenic in almost all vaccine recipients and the antibody response in the vaccine group was still significantly higher than that in the placebo group at 6 months (Table 4). This favorable safety profile provides a reliable basis to proceed with the following phase II/III efficacy trials in Africa.38
Since studies in NHPs had shown that immunogenicity and duration of high-level protection against challenge could be extended by administering a dose of MVA-BN Filo as a booster.34 Another study of ChAd3-EBO-Z with MVA-BN Filo among Malian and US adults began in late November 201439 (ClinicalTrials.gov NCT02231866 and NCT02267109, respectively) (Table 2). It was the first time that ChAd3-EBO-Z was administered to Africans participants. Participants were randomly allocated to receive one-dose of 1 × 1010 or 2.5 × 1010 or 5 × 1010 or 1 × 1011 vp, then boosted participants with one-dose 2 × 108 vp of MVA-BN Filo on day 79-111 post-priming. Most adverse events were mild, without any unexpected serious adverse reactions related to the vaccine. After the prime vaccination, the GMTs peaked at day 28 and then decreased slowly through the next 12 weeks. After boosting with MVA-BN Filo, the GMT rapidly increased by 36 times and persisted at a high level. MVA-BN Filo boosting was well-tolerated and powerfully immunogenic, eliciting significant anamnestic anti-GP antibody and multi-functional CD8 and CD4 T-cell responses irrespective of the dosage of ChAd3-EBO-Z priming or the prime-boost interval (Table 4). Up to date, more and more clinical trials investigated on a priming-boosting regimen of combined usage of different Ebola vaccines are ongoing to determine a more efficient immunization schedule.
MVA-vectored vaccines
In 1997, Gilligan et al.40 first found that MVA-vectored vaccine expressing Ebola virus VP24 was able to prolong the mean lifespan of guinea pigs after the lethal challenge with Ebola Virus. Though this vaccine can achieve a protection of 60% for guinea pigs, it was failed in protecting cynomolgus macaques in challenge model despite the fact that the neutralizing antibodies against EBOV were detected after the vaccinations (Table 3).41 However, a further study demonstrated 100% protection for NHPs receiving a priming dose of ChAd3 vaccine with subsequent boosting by MVA-vectored vaccine.34 So far, there are 2 kinds of MVA-vectored vaccines which have already advanced into clinical trial. One is a multivalent MVA-BN Filo that encodes the GPs from EBOV, SUDV, Marburg virus (MARV), and a NP from TAFV. The other one is a monovalent MVA-vectored vaccine encoding only EBOV GP.
Four phase I clinical trials using a prime-boost regimen with a combination of the Ad26-EBOV vaccine and MVA-BN Filo vaccine are ongoing in different countries (NCT02325050, NCT02313077, NCT02376400, NCT02376426) (Table 2). Besides, 2 phase II random, single-blind and placebo-control trials using the same prime-boost regimen are also ongoing in France and Africa countries, respectively (ClinicalTrials.gov NCT02416453, NCT02564523) (Table 2). Moreover, there are 3 phase III studies, adopting the same prime-boost regimen are recruiting subjects or ongoing now. (ClinicalTrials.gov NCT02543567, NCT02509494, NCT02543268) (Table 2).
In addition, there are 6 clinical trials with ChAd3 vaccine as primer and MVA-BN Filo as booster are ongoing (Table 2). In November 2015, the results from one of 6 previous clinical trials (ClinicalTrials.gov NCT02267109) showed a well tolerance and strong immunogenicity of prime-boost regimen.39 Since administration with MVA-vectored vaccine is capable of extending the duration of protection, more and more studies using MVA-vectored vaccines as booster are been conducted now.
KUN VLPs
Flavivirus Kunjin is an Australian subtype of West Nile virus which is substantially less pathogenic than North American strains of West Nile virus.42 Reynard et al.43 developed KUN VLPs expressing Ebola virus GP, which were capable of infecting and delivering replicon RNA into most mammalian cell types. Later the vaccines were evaluated in guinea pig model in 2009, vaccinating female, 3-week-old Dunkin-Hartley guinea pigs with 1 × 106 or 5 × 106 vp KUN VLPs expressing full-length wild-type or D637L-mutated GPs or GP/Ctr intraperitoneally.43 Then, 20 d after the prime vaccination, a boosting immunization with the same dosage and type of KUN VLPs was administrated. Only 25%∼75% of guinea pigs inoculated with KUN VLPs expressing full-length wild-type or D637L-mutated GPs survived after challenging with a lethal dose of 200 LD50s of recombinant guinea pig-adapted EBOV. However, immunization with KUN VLPs expressing GP/Ctr did not elicit any protection.43 Pyankov et al.44 injected 4 African green monkeys subcutaneously with 109vp KUN VLPs encoding GP/D637L per animal twice with an interval of 4 weeks, only 75% animals survived from the challenged 3 weeks later with 600 PFU of EBOV (Table 3). Because of the limited efficacy, the KUN VLPs is far away from applying in humans currently, further studies with higher dosages of the vaccine may need to be investigated.
Replicative vector-based Ebola vaccines
rVSV-EBOV
The rVSV-EBOV vaccine is generated from a live attenuated recombinant, vesicular stomatitis virus (rVSV) encoding the GP of the EBOV Kikwit 1995 strain.45 The rVSV-EBOV vaccine developed by the Canadian National Microbiology Laboratory was licensed to NewLink Genetics and subsequently sublicensed to Merck, which was responsible for ongoing researches. Early in 2004, a pre-clinical study found that mice were 100% protected from the lethal challenge with 1000 LD50 of mouse-adapted Ebola virus after immunization with rVSV-EBOV vaccine.45 Next year, a complete protection was also proved in monkeys, which were challenged with 1000 PFU of EBOV after inoculating with 107 PFU of rVSV-EBOV.46 Subsequently, targeted at exploring whether vaccine was suitable for emergency immunization after exposure to Ebola or not, Feldmann et al.47 immunized animals with the rVSV-EBOV as late as 24 hours after lethal challenge of Ebola virus and achieved 50% and 100% protection in guinea pigs and mice, respectively. More important, 50% of cynomolgus macaques were survived if administrated with rVSV-EBOV 20 to 30 minutes after Ebola virus infection (Table 3).13 Other preclinical studies also demonstrated a rapid and significant protection in NHPs.12,14, 48-52
The first 3 open-label, uncontrolled, phase I clinical trials of rVSV-EBOV vaccine were conducted in Lambaréné, Kilifi, and Hamburg, respectively, which were designed to assess the safety, and immunogenicity of escalating doses ranging from 3 × 105 to 2 × 107 vp in early 2014 (NCT02283099, NCT02287480, NCT02296983) (Table 1).53 The preliminary results of the rVSV-EBOV vaccine from the above 3 trials, involving a total of 99 participants, demonstrated a good immunogenicity, but a mild to moderate reactions related to vaccination (Table 4).
Simultaneously, another double-blind, randomized, placebo-controlled, phase I trial of the rVSV-EBOV vaccine at dose of 1 × 107 and 5 × 107 vp was conducted in Geneva. A total of 59 participants were administered with one single shot intramuscularly into the deltoid. Of them, 11 (22%) participants without any previous history of joint disease had an onset of arthralgia at a median of 11 d post-vaccination. After magnetic resonance imaging and physical examination, arthritis was confirmed in 9 of 11 participants. The arthritis symptoms in most participants were mild and self-limited with a median duration of 8 d. But at the 6-month visit, there was still one participant who had arthritis with symptoms of swelling on peripheral joints unresolved.53 Moreover, another 3 participants among the 11 patients with arthritis got a mild maculopapular rash involving fingers or toes, which lasted for 7 to 15 d. It indicated that the dissemination and replication of vesicular stomatitis virus can occur and persist for few weeks after immunization. Because of these unexpected adverse reactions, the study was suspended in December 2014. One month later, this study was resumed with a lower dose of rVSV-EBOV vaccine, which was intended to gain a better tolerance of the vaccine by reducing the dosage, since the preliminary data from another trial in Gabon with a lower vaccine doses suggested a better safety profile of the rVSV-EBOV vaccine but still immunogenic. Therefore, another 56 participants were recruited to continue this trial, with 43 of them were randomly assigned to receive lower dose rVSV-EBOV of 3 × 105 vp or placebo and 13 only received open-label rVSV-EBOV at 3 × 105 vp (NCT02287480).54 The results showed that the dose reduction to 3 × 105 vp could decreased the occurrence of viremia, but 13 low-dose vaccinees (25%) still occurred arthralgia after immunization. Additionally, another 2 participants reported purpura on the lower legs and the counts of lymphocyte, neutrophil, and platelet decreased significantly at day 1-3 post-vaccination. Although the adverse reactions in those participants received a lower dose rVSV-EBOV at 3 × 105 vp is comparatively mild to moderate with a lower frequency and a similar seropositivity rates observed on day 28 (94%, 48/51) which is very similar with that in previous study participants received high dose at 1 × 107 or 5 × 107 vp, the post-vaccination antibody response level in terms of GMTs were noted significantly lower than high dose (344.5 vs 1064.2). In general, lowing dosage of the rVSV-EBOV vaccine failed to decrease or preclude the occurrence of vaccine-induced arthritis, dermatitis, and cutaneous vasculitis, and moreover, negatively affected antibody responses.
After the reports of arthritis from the above trials, researchers paid more attention to the occurrence of any adverse reactions related to vaccination in another 2 phase I trials with the rVSV-EBOV, which were proceed in the US with a total of 52 adults at the Walter Reed Army Institute of Research (WRAIR) and the NIH Clinical Center55 (NCT02269423, NCT02280408) (Table 1). But arthritis was neither observed at the WRAIR nor NIH site. The common adverse reactions were injection-site pain, myalgia, fatigue, headache, subjective fever, and chills. Anti-Ebola immune responses were identified in all the participants as well as the VSV viremia with a limit duration. At day 28, GMT of antibodies against EBOV GP were higher in the group receiving 20 million PFU than in the group receiving 3 million PFU (4079 vs. 1300). All these phase I studies with small-scale population facilitated rapid progression to phase II and III trials.
In 2015, a phase III trial was performed in Guinea to assess the efficacy and effectiveness of the rVSV-EBOV vaccine at 2 × 107 vp administered intramuscularly for the prevention of Ebola disease during outbreak.56 This trial used a cluster randomization design with a ring vaccination approach, which was used for smallpox eradication in the 1970s.57 A cluster of individuals at high risk of infection defined as contacts or contacts of contacts because of their social or geographical association with the newly confirmed Ebola patient was randomized to receive one dose of rVSV-EBOV vaccine immediately or 21-day delayed. 4123 participants were assigned to immediate vaccination group, and 3528 participants were assigned to delayed vaccination group. No Ebola disease case was found in 4123 participants receiving rVSV-EBOV vaccine immediately, while 16 cases were determined in those received vaccination with 21 day delay, resulting in a complete protection against Ebola disease. Though 43 serious adverse events were reported, only one serious adverse event was judged to be causally related to vaccination, while assessment of serious adverse events is ongoing. The results of this interim analysis are so encouraging that rVSV-EBOV might become the first licensed vaccine in preventing Ebola virus disease. Besides, it also indicated that ring vaccination strategy is most likely effective to target on the population when deliver during an Ebola virus disease outbreak.
Additionally, according to a case report of a physician who was exposed to Ebola virus in treatment unit, he was vaccinated with rVSV-EBOV 43 hours after the exposure.58 Moderate to severe adverse reactions which are similar to the symptoms of infection of Ebola virus developed 12 hours after vaccination and diminished over 3 to 4 d. Lai et al.58 detected the blood sample of this patient as showing that Ebola virus GP-specific antibodies and T cells were detectable, but antibodies against Ebola viral matrix protein 40 (not in the vaccine) were not detected. Strong innate and Ebola-specific adaptive immune responses were also detected after vaccination. The clinical syndrome and laboratory evidence were consistent with vaccination response, and no evidence of Ebola virus infection was detected. However, it is unsure that this physician was able to get infected with Ebola virus after having a high-risk occupational exposure without intervention. Besides, it is unknown if rVSV-EBOV is safe or effective for post-exposure vaccination in humans.
Though the most promising rVSV-EBOV vaccine was the first one which demonstrated the great protective effect with highly protection, we still need further observation and studies to identify the efficacy and safety of this vaccine.
HPIV3 vaccine
HPIV3 as a negative-sense RNA virus, which can cause generally respiratory disease among children, has been successfully advanced as a vaccine platform against Ebola virus. In 2005, Bukreyev et al.59 inserted a transcription casette encoding the GP gene into HPIV3 independently or together with NP gene to formulate this new vaccine. Guinea pigs were protected from challenging with 103vp Ebola virus on day 28 after a single intranasal inoculation of 105.3 PFU of experimental vaccine. In rhesus macaques challenging model, 2 doses vaccine were able to achieve a 100% protection while one dose showed only an 88% protection after challenging with 1000 PFU of EBOV9 (Table 3). Since the impeding factor showed from an epidemiological investigation60 that PEI to HPIV3 in the human adults may greatly impact the replication and immunogenicity of the vaccine, the vaccine vector was improved by deleting the HPIV3 F and HN genes, which are the main targets for the HPIV3-specific humoral immune response.61 The new attenuated vector expressing EBOV-GP was more efficient in comparison to the previous construct.61 One of the main advantages of the HPIV3-based vector platform is the potential for needle-free administration because of this vaccination via the respiratory route. Safety is another virtue of HPIV3, triggered by restriction of virus only in epithelial cells of respiratory tract, an ongoing phase I clinical trial is carried out in US last year and this is one clinical trial which is given intranasally to human beings for now (NCT02564575) (Table 1).
rCMV-based vaccines
rCMV as a novel vector platform was developed because of the unique potential to re-infect and disseminate through target wildlife populations regardless of prior immunity.62 This vaccine was hypothesized to achieve high vaccine coverage in inaccessible wildlife like apes.63-65 In 2011, Tsuda et al.62 constructed a recombinant mouse CMV vector expressing a CD8+ T cell epitope derived from EBOV NP and mice vaccinated with 2 doses were fully protected against 1000 PFU of mouse-adapted EBOV (Table 3). Since as the major advantage of this platform that once the virus has been established in a host, it can continue to replicate autonomously, this type of vaccine is quite relevant for the control of emerging and re-emerging zoonotic infections.66 This foundation study supported the potential for disseminating rCMV-based vaccines to prevent EBOV transmission in wildlife populations.
rRABV-based vaccines
RABV has been explored as a vaccine platform against Ebola virus recently. For the purpose of reducing neurovirulence to generate Ebola vaccine, Papaneri et al.67 constructed inactivated and live-attenuated bivalent vaccines expressing EBOV GP based on the SAD B19 strain of RABV. The vaccine candidates were avirulent in adult mice and displayed low neurovirulence in suckling mice. Another study also demonstrated that 5 × 105 FFU of this vaccine could induce humoral immunity and conferred protection from both RABV and EBOV after intraperitoneal challenging with 1000 PFU of mouse-adapted EBOV in mice68 (Table 3). In 2013, a study immunized NHPs with a platform based on replication competent RABV, replication-deficient RABV, or chemically inactivated RABV expressing EBOV GP.69 The live replication-competent vaccine provided 100% protection following EBOV challenge while the others provided 50% protection. The results indicated that the protection of immunized animals against EBOV was largely dependent on the quality of humoral immune response against EBOV GP. Hence, utilization of particles containing higher levels of EBOV GP and a boost immunization dose would raise protection rate up to 100% in the animals. Considering of Africa as a high rabies rate region,70,71 it is significant to produce an effective bivalent RABV/Ebola vaccine as a valuable and remarkable public health tool in that region. However, more safety tests against RABV and EBOV should be conducted before advancing the RABV-based vaccines to clinical trials.
Other Ebola vaccines
Inactivated vaccine
As the first attempting for Ebola vaccine using inactivated virus, it was preformed shortly after the discovery of Ebola virus in 1976. Lupton et al.72 used formalin or heat-inactivated virus preparations to immunize guinea pigs that challenged with wild-type EBOV later, the results showed that guinea pigs survived with high protection. However, an additional phenomenon should be noticed that only 29% fatality rate of control group was observed in this study, which leaded to doubts about the validity of this study. Later, inactivated vaccine was proved that can provide 100% protection after the challenge with 10 PFU of mouse-adapted Ebola virus in mouse73 (Table 3). Unfortunately, because of the biological safety hazard and the fact that this inactivated vaccine failed to protect NHPs from challenging of 1000 PFU of EBOV,41 the continue researches have been suspended in 2002 till now (Fig. 2).
DNA vaccines
Early in 1998, Xu et al.74 engineered DNA vaccine expressing the GP or NP gene to immunize guinea pigs and the results showed a complete protection. Another study also demonstrated a protection of 100% against challenge with 30LD50 of mouse-adapted EBOV after immunizing mice with 4 doses of a GP DNA vaccine.75 Subsequently in 2012, a multi-targeting (trivalent vaccine) DNA vaccine expressing GP genes of EBOV, SUDV and MARV was demonstrated to be highly effective in mice without evidence of interference.76 Moreover, prime immunization with DNA vaccine and then boosted with adenoviral vectors was shown to protect NHPs from lethal EBOV challenge.20 DNA vaccine, which induces strong CD4 responses associated with durable immunity, is initially regarded as the most promising and supportive platform against Ebola virus.77 Since numerous pre-clinical studies have already demonstrated a safety and immunogenicity profiles in animals and the protection related to antibody or cellar response directly20,21,24 (Table 3), an initial phase I clinical trial with a 3-plasmid DNA vaccine which encoded the envelope GP from EBOV and SUDV subspecies was conducted in 2003.78 A total of 27 Healthy adult volunteers were enrolled and then arranged into 3 sequential groups to receive placebo or vaccine (5 in the 2-mg dose group, 8 each in the 4-mg and 8-mg dose groups, and 6 in the placebo group respectively). In this study, no serious adverse events were reported, and the experimental vaccine were well-tolerated and safe in healthy adults. Furthermore, Ebola specific humoral responses were successfully detected in all vaccinees and the range of antibody titers was similar to those detected in nonhuman primates.21 The specific antibodies to each antigen can be induced by the vaccine independently and are not cross-reactive.78 However, Ebola virus-specific neutralizing antibody failed to be detected in vaccines as might be expected with DNA vaccination in the absence of boosting with adenoviral vector-based Ebola vaccine.
Moreover, another study published the results of a phase I clinical trial which evaluated the safety, tolerability and immunogenicity of 2 DNA vaccines, one that encodes for MARV Angola GP and the second for EBOV and SUDV wild-type (WT) GP in 2008.79 (NCT00605514) (Table 1) The first Group was enrolled to receive the MARV DNA vaccine and the second group received the EBOV WT DNA vaccine. Besides, the immunization series was a 3-dose priming regimen with an optional single-dose homologous booster in both groups. After whole vaccination, both the EBOV and MARV WT GP vaccines were well tolerated. The WT GP constructs evaluated in this study were immunogenic and induced both humoral and T-cell responses to all 3 GP immunogen inserts. Also additional administration of a fourth dose of DNA as a homologous boost improved the antibody titers and T-cell responses. This study lighted the evaluation of these 2 vaccine candidates in further clinical trial in Africa for immunogenicity and efficacy.
Then, in 2009, a phase I, double-blinded, randomized, placebo-controlled clinical trial was conducted to examine the safety and immunogenicity of the EBOV and MARV vaccines given individually and concomitantly in Kampala, Uganda32 (NCT00997607) (Table 1). As the first clinical trial of Ebola virus and MARV vaccines in Africa, 108 participants were enrolled into the study and randomly assigned (in a ratio of 5:1) to receive at least one injection of either vaccine or placebo. In part 1 of the study, participants were randomly assigned to receive EBOV vaccine and MARV vaccine, or placebo. And in part 2 of the study, participants were randomly assigned to receive either EBOV vaccine in the left arm and MARV vaccine in the right arm or a placebo injection in both arms at each of the 3 injection visits. The results showed that, given separately or together, both vaccines were well tolerated and immunogenic to elicit antigen-specific humoral and cellular immune responses (Table 4). All these findings have contributed to the accelerated development of more potential Ebola vaccines that encode the same WT GP antigens.
One of the advantages of DNA vaccine plasmids is that it can be genetically designed to produce proteins from a pathogen with no risk of infection. Additionally, this stable and easily developed vaccine is inexpensive to produce. Considering the broad immunogenicity of this Ebola virus DNA vaccine, immunization by plasmid DNA delivery is a viable platform and merits further development. Up to date, phase III clinical trials of the DNA platform alone or in combination with replication-defective adenoviral vector vaccines are ongoing.15
VLPs
Generally, as a new complex protein-based vaccine, VLPs was much safer than inactivated vaccine or attenuated vaccine due to its specific characteristic that without viral genome. It is also capable of stimulating response of both humoral immunity and cellular immunity. Bavari et al.80 generated Ebola VLP vaccine by the expression of VP40 alone or along with GP and NP in 2002. Warfield et al.81,82 following demonstrated that mice and guinea pigs immunized with Ebola VLPs and Marburg VLPs respectively, which generated humoral immunity could be totally protected after challenging. In another study performed in 2007, all Ebola VLP-vaccinated NHPs survived 1000 PFU of EBOV challenge after 3 vaccinations. However, an issue that immunization period ups to 6 weeks is too long for achieving emergency needs for rapid immunization in outbreak areas.83 Besides, it is difficult to produce tremendous amounts of VLPs needed for vaccination because of the expensive, time-consuming and laborintensive production period. For addressing this problem, new approach comes out recently by using the baculovirus expression system to produce VLPs in insect cells, which is proved protection of mice.84 Furthermore, Warfield et al.85 adopted the NHPs model to show that one or 2 doses of VLPs vaccine can confer protection from lethal challenge (Table 3). As a vaccine candidate, Ebola VLPs have relatively low biosafety concerns, and their use can bypass issues associated with PEI.
rEBOVΔVP30
rEBOVΔVP30 is a vaccine candidate reported firstly in 200886 and generated by using reverse genetics to delete VP30 from the genome of EBOV artificially.87 Halfmann et al.86 replaced VP30 from the genome of EBOV with neomycin genome and the virus lose the normal ability of replication and production of progeny without changing of morphology. The team further demonstrated that rEBOVΔVP30 was safe in STAT-1 knockout mice and also evaluated the protective efficacy in mice and guinea pigs which were injected with 2 doses and challenged with mouse-or guinea pig–adapted EBOV subsequently88 (Table 3). However this vaccine is replication incompetent, its genome still contains more than 95% of the original EBOV genome, some concerns targeted to the safety issue when the generation of viruses reintegrated VP30 into their genome in nature with the horrible consequence. In 2015, Marzi et al.89 inactivated the vaccine with hydrogen peroxide, the results showed that rEBOVΔVP30 was capable of protecting NHPs against challenging with 1000 PFU of EBOV when given either one or 2 doses. Regardless of the good immunogenicity and efficacy of the rEBOVΔVP30 observed in animal studies, due to the safety concerns of the regaining replication ability, rEBOVΔVP30 was not able to enter the clinical trials for further evaluation.
Conclusions and future directions
In recent 2 years, we have seen a significant accelerated progress in the development of Ebola vaccine, and there were a number of vaccine candidates that performed extremely well through phase I or II clinical trials. Meanwhile some phase III studies with Ebola vaccines are ongoing. Even though the prevalence of Ebola outbreaks had been stopped, and only sporadic cases with Ebola disease had been reported in the past a few months, Ebola virus still attracted tremendous attention worldwide. rVSV-EBOV vaccine is the first vaccine successfully demonstrated a high protective efficacy against Ebola disease in the phase III trial, which may become the first licensed Ebola vaccine. However, there are still several concerns about the rVSV-EBOV vaccine, especially for the safety like immunization induced viremia and arthralgia. According to previous study results, reducing the dosage of rVSV-EBOV vaccine may not be a useful strategy to improve its safety profile. The adverse reactions associated with the rVSV-EBOV vaccine like fever, myalgia, chill and headache especially post-exposure, needs to be further accessed and distinguished because these reactions are similar to symptoms of infection of Ebola virus apparently. Considering the current clinical trials are mostly conducted in subjects aged over 18 y old and non-pregnant women, we should further design and evaluate the vaccine applied on children, elders and pregnant women. Moreover because of the high rates of positive HIV, rabies or malaria and poorly available treatment care, vaccines should not only be designed for healthy adults, but also for those special groups such as HIV infected, malaria infected or people with other baseline diseases, who were more vulnerable to Ebola.
Besides, the persistence of the vaccine protection and the need of booster injection after prime vaccination to against warning of antibodies still need to be further investigated. Although a single shot may be more efficient for emergency immunization, it may not be enough to induce a durable protection against potent infectious agent, whereas a prime-boost combination can induce broader and durable immunity. Moreover, boosting with the heterologous vaccine could prolong durability of protective immunity and induce stronger immune response to antigens than using homogeneous vaccination, which was shown in different clinical trials and suggested as a prospective approach of vaccination by WHO experts.30,33,37
In the past decade, we have witnessed impressive progress in the development of viral vectored vaccine. Recombinant viral vectors are good at delivering heterologous antigens that combine the different favorable features of other vaccine modalities, with minimal disadvantages.90 Compared to simpler vaccines, viral vectored vaccines which are capable of infecting cells and expressing encoded antigens ensures efficient induction of humoral and cellular responses. Particularly, CD8+ T cells are critical for the elimination of intracellular pathogens, which makes it the key advantage over simpler vaccines. However, the main drawback of viral vector vaccines is that the transgene-specific response may be influenced and dampened. Currently, more and more studies adopted the use of higher doses and heterologous prime–boost regimens to overcome the disadvantage.
So far, the Ebola virus were mostly found circulated in undeveloped areas in Africa. Therefore, an ideal Ebola vaccine should also be highly cost-efficient and affordable. Since the cost of boosting vaccination would be higher than a single-shot immunization, a cost-effectiveness study is also in need.
Furthermore, different vaccination strategies should be considered to achieve a high efficient for controlling the transmission of Ebola virus as post-exposure immunization or emergency immunization in susceptible population in Ebola outbreak areas, like ring vaccination. According to the more and more sporadic flare ups infected by survivors, vaccination strategy should be scheduled in case of this phenomena, we should discuss if there is necessary to use the ring vaccination to get more residents protected.
The 2014-2015 Ebola outbreak was catastrophic in Africa, and it debilitated the local health systems, hampered diagnosis and treatment for endemic diseases like malaria, HIV and tuberculosis and resulted in increasing the mortality rates of other diseases indirectly.91 Even though a promising vaccine candidate was confirmed, there still remains large gap between production of Ebola vaccines and the vaccination demands. There is a huge vaccine demanding in undeveloped countries, but the poor medical infrastructure will interfere development of vaccine forward. Because the people most at risk for Ebola are the ones least able to pay for vaccines, this leaves little in the way of market incentives for manufacturers to develop vaccines, unless there are large numbers of people who are at risk in wealthy countries. The key reason we still lack of an Ebola vaccine coming to market now is because that there is no market for Ebola. Besides, the vaccine development that taking a well-known antigen and turning it into a viable vaccine is expensive, complicated and long-term process, hence developing an Ebola vaccine is commercially risky and the only reason we have vaccine candidates now is actually because of a somewhat misguided fear.
Moreover, it is meaningful to take standard precautions against Ebola virus like basic hand hygiene, respiratory hygiene, using of personal protective equipment, safe injection practices and safe burial practices. Hopefully, the most promising licensed vaccine will be produced global in the next a few years and also will contribute to world.
Abbreviations
- EVD
Ebola virus disease
- rVSV-EBOV
recombinant vesicular stomatitis virus-based vaccine
- EBOV
Zaire Ebola virus
- SUDV
Sudan Ebola virus
- BDBV
Bundibugyo Ebola virus
- TAFV
Tai Forest Ebola virus
- RESTV
Reston Ebola virus
- NHPs
non-human primates
- WHO
World Health Organization
- UK
United Kingdom
- US
United States
- GP
glycoprotein
- VP
virion protein
- NP
nucleoprotein
- L
RNA-dependent RNA polymerase
- MVA
modified vaccinia strain Ankara
- VEEV
venezuelan equine encephalitis virus
- ChAd3 vaccine
replication-defective recombinant chimpanzee adenovirus type 3-vectored vaccine
- ChAd3-EBO-Z
recombinant chimpanzee adenovirus type 3-vectored vaccine expressing wild-type GP from EBOV
- cAd3-EBO
recombinant chimpanzee adenovirus type 3-vectored vaccine expressing wild-type GP from EBOV or/and SUDV
- KUN VLPs
Kunjin replicon virus-like particle vaccine
- vp
viral particles
- PFU
plaque-forming units
- FFU
focus-forming units
- IU
infectious units
- HPIV3
human parainfluenza virus type 3
- rCMV
recombinant cytomegalovirus
- RABV
recombinant rabies virus
- VLPs
virus-like particles
- rEBOVΔVP30
recombinant EBOVΔVP30
- VRP
VEEV replicon particles
- rAd5
replication defective human adenovirus 5
- NIH
National Institutes of Health
- PEI
pre-existing immunity
- Ad5-EBOV
recombinant human Ad5 vector based Ebola vaccine
- GMT
geometric mean titer
- MARV
Marburg virus
- MVA-BN Filo
multivalent MVA-BN Filo encoding the GPs from EBOV, SUDV, MARV and a NP from TAFV
- WRAIR
Walter Reed Army Institute of Research
- WT
wild-type
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Rimoin AW, Hotez PJ. NTDs in the heart of darkness: the Democratic Republic of Congo's unknown burden of neglected tropical diseases. PLoS Negl Trop Dis 2013; 7:e2118; PMID:23936557; http://dx.doi.org/ 10.1371/journal.pntd.0002118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Towner JS, Sealy TK, Khristova ML, Albarino CG, Conlan S, Reeder SA, Quan PL, Lipkin WI, Downing R, Tappero JW, et al.. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog 2008; 4:e1000212; PMID:19023410; http://dx.doi.org/ 10.1371/journal.ppat.1000212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mahanty S, Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis 2004; 4:487-98; PMID:15288821; http://dx.doi.org/ 10.1016/S1473-3099(04)01103-X [DOI] [PubMed] [Google Scholar]
- [4].Pourrut X, Kumulungui B, Wittmann T, Moussavou G, Delicat A, Yaba P, Nkoghe D, Gonzalez JP, Leroy EM. The natural history of Ebola virus in Africa. Microbes infect 2005; 7:1005-14; PMID:16002313; http://dx.doi.org/ 10.1016/j.micinf.2005.04.006 [DOI] [PubMed] [Google Scholar]
- [5].Reed DS, Mohamadzadeh M. Status and challenges of filovirus vaccines. Vaccine 2007; 25:1923-34; PMID:17241710; http://dx.doi.org/ 10.1016/j.vaccine.2006.11.037 [DOI] [PubMed] [Google Scholar]
- [6].World Health Organization Ebola virus disease fact sheets. Available at: http://www.who.int/mediacentre/factsheets/fs103/en/. Access: May27, 2016. [Google Scholar]
- [7].World Health Organization Ebola virus disease outbreak. Available at: http://www.who.int/csr/disease/ebola/en/. Access: June10, 2016. [Google Scholar]
- [8].Sridhar S. Clinical development of Ebola vaccines. Ther Adv Vaccines 2015; 3:125-38; PMID:26668751; http://dx.doi.org/ 10.1177/2051013615611017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bukreyev A, Rollin PE, Tate MK, Yang L, Zaki SR, Shieh WJ, Murphy BR, Collins PL, Sanchez A. Successful topical respiratory tract immunization of primates against Ebola virus. J Virol 2007; 81:6379-88; PMID:17428868; http://dx.doi.org/ 10.1128/JVI.00105-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beniac DR, Melito PL, Devarennes SL, Hiebert SL, Rabb MJ, Lamboo LL, Jones SM, Booth TF. The organisation of Ebola virus reveals a capacity for extensive, modular polyploidy. PloS one 2012; 7:e29608; PMID:22247782; http://dx.doi.org/ 10.1371/journal.pone.0029608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Audet J, Kobinger GP. Immune evasion in ebolavirus infections. Viral Immunol 2015; 28:10-8; PMID:25396298; http://dx.doi.org/ 10.1089/vim.2014.0066 [DOI] [PubMed] [Google Scholar]
- [12].Geisbert TW, Daddario-Dicaprio KM, Lewis MG, Geisbert JB, Grolla A, Leung A, Paragas J, Matthias L, Smith MA, Jones SM, et al.. Vesicular stomatitis virus-based ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Pathog 2008; 4:e1000225; PMID:19043556; http://dx.doi.org/ 10.1371/journal.ppat.1000225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Geisbert TW, Daddario-DiCaprio KM, Williams KJ, Geisbert JB, Leung A, Feldmann F, Hensley LE, Feldmann H, Jones SM. Recombinant vesicular stomatitis virus vector mediates postexposure protection against Sudan Ebola hemorrhagic fever in nonhuman primates. J Virol 2008; 82:5664-8; PMID:18385248; http://dx.doi.org/ 10.1128/JVI.00456-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Geisbert TW, Geisbert JB, Leung A, Daddario-DiCaprio KM, Hensley LE, Grolla A, Feldmann H. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J Virol 2009; 83:7296-304; PMID:19386702; http://dx.doi.org/ 10.1128/JVI.00561-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].ClinicalTrial.gov Clinical trials found for Ebola vaccine. Available at: https://clinicaltrials.gov/ct2/results?term=Ebola+vaccine&Search=Search. Access: June10, 2016. [Google Scholar]
- [16].Basler CF, Wang X, Muhlberger E, Volchkov V, Paragas J, Klenk HD, Garcia-Sastre A, Palese P. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc Natl Acad Sci U S A 2000; 97:12289-94; PMID:11027311; http://dx.doi.org/ 10.1073/pnas.220398297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pushko P, Bray M, Ludwig GV, Parker M, Schmaljohn A, Sanchez A, Jahrling PB, Smith JF. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 2000; 19:142-53; PMID:10924796 [DOI] [PubMed] [Google Scholar]
- [18].Wilson JA, Hart MK. Protection from Ebola Virus Mediated by Cytotoxic T Lymphocytes Specific for the Viral Nucleoprotein. J Virol 2001; 75:2660-4; PMID:11222689; http://dx.doi.org/ 10.1128/JVI.75.6.2660-2664.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Herbert AS, Kuehne AI, Barth JF, Ortiz RA, Nichols DK, Zak SE, Stonier SW, Muhammad MA, Bakken RR, Prugar LI, et al.. Venezuelan Equine Encephalitis Virus Replicon Particle Vaccine Protects Nonhuman Primates from Intramuscular and Aerosol Challenge with ebolavirus. J Virol 2013; 87:4952-64; PMID:23408633; http://dx.doi.org/ 10.1128/JVI.03361-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature 2000; 408:605-9; PMID:11117750; http://dx.doi.org/ 10.1038/35046108 [DOI] [PubMed] [Google Scholar]
- [21].Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZY, Roederer M, Koup RA, Jahrling PB, Nabel GJ. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 2003; 424:681-4; PMID:12904795; http://dx.doi.org/ 10.1038/nature01876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sullivan NJ, Hensley L, Asiedu C, Geisbert TW, Stanley D, Johnson J, Honko A, Olinger G, Bailey M, Geisbert JB, et al.. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med 2011; 17:1128-31; PMID:21857654; http://dx.doi.org/ 10.1038/nm.2447 [DOI] [PubMed] [Google Scholar]
- [23].Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, Mulangu S, Hu Z, Andrews CA, Sheets RA, et al.. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine 2010; 29:304-13; PMID:21034824; http://dx.doi.org/ 10.1016/j.vaccine.2010.10.037 [DOI] [PubMed] [Google Scholar]
- [24].Sullivan NJ, Geisbert TW, Geisbert JB, Shedlock DJ, Xu L, Lamoreaux L, Custers JH, Popernack PM, Yang ZY, Pau MG, et al.. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med 2006; 3:e177; PMID:16683867; http://dx.doi.org/ 10.1371/journal.pmed.0030177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E, et al.. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 2010; 28:950-7; PMID:16683867; http://dx.doi.org/ 10.1371/journal.pmed.0030177 [DOI] [PubMed] [Google Scholar]
- [26].Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, et al.. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol 2004; 172:6290-7; PMID:15128818 [DOI] [PubMed] [Google Scholar]
- [27].Kobinger GP, Feldmann H, Zhi Y, Schumer G, Gao G, Feldmann F, Jones S, Wilson JM. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology 2006; 346:394-401; PMID:16356525; http://dx.doi.org/ 10.1016/j.virol.2005.10.042 [DOI] [PubMed] [Google Scholar]
- [28].Geisbert TW, Bailey M, Hensley L, Asiedu C, Geisbert J, Stanley D, Honko A, Johnson J, Mulangu S, Pau MG, et al.. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J Virol 2011; 85:4222-33; PMID:21325402; http://dx.doi.org/ 10.1128/JVI.02407-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zahn R, Gillisen G, Roos A, Koning M, van der Helm E, Spek D, Weijtens M, Grazia Pau M, Radosevic K, Weverling GJ, et al.. Ad35 and ad26 vaccine vectors induce potent and cross-reactive antibody and T-cell responses to multiple filovirus species. PloS one 2012; 7:e44115; PMID:23236343; http://dx.doi.org/ 10.1371/journal.pone.0044115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Milligan ID, Gibani MM, Sewell R, Clutterbuck EA, Campbell D, Plested E, Nuthall E, Voysey M, Silva-Reyes L, McElrath MJ, et al.. Safety and Immunogenicity of Novel Adenovirus Type 26- and Modified Vaccinia Ankara-Vectored Ebola Vaccines: A Randomized Clinical Trial. Jama 2016; 315:1610-23; PMID:27092831; http://dx.doi.org/ 10.1001/jama.2016.4218 [DOI] [PubMed] [Google Scholar]
- [31].Zhu FC, Hou LH, Li JX, Wu SP, Liu P, Zhang GR, Hu YM, Meng FY, Xu JJ, Tang R, et al.. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet 2015; 385:2272-9; PMID:25817373; http://dx.doi.org/ 10.1016/S0140-6736(15)60553-0 [DOI] [PubMed] [Google Scholar]
- [32].Kibuuka H, Berkowitz NM, Millard M, Enama ME, Tindikahwa A, Sekiziyivu AB, Costner P, Sitar S, Glover D, Hu Z, et al.. Safety and immunogenicity of Ebola virus and Marburg virus glycoprotein DNA vaccines assessed separately and concomitantly in healthy Ugandan adults: a phase 1b, randomised, double-blind, placebo-controlled clinical trial. Lancet 2015; 385:1545-54; PMID:25540891; http://dx.doi.org/ 10.1016/S0140-6736(14)62385-0 [DOI] [PubMed] [Google Scholar]
- [33].Rampling T, Ewer K, Bowyer G, Wright D, Imoukhuede EB, Payne R, Hartnell F, Gibani M, Bliss C, Minhinnick A, et al.. A Monovalent Chimpanzee Adenovirus Ebola Vaccine - Preliminary Report. N Engl J Med 2015; 374(17):1635-46; PMID:25629663; http://dx.doi.org/ 10.1056/NEJMoa1411627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stanley DA, Honko AN, Asiedu C, Trefry JC, Lau-Kilby AW, Johnson JC, Hensley L, Ammendola V, Abbate A, Grazioli F, et al.. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 2014; 20:1126-9; PMID:25194571; http://dx.doi.org/ 10.1038/nm.3702 [DOI] [PubMed] [Google Scholar]
- [35].Ledgerwood JE, DeZure AD, Stanley DA, Novik L, Enama ME, Berkowitz NM, Hu Z, Joshi G, Ploquin A, Sitar S, et al.. Chimpanzee Adenovirus Vector Ebola Vaccine - Preliminary Report. N Engl J Med 2014; PMID:25426834; http://dx.doi.org/ 10.1056/NEJMoa1410863 [DOI] [PubMed] [Google Scholar]
- [36].Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R, et al.. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Translational Med 2012; 4:115ra1; PMID:22218690; http://dx.doi.org/ 10.1126/scitranslmed.3003155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ewer K, Rampling T, Venkatraman N, Bowyer G, Wright D, Lambe T, Imoukhuede EB, Payne R, Fehling SK, Strecker T, et al.. A Monovalent Chimpanzee Adenovirus Ebola Vaccine Boosted with MVA. N Engl J Med 2016; 374:1635-46; PMID:25629663; http://dx.doi.org/ 10.1056/NEJMoa1411627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].De Santis O, Audran R, Pothin E, Warpelin-Decrausaz L, Vallotton L, Wuerzner G, Cochet C, Estoppey D, Steiner-Monard V, Lonchampt S, et al.. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis 2016; 16:311-20; PMID:26725450; http://dx.doi.org/ 10.1016/S1473-3099(15)00486-7 [DOI] [PubMed] [Google Scholar]
- [39].Tapia MD, Sow SO, Lyke KE, Haidara FC, Diallo F, Doumbia M, Traore A, Coulibaly F, Kodio M, Onwuchekwa U, et al.. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2016; 16:31-42; PMID:26546548; http://dx.doi.org/ 10.1016/S1473-3099(15)00362-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chepurnov AA, Ternovoi VA, Dadaeva AA, Dmitriev IP, Sizikova LP, Volchkov VE, Kudoiarova NM, Rudzevich TN, Netesov SV. Immunobiological properties of vp24 protein of Ebola virus expressed by recombinant vaccinia virus. Voprosy virusologii 1997; 42:115-20; PMID:9297340 [PubMed] [Google Scholar]
- [41].Geisbert TW, Pushko P, Anderson K, Smith J, Davis KJ, Jahrling PB. Evaluation in nonhuman primates of vaccines against Ebola virus. Emerg Infect Dis 2002; 8:503-7; PMID:11996686; http://dx.doi.org/ 10.3201/eid0805.010284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pijlman GP, Suhrbier A, Khromykh AA. Kunjin virus replicons: an RNA-based, non-cytopathic viral vector system for protein production, vaccine and gene therapy applications. Exp Opin Biol Ther 2006; 6:135-45; PMID:16436039; http://dx.doi.org/ 10.1517/14712598.6.2.135 [DOI] [PubMed] [Google Scholar]
- [43].Reynard O, Mokhonov V, Mokhonova E, Leung J, Page A, Mateo M, Pyankova O, Georges-Courbot MC, Raoul H, Khromykh AA, et al.. Kunjin virus replicon-based vaccines expressing Ebola virus glycoprotein GP protect the guinea pig against lethal Ebola virus infection. J Infect Dis 2011; 204 Suppl 3:S1060-5; PMID:21987742; http://dx.doi.org/ 10.1093/infdis/jir347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pyankov OV, Bodnev SA, Pyankova OG, Solodkyi VV, Pyankov SA, Setoh YX, Volchkova VA, Suhrbier A, Volchkov VV, Agafonov AA, et al.. A Kunjin Replicon Virus-like Particle Vaccine Provides Protection Against Ebola Virus Infection in Nonhuman Primates. J Infect Dis 2015; 212 Suppl 2:S368-71; PMID:25732811; http://dx.doi.org/ 10.1093/infdis/jiv019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Garbutt M, Liebscher R, Wahl-Jensen V, Jones S, Moller P, Wagner R, Volchkov V, Klenk HD, Feldmann H, Stroher U. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol 2004; 78:5458-65; PMID:15113924; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, et al.. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med 2005; 11:786-90; PMID:15937495; http://dx.doi.org/ 10.1038/nm1258 [DOI] [PubMed] [Google Scholar]
- [47].Feldmann H, Jones SM, Daddario-DiCaprio KM, Geisbert JB, Stroher U, Grolla A, Bray M, Fritz EA, Fernando L, Feldmann F, et al.. Effective post-exposure treatment of Ebola infection. PLoS Pathog 2007; 3:e2; PMID:17238284; http://dx.doi.org/ 10.1371/journal.ppat.0030002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Qiu X, Fernando L, Alimonti JB, Melito PL, Feldmann F, Dick D, Stroher U, Feldmann H, Jones SM. Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. PloS one 2009; 4:e5547; PMID:19440245; http://dx.doi.org/ 10.1371/journal.pone.0005547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tuffs A. Experimental vaccine may have saved Hamburg scientist from Ebola fever. BMJ 2009; 338:b1223; PMID:19307268 [DOI] [PubMed] [Google Scholar]
- [50].Geisbert TW, Hensley LE, Geisbert JB, Leung A, Johnson JC, Grolla A, Feldmann H. Postexposure treatment of Marburg virus infection. Emerg Infect Dis 2010; 16:1119-22; PMID:20587184; http://dx.doi.org/ 10.3201/eid1607.100159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tsuda Y, Safronetz D, Brown K, LaCasse R, Marzi A, Ebihara H, Feldmann H. Protective efficacy of a bivalent recombinant vesicular stomatitis virus vaccine in the Syrian hamster model of lethal Ebola virus infection. J Infect Dis 2011; 204 Suppl 3:S1090-7; PMID:21987746; http://dx.doi.org/ 10.1093/infdis/jir379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wong G, Audet J, Fernando L, Fausther-Bovendo H, Alimonti JB, Kobinger GP, Qiu X. Immunization with vesicular stomatitis virus vaccine expressing the Ebola glycoprotein provides sustained long-term protection in rodents. Vaccine 2014; 32:5722-9; PMID:25173474; http://dx.doi.org/ 10.1016/j.vaccine.2014.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Agnandji ST, Huttner A, Zinser ME, Njuguna P, Dahlke C, Fernandes JF, Yerly S, Dayer JA, Kraehling V, Kasonta R, et al.. Phase 1 Trials of rVSV Ebola Vaccine in Africa and Europe - Preliminary Report. N Engl J Med 2016; 374:1647-60; PMID:25830326; http://dx.doi.org/ 10.1056/NEJMoa1502924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Huttner A, Dayer JA, Yerly S, Combescure C, Auderset F, Desmeules J, Eickmann M, Finckh A, Goncalves AR, Hooper JW, et al.. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis 2015; 15:1156-66; PMID:26248510; http://dx.doi.org/ 10.1016/S1473-3099(15)00154-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Regules JA, Beigel JH, Paolino KM, Voell J, Castellano AR, Munoz P, Moon JE, Ruck RC, Bennett JW, Twomey PS, et al.. A Recombinant Vesicular Stomatitis Virus Ebola Vaccine - Preliminary Report. N Engl J Med 2015; PMID:25830322; http://dx.doi.org/ 10.1056/NEJMoa1414216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, Carroll MW, Doumbia M, Draguez B, Duraffour S, et al.. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015; 386:857-66; PMID:26248676; http://dx.doi.org/ 10.1016/S0140-6736(15)61117-5 [DOI] [PubMed] [Google Scholar]
- [57].Wells CR, Tchuenche JM, Meyers LA, Galvani AP, Bauch CT. Impact of imitation processes on the effectiveness of ring vaccination. Bull Mathematical Biol 2011; 73:2748-72; PMID:21409511; http://dx.doi.org/ 10.1007/s11538-011-9646-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lai L, Davey R, Beck A, Xu Y, Suffredini AF, Palmore T, Kabbani S, Rogers S, Kobinger G, Alimonti J, et al.. Emergency postexposure vaccination with vesicular stomatitis virus-vectored Ebola vaccine after needlestick. Jama 2015; 313:1249-55; PMID:25742465; http://dx.doi.org/ 10.1001/jama.2015.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bukreyev A, Yang L, Zaki SR, Shieh WJ, Rollin PE, Murphy BR, Collins PL, Sanchez A. A single intranasal inoculation with a paramyxovirus-vectored vaccine protects guinea pigs against a lethal-dose Ebola virus challenge. J Virol 2006; 80:2267-79; PMID:16474134; http://dx.doi.org/ 10.1128/JVI.80.5.2267-2279.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yano T, Fukuta M, Maeda C, Akachi S, Matsuno Y, Yamadera M, Kobayashi A, Nagai Y, Kusuhara H, Kobayashi T, et al.. Epidemiological investigation and seroprevalence of human parainfluenza virus in Mie Prefecture in Japan during 2009-2013. Jpn J Infect Dis 2014; 67:506-8; PMID:25410571 [PubMed] [Google Scholar]
- [61].Bukreyev A, Marzi A, Feldmann F, Zhang L, Yang L, Ward JM, Dorward DW, Pickles RJ, Murphy BR, Feldmann H, et al.. Chimeric human parainfluenza virus bearing the Ebola virus glycoprotein as the sole surface protein is immunogenic and highly protective against Ebola virus challenge. Virology 2009; 383:348-61; PMID:19010509; http://dx.doi.org/ 10.1016/j.virol.2008.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tsuda Y, Caposio P, Parkins CJ, Botto S, Messaoudi I, Cicin-Sain L, Feldmann H, Jarvis MA. A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS Negl Trop Dis 2011; 5:e1275; PMID:21858240; http://dx.doi.org/ 10.1371/journal.pntd.0001275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Klyushnenkova EN, Kouiavskaia DV, Parkins CJ, Caposio P, Botto S, Alexander RB, Jarvis MA. A cytomegalovirus-based vaccine expressing a single tumor-specific CD8+ T-cell epitope delays tumor growth in a murine model of prostate cancer. J Immunother 2012; 35:390-9; PMID:22576344; http://dx.doi.org/ 10.1097/CJI.0b013e3182585d50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tierney R, Nakai T, Parkins CJ, Caposio P, Fairweather NF, Sesardic D, Jarvis MA. A single-dose cytomegalovirus-based vaccine encoding tetanus toxin fragment C induces sustained levels of protective tetanus toxin antibodies in mice. Vaccine 2012; 30:3047-52; PMID:22414558; http://dx.doi.org/ 10.1016/j.vaccine.2012.02.043 [DOI] [PubMed] [Google Scholar]
- [65].Xu G, Smith T, Grey F, Hill AB. Cytomegalovirus-based cancer vaccines expressing TRP2 induce rejection of melanoma in mice. Biochem Biophys Res Commun 2013; 437:287-91; PMID:23811402; http://dx.doi.org/ 10.1016/j.bbrc.2013.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ohimain EI. Recent advances in the development of vaccines for Ebola virus disease. Virus Res 2016; 211:174-85; PMID:26596227; http://dx.doi.org/ 10.1016/j.virusres.2015.10.021 [DOI] [PubMed] [Google Scholar]
- [67].Papaneri AB, Wirblich C, Cann JA, Cooper K, Jahrling PB, Schnell MJ, Blaney JE. A replication-deficient rabies virus vaccine expressing Ebola virus glycoprotein is highly attenuated for neurovirulence. Virology 2012; 434:18-26; PMID:22889613; http://dx.doi.org/ 10.1016/j.virol.2012.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Blaney JE, Wirblich C, Papaneri AB, Johnson RF, Myers CJ, Juelich TL, Holbrook MR, Freiberg AN, Bernbaum JG, Jahrling PB, et al.. Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and Ebola viruses. J Virol 2011; 85:10605-16; PMID:21849459; http://dx.doi.org/ 10.1128/JVI.00558-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Blaney JE, Marzi A, Willet M, Papaneri AB, Wirblich C, Feldmann F, Holbrook M, Jahrling P, Feldmann H, Schnell MJ. Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine. PLoS Pathog 2013; 9:e1003389; PMID:23737747; http://dx.doi.org/ 10.1371/journal.ppat.1003389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Cleaveland S, Fevre EM, Kaare M, Coleman PG. Estimating human rabies mortality in the United Republic of Tanzania from dog bite injuries. Bulletin of the World Health Organization 2002; 80:304-10; PMID:12075367 [PMC free article] [PubMed] [Google Scholar]
- [71].Knobel DL, du Toit JT, Bingham J. Development of a bait and baiting system for delivery of oral rabies vaccine to free-ranging African wild dogs (Lycaon pictus). J Wildlife Dis 2002; 38:352-62; PMID:12038135; http://dx.doi.org/ 10.7589/0090-3558-38.2.352 [DOI] [PubMed] [Google Scholar]
- [72].Lupton HW, Lambert RD, Bumgardner DL, Moe JB, Eddy GA. Inactivated vaccine for Ebola virus efficacious in guineapig model. Lancet 1980; 2:1294-5; PMID:6108462 [DOI] [PubMed] [Google Scholar]
- [73].Rao M, Bray M, Alving CR, Jahrling P, Matyas GR. Induction of immune responses in mice and monkeys to Ebola virus after immunization with liposome-encapsulated irradiated Ebola virus: protection in mice requires CD4(+) T cells. J Virol 2002; 76:9176-85; PMID:12186901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Xu L, Sanchez A, Yang Z, Zaki SR, Nabel EG, Nichol ST, Nabel GJ. Immunization for Ebola virus infection. Nat Med 1998; 4:37-42; PMID:9427604 [DOI] [PubMed] [Google Scholar]
- [75].Vanderzanden L, Bray M, Fuller D, Roberts T, Custer D, Spik K, Jahrling P, Huggins J, Schmaljohn A, Schmaljohn C. DNA vaccines expressing either the GP or NP genes of Ebola virus protect mice from lethal challenge. Virology 1998; 246:134-44; PMID:9657001 [DOI] [PubMed] [Google Scholar]
- [76].Grant-Klein RJ, Van Deusen NM, Badger CV, Hannaman D, Dupuy LC, Schmaljohn CS. A multiagent filovirus DNA vaccine delivered by intramuscular electroporation completely protects mice from ebola and Marburg virus challenge. Hum Vaccin Immunother 2012; 8:1703-6; PMID:22922764; http://dx.doi.org/ 10.4161/hv.21873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ebihara H, Zivcec M, Gardner D, Falzarano D, LaCasse R, Rosenke R, Long D, Haddock E, Fischer E, Kawaoka Y, et al.. A Syrian golden hamster model recapitulating ebola hemorrhagic fever. J Infect Dis 2013; 207:306-18; PMID:23045629; http://dx.doi.org/ 10.1093/infdis/jis626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Martin JE, Sullivan NJ, Enama ME, Gordon IJ, Roederer M, Koup RA, Bailer RT, Chakrabarti BK, Bailey MA, Gomez PL, et al.. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin Vaccine Immunol 2006; 13:1267-77; PMID:16988008; http://dx.doi.org/ 10.1128/CVI.00162-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Sarwar UN, Costner P, Enama ME, Berkowitz N, Hu Z, Hendel CS, Sitar S, Plummer S, Mulangu S, Bailer RT, et al.. Safety and immunogenicity of DNA vaccines encoding Ebolavirus and Marburgvirus wild-type glycoproteins in a phase I clinical trial. J Infect Dis 2015; 211:549-57; PMID:25225676; http://dx.doi.org/ 10.1093/infdis/jiu511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bavari S, Bosio CM, Wiegand E, Ruthel G, Will AB, Geisbert TW, Hevey M, Schmaljohn C, Schmaljohn A, Aman MJ. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J Exp Med 2002; 195:593-602; PMID:11877482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Warfield KL, Bosio CM, Welcher BC, Deal EM, Mohamadzadeh M, Schmaljohn A, Aman MJ, Bavari S. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci U S A 2003; 100:15889-94; PMID:14673108; http://dx.doi.org/ 10.1073/pnas.2237038100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Warfield KL, Swenson DL, Negley DL, Schmaljohn AL, Aman MJ, Bavari S. Marburg virus-like particles protect guinea pigs from lethal Marburg virus infection. Vaccine 2004; 22:3495-502; PMID:15308377; http://dx.doi.org/ 10.1016/j.vaccine.2004.01.063 [DOI] [PubMed] [Google Scholar]
- [83].Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis 2007; 196 Suppl 2:S430-7; PMID:17940980 [DOI] [PubMed] [Google Scholar]
- [84].Sun Y, Carrion R Jr., Ye L, Wen Z, Ro YT, Brasky K, Ticer AE, Schwegler EE, Patterson JL, Compans RW, et al.. Protection against lethal challenge by Ebola virus-like particles produced in insect cells. Virology 2009; 383:12-21; PMID:18986663; http://dx.doi.org/ 10.1016/j.virol.2008.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Warfield KL, Dye JM, Wells JB, Unfer RC, Holtsberg FW, Shulenin S, Vu H, Swenson DL, Bavari S, Aman MJ. Homologous and heterologous protection of nonhuman primates by Ebola and Sudan virus-like particles. PloS one 2015; 10:e0118881; PMID:25793502; http://dx.doi.org/ 10.1371/journal.pone.0118881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Halfmann P, Kim JH, Ebihara H, Noda T, Neumann G, Feldmann H, Kawaoka Y. Generation of biologically contained Ebola viruses. Proc Natl Acad Sci U S A 2008; 105:1129-33; PMID:18212124; http://dx.doi.org/ 10.1073/pnas.0708057105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hoenen T, Groseth A, de Kok-Mercado F, Kuhn JH, Wahl-Jensen V. Minigenomes, transcription and replication competent virus-like particles and beyond: reverse genetics systems for filoviruses and other negative stranded hemorrhagic fever viruses. Antiviral Research 2011; 91:195-208; PMID:21699921; http://dx.doi.org/ 10.1016/j.antiviral.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Halfmann P, Ebihara H, Marzi A, Hatta Y, Watanabe S, Suresh M, Neumann G, Feldmann H, Kawaoka Y. Replication-deficient ebolavirus as a vaccine candidate. J Virol 2009; 83:3810-5; PMID:19211761; http://dx.doi.org/ 10.1128/JVI.00074-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Marzi A, Halfmann P, Hill-Batorski L, Feldmann F, Shupert WL, Neumann G, Feldmann H, Kawaoka Y. Vaccines. An Ebola whole-virus vaccine is protective in nonhuman primates. Science 2015; 348:439-42; PMID:25814063; http://dx.doi.org/ 10.1126/science.aaa4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ewer KJ, Lambe T, Rollier CS, Spencer AJ, Hill AV, Dorrell L. Viral vectors as vaccine platforms: from immunogenicity to impact. Curr Opin Immunol 2016; 41:47-54; PMID:27286566; http://dx.doi.org/ 10.1016/j.coi.2016.05.014 [DOI] [PubMed] [Google Scholar]
- [91].Parpia AS, Ndeffo-Mbah ML, Wenzel NS, Galvani AP. Effects of Response to 2014-2015 Ebola Outbreak on Deaths from Malaria, HIV/AIDS, and Tuberculosis, West Africa. Emerg Infect Dis 2016; 22:433-41; PMID:26886846; http://dx.doi.org/ 10.3201/eid2203.150977 [DOI] [PMC free article] [PubMed] [Google Scholar]


