Abstract
Astrocytes secrete vasodilator and vasoconstrictor factors via end feet processes, altering blood flow to meet neuronal metabolic demand. Compared to what is known about the ability of astrocytes to release factors that dilate local cerebral vasculature, very little is known regarding the source and identity of astrocyte derived constricting factors. The present study investigated if astrocytes express CYP 4A ω-hydroxylase and metabolize arachidonic acid (AA) to 20-hydroxyeicotetraenoic acid (20-HETE) that regulates KCa channel activity in astrocytes and cerebral arterial myocyte contractility. Here we report that cultured astrocytes express CYP 4A2/3 ω-hydroxylase mRNA and CYP 4A protein and produce 20-HETE and the CYP epoxygenase metabolites epoxyeicosatrienoic acids (EETs) when incubated with AA. The production of 20-HETE and EETs was enhanced following stimulation of metabotropic glutamate receptors (mGluR) on the astrocytes. Exogenous application of 20-HETE attenuated, whereas inhibition of 20-HETE production with HET-0016 increased the open state probabilities (NPo) of 71 pS and 161 pS KCa single-channel currents recorded from astrocytes. Exposure of isolated cerebral arterial myocytes to conditioned media from cultured astrocytes caused shortening of the length of freshly isolated cerebral arterial myocytes that was not evident following inhibition of astrocyte 20-HETE synthesis and action. These findings suggest that astrocytes not only release vasodilator EETs in response to mGluR stimulation but also synthetize and release the cerebral arterial myocyte constrictor 20-HETE that also functions as an endogenous inhibitor of the activity of two types of KCa channel currents found in astrocytes.
Keywords: Astrocyte, Neurovascular unit, Arachidonic acid, CYP 4A ω-hydroxylase, 20-HETE, Cerebral circulation
1. Introduction
Astrocytes are the most abundant cell types in the brain and are known to provide structural and metabolic support to neurons. Astrocytes send foot processes to neurons and adjacent cerebral microvessels, and communicate with each other through gap junctions. They couple neuronal activity to local blood flow as a component of the neurovascular unit (NVU) [1,2,3]. A variety of membrane-derived lipids is emerging as important regulators of cerebrovascular function under physiological and pathological conditions [4–12]. Enzymes of the cytochrome P450 (CYP) gene family are expressed in different cell types in the brain and catalyze conversion of AA released by phospholipase A2 to a variety of fatty acid signaling molecules [5–7,12–16]. We have previously reported that brain astrocytes express the CYP 2C11 and 4X1 epoxygenases, and the CYP 4F5 and 4F6 ω-hydroxylases in addition to the presence of cyclooxygenases (COX) [5–7,17,18]. These enzymes metabolize AA to epoxyeicosatrienoic acids (EETs) and prostaglandins (PGs) [6,7,10,18–20]. The EETs can activate Ca2+-activated K+ channel (KCa) channel currents in adjacent arteriolar muscle cells to hyperpolarize the membrane potential and elicit cerebral vasodilation [21,22]. Since the production and release of EETs by astrocytes is increased in response to elevated neuronal activity, the EETs are now regarded as one of the astrocyte-derived vasodilator factors along with NO, adenosine, prostaglandins and K+ that mediate functional hyperemia in the brain [21–25]. EETs also increase the activity of two types of KCa channel currents in astrocytes and initiate signaling cascades that regulate astrocyte function [26]. In addition to the CYP epoxygenases catalyzing formation of EETs from AA, earlier studies from our laboratory identified the expression of CYP ω-hydroxylase isoforms that catalyze the ω-hydroxylation of AA to 20-hydroxeicosatetraenoic acid (20-HETE) in different cell types in the brain [4,6–8,11,27]. The CYP 4A11, CYP 4A22, CYP 4F2 and CYP 4F3 isoforms are all expressed in man and have been reported to catalyze the formation of 20-HETE from arachidonic acid [28]. CYP4A10, 4A12a, 4A12b and 4A14 are the isoforms expressed in mice. CYP4A12a and b convert arachidonic acid to 20-HETE, while the activity of the 4A10 and 4A14 isoforms are very low [29]. The corresponding isoforms expressed in the rat are CYP4A1, CYP4A2, CYP4A3 and CYP4A8. Of these, CYP4A2 and 4A3 are most avidly expressed and all 4 isoforms metabolize arachidonic acid to 20-HETE [28]. 20-HETE is a potent vasoconstrictor that is formed in cerebral arterial muscle cells and acts through PKC-dependent inhibition of KCa channel currents with subsequent membrane depolarization and activation of L-type Ca2+, TRPV1 and TRPC6 channels to promote influx of Ca2+ [14–16,30,31]. 20-HETE mediates pressure-dependent myogenic cerebral arterial constriction, which is responsible for autoregulation of cerebral blood flow during increases in mean arterial pressure [8,13].
A previous study [32] suggested that a rise in [Ca2+]i in astrocytes in rat and mouse brain slice preparations can induce constriction of adjacent cerebral arterioles and that this effect was attenuated by HET0016, an inhibitor of 20-HETE synthesis [32]. From these findings, the authors proposed that a rise in astrocyte [Ca2+]i activates PLA2-dependent AA release which is catalytically converted to 20-HETE by CYP 4A ω-hydroxylase in adjacent cerebral arteriolar smooth muscle to trigger vasoconstriction. However, no evidence was indicated if rat brain astrocytes express CYP 4A ω-hydroxylase or can metabolize AA to 20-HETE. The present study investigated whether cultures of neonatal rat brain astrocytes that are devoid of neurons express message and protein for CYP 4A isoforms and produce 20-HETE when incubated with AA. We have also examined contribution of 20-HETE to the actions of astrocyte conditioned media on reactivity of freshly isolated cerebral arterial myocytes, and the impact of inhibition of endogenous 20-HETE synthesis and application of exogenous 20-HETE on the open state probabilities of KCa channel currents recorded from cell-attached patches of cultured rat brain astrocytes. The present findings demonstrate that rat brain astrocytes express CYP 4A ω-hydroxylase message and protein, produce and release 20-HETE that could function as endogenous inhibitor of astrocyte KCa channel currents and influence the tone of cerebral arterial myocytes.
2. Methods
The animal protocols used in this study were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
2.1. Cell culture
Sprague Dawley rat pups of 2–3 days of age were anesthetized with diethyl-ether, decapitated and the brain removed for preparation of astrocyte cultures as previously described [5]. Briefly, the brain was dissected free of meninges, and the cerebral cortices and hippocampus were isolated and cut into small pieces, and transferred to sterile dish containing 20 U/ml papain (Worthington Biochemical Corp) and cysteine (0.15 mg/ml; Sigma) dissolved in Earle’s balanced salt solution (Gibco BRL) and incubated at 37 °C for 40 min with gentle agitation. Digestion was stopped by washing three times with an astrocyte growth medium containing DMEM, 10% fetal bovine serum (FBS), 25 units/ml penicillin, 25 μg/ml streptomycin and 0.1% gentamicin (Invitrogen, Carlsbad, CA). The tissue was then dissociated by trituration with flame-narrowed Pasteur pipette and cell suspension was diluted with feeding medium and seeded at an initial density of approximately 2 × 105 cells per square centimeter. The cells were incubated at 37 °C in a 95%/5% mixture of atmospheric air and CO2. The medium was changed after 2 days and subsequently twice a week. Confluent monolayers of brain astrocytes were studied.
2.2. CYP 4A mRNA analysis
Real-time PCR was used to measure the expression of CYP 4A2 and 4A3 mRNA in primary cultures of rat brain astrocytes. Confluent cultured astrocytes were serum starved for 12 h in medium containing low-glucose (1 g/l) DMEM, 1% horse serum, 25 units/ml penicillin and 25 μg/ml streptomycin (Invitrogen Corporation, Carlsbad, CA). The cultured astrocytes were rinsed twice with DPBS (Dulbecco’s Phosphate Buffered Saline), lysed on ice with a R-I buffer (Axygen Biosciences, Union City, CA, USA), which nullifies endogenous RNase activity, and collected in 1.5 ml tubes. Total RNA was isolated using the AxyPrep Multisource Total RNA Miniprep Kit (Axygen Biosciences). The samples were treated with DNase I (ThermoFisher Scientific Incorporated, Waltham, MA) to prevent contamination of the RNA with genomic DNA. RNA integrity was checked by electrophoresis on a formaldehyde agarose gel and the concentration of RNA was quantified using a NanoDrop 1000 Spectrophotometer (ThermoFisher Scientific Incorporated). For each sample, 1 μg of RNA was reverse transcribed in a 20 μl reaction using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). For RT-PCR, 5 ng of the cDNA was amplified in a 25 μl reaction containing iQ SYBR Green Supermix (Bio-Rad Laboratories) using an iCycler thermal cycler and iQ5 detection module (Bio-Rad Laboratories). After denaturing of the samples for 3 min at 95 °C, the reactions were amplified for 40 cycles for 30 s at 95 °C, 30 s at the annealing temperature (56 °C for Polr2 and 56.5 °C for CYP 4A2/3). The primers were obtained from Operon Biotechnologies (Huntsville, AL). The primer sequences for Polr2 were: forward 5′ ctgatgcgggtgctgagtcagaagg3′ and reverse 5′ gcggttgaccccatgacgagtg3′. The sequences for the CYP 4A2/3 primers were taken from a previous publication [33]. The forward primer was: 5′ GTC CCC ATG CCA AGA CTT GT 3′ and the reverse primer was 5′ GTC TGG AGT AAA AGC TTT GGA GCT 3′. The linearity of amplification for both primer sets was verified by analysis of serially diluted cDNA. Product specificity was confirmed by melt curve analysis and agarose gel electrophoresis. Sequence analysis of the product formed could not differentiate between 4A2 vs. 4A3. Recent data indicates that the most likely CYP 4A isoform for the formation of 20-HETE in rat cerebral arteries is 4A3 [34,35]. Relative quantification of expression was determined by measuring the threshold cycle (Ct) values of each sample using the 2−ΔΔCt method [36]. Relative abundance of CYP 4A2/3 was normalized to Polr2 mRNA expression. Data are presented as mean ± SEM.
2.3. Western blot analysis
Microsomes were prepared from cultured hippocampal astrocytes or Sprague Dawley rat liver using differential centrifugation methods as described previously [5,15,16]. Briefly, cultured astrocytes or liver were separately homogenized in RIPA buffer (150 mM NaCl, 1.0% NP-40, 0.5% deoxycholate, 1 mM EDTA, 50 mM Tris pH 8.0) containing protease inhibitor cocktail (PharMingen, San Diego, CA, USA) composed of benzamidine HCl (16 μg/ml), phenanthroline (10 μg/ml), aprotinin (10 μg/ml), leupeptin (10 μg/ml), pepstatin A (10 μg/ml) and 50 mM PMSF. The homogenates were centrifuged for 10 min at 3500g, 30 min at 9000g, and 60 min at 100000g to obtain the microsomal pellet, and the pellet was resuspended in RIPA buffer. The protein concentration of the microsomes was quantified using the Bio-Rad protein assay method (Bio-Rad Laboratories, Richmond, CA). An aliquot (10 μg of protein) of the astrocyte or rat liver microsomal proteins was added to 2 × Laemelli sodium dodecyl sulfate (SDS) sample buffer, boiled for 5 min, and loaded onto 10% SDS-polyacrylamide gel (4% stacking, 10% resolving) (Bio-Rad Ready-gels). Following separation, the proteins were transferred to nitrocellulose membranes (Bio-Rad), and probed using CYP 4A ω-hydroxylase polyclonal rabbit antibody (PA3-033, ThermoFisher Scientific) (1:500 dilution in TBS-T containing 2% nonfat dry milk) for 2 h at room temperature and a goat anti-rabbit HRP secondary antibody (#1662408, Bio-Rad) (1:1000). The blot was developed by exposure to ECL reagent (Amersham Biosciences, UK). Blot was exposed to Hyperfilm ECL from Amersham Biosciences. Immunoreactive bands around 52 kD, corresponding to the size of the CYP 4A ω-hydroxylases, were identified using prestained molecular weight marker proteins (#161-0374, Bio-Rad).
2.4. Immunofluorescence microscopy
Confluent cultures of neonate rat brain astrocytes grown on coverslips were rinsed in phosphate buffered saline (PBS) at room temperature (RT) and fixed with 4% paraformaldehyde for 10 min. The astrocytes were rinsed in PBS at RT and incubated in blocking solution (5% BSA in PBS) in a humidified chamber for 30 min. To examine and rule out presence of neuronal cells, the adherent astrocytes on the cover slips were incubated with the astrocyte marker rabbit polyclonal anti-GFAP antibody (1:200) Novus Biologicals (5C10) (NBPI-05197) or with the specific neuronal marker anti-NeuN antibody [1B7] (1:500) (abcam ab104224) and rabbit polyclonal anti-CYP 4A antibody (1:200) (PA3-033, ThermoFisher Scientific) overnight, followed by incubation with a secondary goat anti-rabbit Alexa Fluor-488 antibody (A-11039, ThermoFisher Scientific) at a dilution of 1:400 (1 h; RT)). The nuclei (blue) were counter stained with I 4′,6-diamidino-2-phenylindole (DAPI) (D1306, ThermoFisher Scientific). The cultured astrocytes were subsequently rinsed three times in PBS and images were taken at a magnification of 20× using Nikon E-600 fluorescent microscope equipped with FITC (fluorescein-isotiocyanate) filters, with excitation at 492 nm and emission at 520 nm.
2.5. Liquid chromatographic–mass spectrometric measurements of eicosanoid production
Confluent primary cultures of rat brain astrocytes free of neuronal cells, were serum starved for 24 h by replacing the 10% fetal bovine serum with 0.1% fetal bovine serum. The cultured astrocytes were incubated at 37 °C with 40 μM AA in potassium phosphate buffer (0.1 M, pH 7.4) containing an NADPH-generating system (20 mM isocitrate and 0.1 units/ml isocitrate dehydrogenase), 1 mM EDTA, 10 mM MgCl2, at 37 °C [15,37] in an incubator in a volume of 5 ml of assay buffer for 60 min. The astrocytes were then stimulated with the specific mGluR agonist RS-3,5-dihydroxyphenylglycine (DHPG, 100 μM) or vehicle and incubated at 37 °C for an additional 30 min. Following incubation, the astrocytes were separated from media, and the media collected and kept on ice. The astrocytes in the culture dish were washed twice with 2 ml of HEPES buffered saline, and then scraped in 5 ml of HEPES buffered saline placed on ice. An aliquot (50 μl) from each sample was transferred to 1.5 ml tubes for quantification of protein concentration as stated above. The cell and media samples were spiked with 2 ng of the following internal standards: deuterated 2H8-EETs (for EETs), 2H8-DHETs (for DHETs), and 2H2-0-HETE (for 20-HETE). The samples were acidified to pH 3.5 and extracted twice with 3 ml of diethyl ether with gentle vortexing for 1 min and then centrifuged at 5000g for 5 min. The diethyl ether layer was then transferred to a clean 10 ml glass centrifuge tube and dried under a flow of nitrogen gas. The dried samples were then reconstituted in 200 μl of acetonitrile for analysis by LC–MS in the negative ion mode. For quantitative measurements, the m/z 319, 327, 337, 345, 319, and 321 ions were used for EETs, [2H8] EETs, DHETs, [2H8] DHETs, and for 20-HETE, [2H2]-20-HETE, respectively. The standard curves were typically constructed over the range of 1–100 pg per injection. The concentrations of the eicosanoids in the samples were calculated by comparing the ratios of their peak areas to standard curves as previously described [27].
2.6. Patch camp recording of single-channel KCa currents
Single-channel KCa currents were recorded at room temperature from cell-attached membrane patches of primary cultures of neonate rat brain astrocytes using the patch-clamp technique as described previously [38,39]. Briefly, recording pipettes were fabricated from borosilicate glass, pulled on a 2-stage micropipette puller (P-97), and heat-polished under a microscope (MF-83 heat polisher; Narishige, Tokyo, Japan). The recording pipettes were mounted on a three-way hydraulic micromanipulator (Narishige) for placement of the tips on the cell membrane. High-resistance seals (>1 GΩ) were established by applying a slight suction between fire-polished pipette tips (3–10 MΩ) and cell membranes. The offset potentials between pipette and bath solution were corrected with an offset circuit before each experiment. Single-channel currents were recorded using an Axopatch 200B amplifier (Molecular Devices, CA). The amplifier output was low-pass filtered at 1 kHz. Current signals were digitized at a sampling rate of 10 kHz (Digidata 1440A, Molecular Devices, CA). Single-channel currents were analyzed using a pClamp software package (pClamp version 10.4; molecular Devices, CA) to determine event frequency, mean current amplitudes, and open state probability. The mean open state probability (NPo) was expressed as NPo = I/i, where I is the time averaged current, N is the number of channels, i is the amplitude of the unitary current, and Po is the probability of a channel being open.
2.6.1. Recording solutions
Single-channel KCa currents were recorded using a pipette solution containing (in mM): 145 KCl, 1.8 CaCl2, 1.1 MgCl2, and 5HEPES with pH adjusted to 7.2 with KOH. The bath solution was composed of (in mM): 145 KCl, 1.8 CaCl2, 1.1 MgCl2, 5HEPES, and 10 ethyleneglycol-bis (β-aminoethyl ether)-N, N, N′, N′-tetraacetic acid (EGTA), with pH adjusted to 7.2 with KOH. The 1 ml bath solution was continuously refreshed with external solution at a rate of 2 ml/min by gravitational flow. Single-channel KCa currents were recorded using a patch potential of +60 mV and the effects of different concentrations of 20-HETE (100, and 300 nM) or HET0016 (50 nM) were determined by adding them to the bath.
2.8. Cerebral arterial myocyte response to astrocyte conditioned media
The culture media was removed from cultured rat brain astrocytes and replaced with 3 ml of Dulbecco’s Phosphate Buffered Saline (DPBS, Gibco) containing 40 μM cold AA as detailed in the Section 2.5, and treated with 1 μM indomethacin, 40 μM MSPPOH and 10 μM 14,15-EEZE for 60 min to inhibit endogenous production of cyclooxygenase and epoxygenase metabolites, and action of preformed and released EETs, respectively. Then the mGluR agonist DHPG (100 μM) was added to the cultured astrocytes followed by incubation at 37 °C for 30 min. Such treatment conditions were felt to assure absence of contribution of other fatty acid metabolites to the action of astrocyte-conditioned media to be studied. In a separate study, the cultured astrocytes incubated as detailed above were also additionally treated with the 20-HETE synthesis inhibitor HET0016 (50 nM) and the 20-HETE antagonist WIT002 (1 μM) to prevent the effect of preformed and released 20-HETE to identify involvement of CYP4A ω-hydroxylase-derived 20-HETE in the effects of astrocyte conditioned media on cerebral arterial myocyte length. The astrocyte-conditioned media was then collected from the two sets of incubations and the effects examined separately on freshly dissociated rat cerebral arterial myocytes that were kept at 37 °C and pretreated with 50 nM HET0016 to inhibit arterial muscle 20-HETE synthesis or the vehicle control. Viability of the isolated arterial myocytes was examined at the end of the experiment by exposure of the cells to 60 mM KCl at 37 °C. Images of the arterial myocytes were captured at 30 s intervals for a period of 5 min using a Nikon Eclipse E600FN microscope with an attached Cascade 1 K camera (Photometrics) and NIS-Elements AR software (Nikon) to analyze responses of cerebral arterial myocytes to the different treatment conditions.
2.9. Drugs and chemicals
All chemicals were analytical grade and were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, U.S.A.). Arachidonic acid was obtained from BIOMOL (Plymouth, PA). 20-HETE (20-hydroxy-5Z,8Z,11Z,14Z-eicosatetraenoic acid), HET0016 N-hydroxy-N′-(4-butyl-2-methylphenyl)formamidine, 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EE-5(Z)-E), MSPPOH and indomethacin was purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). RS-3,5-dihydroxyphenylglycine (DHPG) was obtained from Tocris Bioscience (Bristol, BS11 0QL United Kingdom).
2.10. Statistical analysis
Data are presented as mean values ± SEM. Differences in mean values between groups were assessed using Student’s t test or one-way analysis of variance (ANOVA) for multiple comparisons. A P < 0.05 was considered statistically significant.
3. Results
3.1. Expression of CYP 4A ω-hydroxylase in cultured rat brain astrocytes
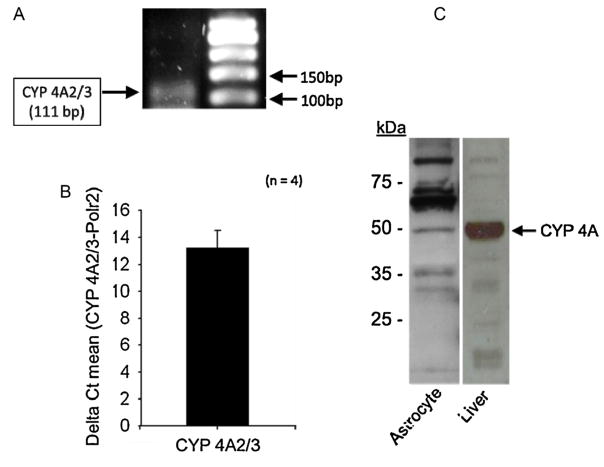
The results of RT-PCR analysis of the expression of CYP 4A2/3 mRNA in cultured rat brain astrocytes revealed that CYP 4A2/3 message was highly expressed since the Threshold cycle (Ct) averaged 13.5 ± 2.60 (4 independent experiments) (Fig. 1A and B). Furthermore, separation of an aliquot of the RT-PCR product on a 1.5% agarose gel, revealed a band corresponding to the expected size for CYP 4A2/3 as depicted in Fig. 1A. This finding indicates that CYP 4A2/3 message is expressed in cultured rat brain astrocytes. Similarly, the results of our western blot experiments using polyclonal rabbit antibody against CYP 4A ω-hydroxylase that cross reacts with all CYP 4A ω-hydroxylase isoforms detected expression of a band of expected molecular size (~52 kDa) for the CYP 4A protein in both astrocyte and rat liver microsomes (Bio-Rad) (Fig. 1C). The appearance of nonspecific bands could be glycosylated or acylated posttranslational modifications of the CYP 4A isoforms that have been described previously [40].
Fig. 1.
Expression of the transcript and protein for CYP 4A2/3 ω-hydroxylase in rat brain astrocytes. (A and B) Identification by RT-PCR analysis of presence of CYP 4A2/3 ω-hydroxylase transcript in cultured rat brain astrocytes (n = 4). (C) Detection of CYP 4A ω-hydroxylase protein in microsomes prepared from cultured rat brain astrocytes and liver used as control. (n = 3 independent experiments for each group).
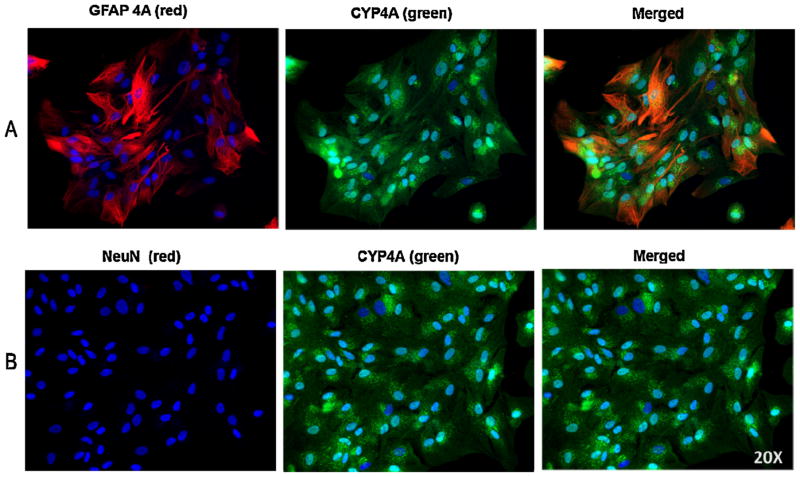
Additional experiments were performed to verify purity of rat brain astrocyte cultures used in this study. The cultured cells were double stained with anti-CYP 4A antibody and anti-GFAP antibody or with the specific neuronal marker anti-NeuN antibody. The cultured cells exhibited positive staining with both anti-GFAP antibody and anti-CYP 4A antibody and were not stained with the neuronal marker anti-NeuN antibody (Fig. 2). The appearance of cells stained with DAPI that are not staining with GFAP could be due to transient changes in GFAP expression in brain cells that is known to occur during development [41]; however, we cannot exclude the presence of some oligodendrocytes or microglia. These findings reveal the absence of neuronal cells in the cultured rat brain astrocytes and confirm expression of CYP 4A ω-hydroxylase protein in these GFAP positive cells.
Fig. 2.
Double staining with anti-NeuN and anti-CYP 4A, and anti-GFAP and anti-CYP 4A. (A) Double-staining of cultured rat brain astrocytes with polyclonal antibodies against GFAP and CYP 4A ω-hydroxylase resulted in positive immunostaining for GFAP (Red, left panel) and CYP 4A ω-hydroxylase (green, middle panel) which is confirmed in the corresponding merged image of GFAP and CYP 4A staining (right panel) demonstrating capacity of cultured astrocytes to express CYP 4A ω-hydroxylase protein that catalyze the formation of 20-HETE from AA. (B) Double labeling of the cultured astrocytes by incubation with the neuronal marker anti-NeuN antibody and anti-CYP 4A ω-hydroxylase displayed negative immunostaining signal with anti-NeuN antibody (right panel) while positively stained with anti-CYP 4A ω-hydroxylase (middle panel). Nuclei were stained with DAPI (blue). These findings clearly demonstrated the absence of neuronal cells in the GFAP positive cultures of rat brain astrocytes that express CYP 4Aω-hydroxylase (n = 3 independent trials).
3.2. Production of CYP ω-hydroxylase and CYP epoxygenase metabolites of AA in cultured rat brain astrocytes
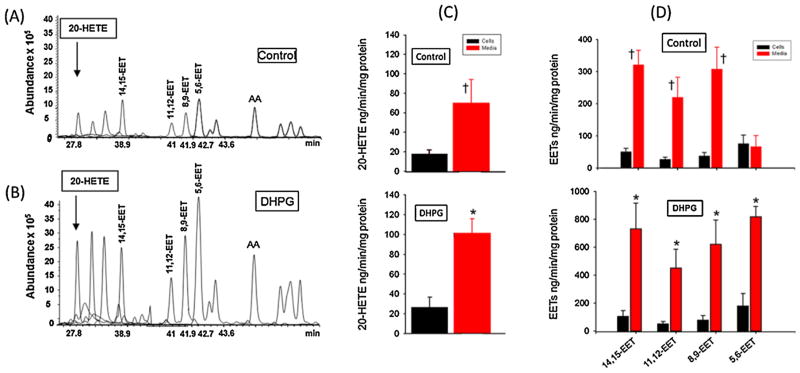
Fig. 3A and B present a representative (LC–ESI–MS) ion chromatogram of 20-HETE, 14,15-EET, 11,12-EET, 8,9-EET and 5,6-EET detected in samples of media and rat brain astrocytes incubated with AA under control condition (A) and following stimulation of metabotropic glutamate receptor (mGluR) with 100 μM DHPG (B). As shown in Fig. 3C and D, extracts of the cells (black bars) and the media (red bars) revealed the presence of 20-HETE (Fig. 3C) and all four regioisomers of epoxyeicosatrienoic acids (EETs) (14,15-, 11,12-, 8,9- and 5,6-EET) (Fig. 3D) under both control conditions (upper panels) and following stimulation with the mGluR agonist DHPG (100 μM) (lower panels), respectively (n = 3–4 independent experiments). The amount of 20-HETE released into the media (red bars) increased after stimulation with the mGluR agonist DHPG (100 μM) (*p < 0.05, Fig. 3C, lower panel). In contrast, the level of 20-HETE in the cells (black bars) did not increase following stimulation with the mGluR agonist DHPG (100 μM) (Fig. 3C, lower panel, n = 3–4 independent experiments).
Fig. 3.
Representative Liquid chromatographic-electrospray ionization-mass spectrometric (LC–ESI–MS) chromatogram of CYP metabolites of AA separated using linear gradient mobile phase (flow rate of 200 μl/min) under vehicle control conditions (Panel A) and after stimulation of the astrocytes with the mGluR agonist DHPG (100 μM). Panel B. Stimulation of mGluR increased the abundance of 20-HETE and the four regiosomers of EETs (n = 3–4 independent experiments). Panel C presents 20-HETE levels normalized to cellular protein concentration under vehicle control treatment condition and in response to stimulation of astrocytes with the mGluR agonist 100 μM DHPG as determined by LC–MS analysis of extracts of cells (astrocytes, black bars) and media (red bars) separated from these cells. The concentration of 20-HETE is greater in the media compared to that measured in the cells either under control (upper panel) conditions or following stimulation of the mGluR in astrocytes (lower panel). Panel D presents levels of 14,15-EET, 11,12-EET, 8,9-EET and 5,6-EET normalized to cellular protein concentration under control conditions (upper panel) and in response to stimulation of astrocytes with the mGluR agonist 100 μM DHPG (lower panel) in extracts from cells (astrocytes, black bars) and media (red bars) separated from these cells. The concentrations of released 20-HETE or EETs isomers are greater in the media under control condition (†P < 0.05), and in that following stimulation by the mGluR agonist DHPG as compared to that measured in the cells (*P < 0.05). Data are mean ± SEM of 3–4 independent experiments for both A and B panels.
The amount of EETs (14,15-, 11,12-, 8,9- and 5,6-EET) measured in samples of cells (black bars) and media (red bars) of cultured brain astrocytes incubated with AA under control condition and in response to stimulation with the mGluR agonist DHPG (100 μM) is depicted in Fig. 3D, upper and lower panels, respectively. The levels of the EETs in the media (red bars) increased significantly following stimulation with the mGluR agonist DHPG (100 μM) (*P < 0.05, Fig. 3D, lower panel). The levels of EETs in cell extract (black bars, Fig. 3D, upper and lower panels) were not significantly altered following mGluR stimulation with 100 μM DHPG. Taken together, these results indicate that rat brain astrocytes synthetize and release both EETs and 20-HETE and the release of these metabolites increases following stimulation with the mGluR agonist.
3.3. Effect of 20-HETE on astrocyte KCa single-channel current recorded from cell-attached patches
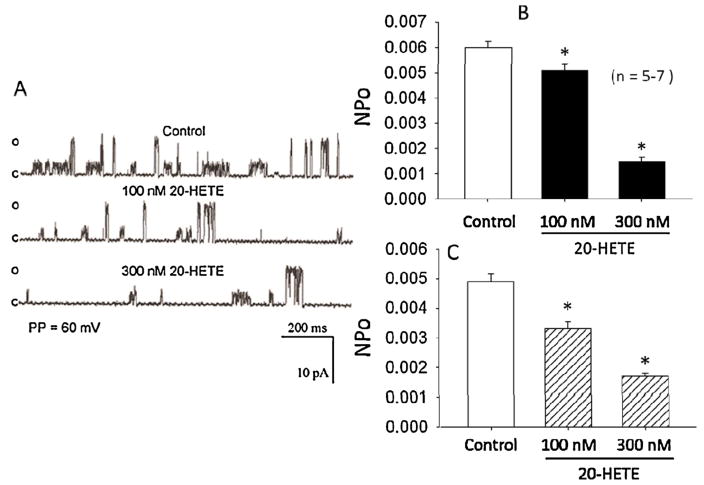
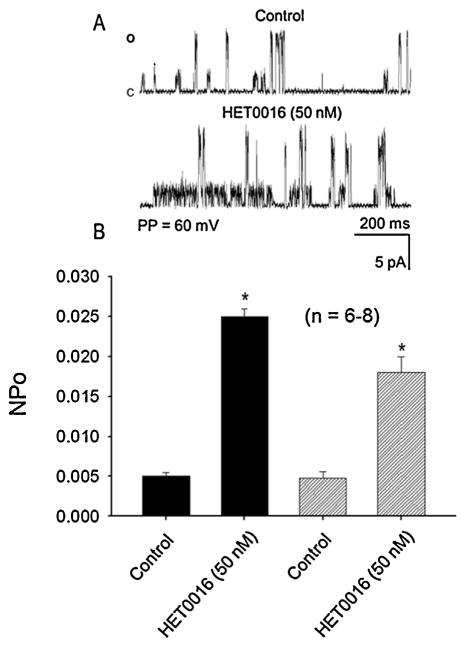
Previous studies from our laboratory characterized two types of single-channel KCa currents in cultured rat brain astrocytes with unitary conductances of 71 pS and 161 pS [38]. The effects of endogenous or exogenous 20-HETE on the activity of the KCa channels in astrocytes has never been reported. In the present study, we examined the effects of exogenous 20-HETE on activities of single-channel KCa currents recorded from cell-attached patches of cultured astrocytes at a patch potential of +60 mV using symmetrical KCl (145 mM) recording solution. As depicted in Fig. 4 application of 100 nM or 300 nM 20-HETE to the solution bathing the cell-attached patches reduced the opening frequencies of both the 71 pS and 161 pS single-channel KCa currents recorded in the cell-attached configuration (panel A). Application of 20-HETE (at 100 nM or 300 nM) also significantly reduced the open state probabilities (NPo) of the 71 pS KCa single-channel current from 0.0047 ± 0.00033 to 0.0035 ± 0.00028 and to 0.0021 ± 0.0005 (panel B); and that of the 161 pS KCa single-channel current from 0.0062 ± 0.00045 to 0.0055 ± 0.00031 and to 0.00193 ± 0.00021 (panel C), respectively (*p < 0.05, n = 5–7 cells). Treatment of the astrocytes with HET0016 induced a significant increase in the NPo of the 71 pS single-channel KCa currents rom 0.0051 ± 0.0004 to 0.0250 ± 0.00010 and that of the 161 pS single-channel KCa currents from 0.0049 ± 0.00063 to 0.0190 ± 0.00080 (Fig. 5, *P < 0.05, n = 6–8 cells).
Fig. 4.
Effects of exogenously applied 20-HETE on the openings of 71 pS and 161 pS single-channel KCa currents recorded from cell-attached patches of cultured rat brain astrocytes using symmetrical 145 mM KCl recording solution at a patch potential of +60 mV. (A) Representative single-channel current openings of the two KCa channel types recorded under control condition and after treatment with either 100 nM or 300 nM 20-HETE. (B) Bar graphs depicting summary of the effects of 100 nM and 300 nM 20-HETE on mean NPo of 71 pS (B) and 161 pS (C) KCa single-channel currents. Application of 20-HETE induced a significant concentration-dependent reduction in the opening frequencies and NPo values of the 71 pS and 161 pS single-channel KCa currents (n = 5–7 cell-attached membrane patches; *p < 0.05).
Fig. 5.
Effect of an inhibitor of the synthesis of 20-HETE, HET0016 (50 nM), on the openings of the 71 pS and 161 pS single-channel KCa currents recorded form cell-attached patches of cultured rat brain astrocytes. Pretreatment with HET0016 (50 nM) induced a significant increase in the NPo and opening frequencies of both the 71 pS and 161 pS single channel currents recorded from cell-attached patches of astrocytes at a patch potential of +60 mV using symmetrical KCl (145 mM) solution. (n = 6–8 cells, *P < 0.05).
3.4. Effects of astrocyte media on freshly dissociated cerebral arterial myocytes
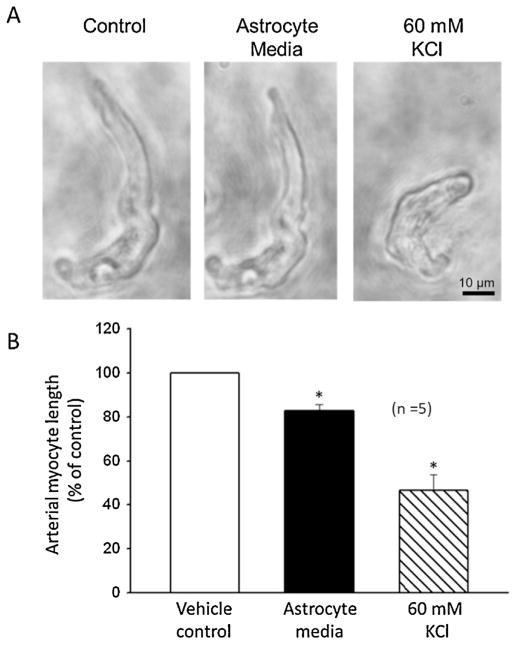
The effect of astrocyte-conditioned media separated from cultured astrocytes pretreated with cyclooxygenase and CYP epoxygenase inhibitors to exclude possible contribution of endogenously produced cyclooxygenase and CYP epoxygenase metabolites of arachidonic acid was examined on freshly isolated cerebral myocytes. We found that exposure of freshly dissociated cerebral arterial myocytes to conditioned media separated after stimulation of the cells with the mGluR agonist DHPG (100 μM) induced shortening of arterial myocyte length as depicted in Fig. 6A and B. Furthermore, the isolated cerebral arterial myocytes pretreated with astrocyte media markedly constricted following application of 60 mM KCl revealing viability of the arterial myocytes under these experimental conditions (Fig. 6A and B).
Fig. 6.
Responses of cerebral arterial myocytes to astrocyte conditioned media exposure. (A) Images of single freshly isolated cerebral arterial myocytes following treatment with vehicle (control), astrocyte conditioned media and 60 mM KCl. (B) Bar graphs depicting summary of the effects of application of vehicle, astrocyte media or 60 mM KCl on arterial myocyte length. Each data point represent mean ± SEM of percent of control absolute cell length in micrometers in response to treatment with vehicle, astrocyte media or 60 mM KCl. * denote significant difference from control value at P < 0.05, n = 5).
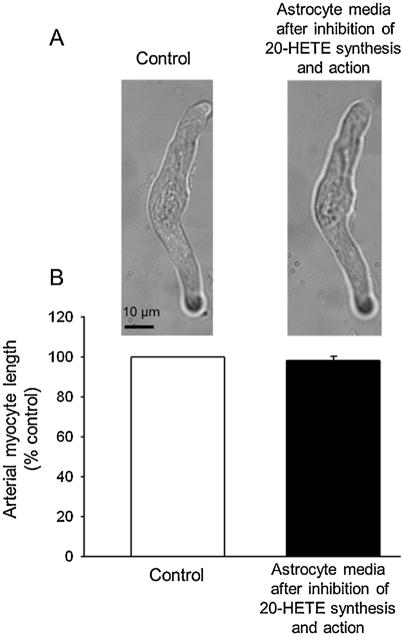
Additional studies were also undertaken to examine if the astrocyte conditioned media induced shortening of the freshly isolated cerebral arterial myocytes was down stream of CYP 4A ω-hydroxylase activity in astrocyte. In these studies, the cultured astrocytes incubated as detailed above, were additionally pretreated with the 20-HETE synthase inhibitor HET0016 (50 nM) and the 20-HETE antagonist 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (WIT002) (1 μM) to inhibit 20-HETE production and to prevent action of preformed and released 20-HETE. Following incubation for 60 min, the cultured astrocytes were stimulated with the mGluR agonist DHPG (100 μM) for 30 min and the effect of separated astrocyte conditioned media on isolated cerebral arterial myocyte length was examined. As shown in Fig. 7A and B application of the conditioned media separated from cultured astrocytes, pretreated with 20-HETE synthase inhibitor HET 0016 and the 20-HETE antagonist WIT002, failed to induce shortening of freshly isolated cerebral arterial myocytes as compared to that of the effects of vehicle treated arterial myocytes. These findings revealed that the factor released from astrocytes following mGluR stimulation into the media causing shortening of cerebral arterial myocytes appears to be the CYP 4A ω-hydroxylase-derived 20-HETE, which is synthetized and released from the cultured rat brain astrocytes into the media.
Fig. 7.
Responses of cerebral arterial myocytes to astrocyte conditioned media following inhibition of 20-HETE synthesis and 20-HETE action. (A) Images of single freshly isolated cerebral arterial myocytes in response to astrocyte media separated after incubation of the astrocyte cultures with vehicle alone (control), after inhibition of 20-HETE synthesis with HET-0016 (50 nM) and 20-HETE action by the 20-HETE antagonist WIT002 (1 μM). (B) Bar graphs summarizing effects of application of the vehicle control, astrocyte media after inhibition of 20-HETE synthesis with HET0016 and blockade of action of released 20-HETE with WIT002 (1 μM) on arterial myocyte length. Each data point represent mean ± SEM of percent of control absolute cell length in micrometers in response to treatment with vehicle control and the astrocyte conditioned media. *Denotes absence of statistically significant difference from control (P > 0.05, n = 6).
4. Discussion
An increase in neuronal metabolic demand matched by activity-dependent increase in nutritive cerebral blood flow referred to as functional hyperemia is facilitated by neurovascular coupling by astrocytes [17,22,25,42]. Growing evidence indicates that astrocytes produce and release mediators that can increase or decrease nutritive blood flow to different areas of the brain to match metabolic demand in neurons. Despite considerable progress in identifying and defining astrocyte derived vasodilators contributing to functional hyperemia, identification of the factors promoting vasoconstriction has lagged far behind. A recent report using brain slices indicated that elevations in intracellular Ca2+ induced PLA2-dependent release of arachidonic acid from brain astrocytes which then diffuses to adjacent cerebral arterial muscle cells and metabolized by CYP 4A ω-hydroxylase enzymes to 20-HETE to elicit cerebral arterial vasoconstriction [32]. The appearance of time delay of several seconds between the increase in [Ca2+]i in the astrocyte endfeet and the vasoconstrictor response in this previously reported study [32] was speculated by these authors to indicate possible release and diffusion of astrocyte produced 20-HETE to the perivascular space. However, it remains to be determined whether astrocytes express the CYP4A enzymes and can produce 20-HETE.
The results of the present studies demonstrate that neonate rat brain astrocytes in culture express message and protein for CYP 4A2/3 and synthesize 20-HETE when incubated with AA. We found that the production and release of 20-HETE in rat brain astrocytes is enhanced by activation of metabotropic glutamate receptors. To our knowledge, this is the first demonstration that rat brain astrocytes express CYP 4A ω-hydroxylase and can produce 20-HETE. The production of 20-HETE by astrocytes may be very significant since it indicates that rat brain astrocytes possess the enzymatic machinery to not only release vasodilators but also elicit a tonic vasoconstrictor influence on regional blood flow in response to changes in neuronal activity. This capacity broadens the dynamic range over which astrocytes can adjust cerebral blood flow in response to changes in neuronal activity. This new observation may lead to the development of an alternative hypothesis that could compliment and support the previous report that elevations in intracellular Ca2+ in astrocytes releases AA that promote 20-HETE evoked cerebral vasoconstriction [32]. For example acute hypoxia is known to increase the production of the vasodilatory EETs and to decrease the levels of 20-HETE. Both of these events contribute to the hypoxia evoked cerebral vasodilation and an increase in CBF required to facilitate delivery of O2 and glucose to different brain regions with increased energy demand [25,26,43].
Basal level of 20-HETE production has become very important for the maintenance of cerebral arterial tone and autoregulation cerebral blood flow [13], whereas elevations in production and actions of 20-HETE have been associated with ischemic stroke and hemorrhagic cerebral vasospasm both in experimental model animals [11,13,44,45] and patients [46,47]. Because astrocytes out number neurons more than two to one in the brain, the capacity of astrocytes to synthetize and release 20-HETE could have a significant impact on susceptibility of neurons and other brain cell types to the adverse effects of a variety of pathological insults. For instance, an increased production of 20-HETE was found to induce oxidative stress through NADPH oxidase-dependent generation of reactive oxygen species [29,48], whereas increased production and action of 20-HETE was reported to promote delayed vasospasm following subarachnoid hemorrhage (SAH) [44,45,47,49,50] and to increase infarct volume evoked by ischemia reperfusion injury [11]. Moreover the use of specific inhibitors of the synthesis or actions of 20-HETE has been indicated as possible mode of therapy for reduction of brain ischemic infarcts and vasospasm resulting from ischemia and SAH, respectively, as determined using experimental model animals [11,45,46,50,51], whereas administration of 20-HETE or its pharmacological agonists exacerbate brain tissue or neuronal damage. Of interest is the recently reported finding [52] that subarachnoid blood can convert neurally evoked vasodilation to vasoconstriction that resulted in dysfunction of the neurovascular unit, and was suspected to result from changes in endogenous production and vasoconstrictor action of 20-HETE the cellular sources of which may involve brain astrocytes as could be inferred from the findings of the present studies.
Given that mGluR activation in astrocytes induces an increase in [Ca2+]i and release of arachidonic acid [19], and as also demonstrated in the present study that such stimulus can also increase production and release of the vasoconstrictor 20-HETE from astrocytes, it could be presumed that not only arachidonic acid but also its metabolites, including 20-HETE and the EETs could be released from astrocytes in the perivascular space to mediate changes in cerebrovascular function under physiological or pathophysiological conditions. Furthermore, as astrocytes also express COX enzymes, a rise in astrocyte [Ca2+]i induced release of arachidonic acid during increased neuronal activity could also be converted to dilator prostaglandins that could be released to promote the astrocyte-mediated functional hyperemic response. The potential of astrocytes to metabolically generate a vasodilation signal mediated by dilatory factors including prostaglandins and EETs, K+ etc. [18,25,53], and a vasoconstriction influence by releasing the COX metabolite prostaglandin E2 [49] and/or 20-HETE as demonstrated in the present study, may suggest that astrocytes could have the capacity to deliver a dynamic homeostatic signaling mechanism driven by the CYP metabolites of AA to regulate changes in cerebral blood flow in response to changes in neuronal metabolic demand under physiological or pathophysiological conditions [17–20,43].
The results of the present study also indicate that exogenously applied or endogenous formation of 20-HETE induces inhibition of the openings of two types of KCa channel currents in cultured brain astrocytes. The observed inhibitory action of 20-HETE on astrocyte KCa channel currents in the present study contrasted with the known activation of KCa channel currents in astrocytes by EETs [26,38]. The functional outcome of opposing effects resulting from inhibition of the two types of KCa channel currents in astrocytes by 20-HETE versus the reported activation of these channels by EETs may serve to modulate the steady state level activities of these KCa channel types under physiological conditions [25,26]. It is also possible that an inhibitory action of 20-HETE on the astrocyte KCa channel currents could reduce or even attenuate the increase in K+ released from astrocytes following neuronal activation that results in hyperpolarization and vasodilation by increasing the opening of inward-rectifying K+ channels in cerebral arterial muscle [54]. On the other hand, the present finding that treatment of astrocytes with the selective 20-HETE synthase inhibitor HET0016 (50 nM) increased the open state probabilities (NPo) of the two types of KCa channel currents in astrocytes and prevented the astrocyte media induced shortening of arterial myocytes (Fig. 7A and B), could identify 20-HETE as an endogenous inhibitor of activities of these two astrocytic KCa channel types as well as an astrocyte released vasoconstrictor factor. Given that the KCa channels expressed in astrocytes are unique due to the existence and association of the auxiliary β4-subunit KCNMβ4 [38], 20-HETE appears to cause inhibition of the activities of this new class of KCa channels. The resulting phenotype could be similar or different from the known inhibitory action of 20-HETE on arterial muscle KCa channel current the underlying mechanism of which awaits further investigation. However, it is important to note that the net effect could be complex as it could reflect differences in the production of the EETs versus 20-HETE in the astrocytes, the ability of released AA to stimulate formation of 20-HETE in cerebral arterial myocytes and the metabolism of released EETs by epoxide hydrolase and 20-HETE by COX and CYP2E1 to the carboxylic acid [12,42,53,55].
A well-recognized functional feature of astrocytes is their ability to exhibit intrinsic oscillations in intracellular Ca2+ due to release from internal stores or stimulation by neurotransmitters released during increased neuronal activity, which in addition to regulating astrocyte function could also trigger generation of signals within astrocyte that pass through the astrocyte foot processes to modulate the function in adjacent brain cell types [56–58]. In the context of the findings of the present investigation, it is possible that such increase in astrocyte [Ca2+]I, could induce Ca2+ waves through the astrocyte syncytium and activate PLA2 to cause release of AA from membrane phospholipids of astrocytes as has been previously reported [32]. AA that is released may be metabolized to 20-HETE in astrocytes, which upon release via the astrocyte foot processes could induce constriction of nearby cerebral arterial myocytes to modulate cerebrovascular tone and function.
5. Conclusion
The findings of the present studies demonstrate that rat brain astrocytes express CYP 4A ω-hydroxylase and produce 20-HETE, and the release of this substance is enhanced by stimulation of astrocyte mGluR. 20-HETE functions as an endogenous inhibitor of the two types of KCa single-channel currents expressed in astrocytes. Exposure of freshly isolated cerebral arterial myocytes to conditioned media from cultured astrocytes in the presence of COX and epoxygenase inhibitors caused shortening, which was not evident for the effects of conditioned media separated from astrocytes pretreated with the 20-HETE synthesis inhibitor HET-0016 and the 20-HETE antagonist WIT002 (1 μM) (Fig. 7A and B). These findings suggest that astrocytes not only generate vasodilatory factors to induce vasodilation in response to an increase in neuronal activity, but also have the capacity to produce and release the vasoconstrictor 20-HETE under certain conditions.
Acknowledgments
This work was supported by NIH/NHLBI R01HL033833, R01 HL092105, R01 HL105997 and by a Research Career Scientist Award from the Clement Zablocki VA Medical Center.
Abbreviations
- 20-HETE
20-hydroxyeicosatetraenoic acid
- CYP 4A enzyme
cytochrome P450 4A ω-hydroxylase
- EETs
epoxyeicosatrienoic acids
- AA
arachidonic acid
- HET0016
((N-hydroxy-N′-(4-butyl-2-methylphenyl)formamidine
- CBF
cerebral blood flow
- LC–ESI–MS
Liquid chromatographic–electrospray ionization–mass spectrometric
- LC–MS
liquid chromatography–mass spectroscopy
- KCa
calcium-activated K+ channel
- NPo
channel open state probability
- mGluR
metabotropic glutamate receptor
- GFAP
glial fibrillary acidic protein
- SAH
subarachnoid hemorrhage
References
- 1.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26(10):523–530. doi: 10.1016/j.tins.2003.08.008. http://dx.doi.org/10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91(22):10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahl M, Schilling L. Regulation of cerebral blood flow—a brief review. Acta Neurochir Suppl. 1993;59:3–10. doi: 10.1007/978-3-7091-9302-0_1. [DOI] [PubMed] [Google Scholar]
- 4.Yang ZJ, Carter EL, Kibler KK, Kwansa H, Crafa DA, Martin LJ, Roman RJ, Harder DR, Koehler RC. Attenuation of neonatal ischemic brain damage using a 20-HETE synthesis inhibitor. J Neurochem. 2012;121(1):168–179. doi: 10.1111/j.1471-4159.2012.07666.x. http://dx.doi.org/10.1111/j.1471-4159.2012.07666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27(5):971–979. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- 6.Bylund J, Harder AG, Maier KG, Roman RJ, Harder DR. Leukotriene B4 omega-side chain hydroxylation by CYP4F5 and CYP4F6. Arch Biochem Biophys. 2003;412(1):34–41. doi: 10.1016/s0003-9861(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 7.Bylund J, Zhang C, Harder DR. Identification of a novel cytochrome P450, CYP4×1, with unique localization specific to the brain. Biochem Biophys Res Commun. 2002;296(3):677–684. doi: 10.1016/s0006-291x(02)00918-x. [DOI] [PubMed] [Google Scholar]
- 8.Harder DR, Narayanan J, Gebremedhin D. Pressure-induced myogenic tone and role of 20-HETE in mediating autoregulation of cerebral blood flow. Am J Physiol Heart Circ Physiol. 2011;300(5):H1557–H1565. doi: 10.1152/ajpheart.01097.2010. http://dx.doi.org/10.1152/ajpheart.01097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGiff JC. Cytochrome P-450 metabolism of arachidonic acid. Annu Rev Pharmacool Toxicol. 1991;31:339–369. doi: 10.1146/annurev.pa.31.040191.002011. http://dx.doi.org/10.1146/annurev.pa.31.040191.002011. [DOI] [PubMed] [Google Scholar]
- 10.Rapoport SI. Arachidonic acid and the brain. J Nutr. 2008;138(12):2515–2520. doi: 10.1093/jn/138.12.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renic M, Kumar SN, Gebremedhin D, Florence MA, Gerges NZ, Falck JR, Harder DR, Roman RJ. Protective effect of 20-HETE inhibition in a model of oxygen-glucose deprivation in hippocampal slice cultures. Am J Physiol Heart Circ Physiol. 2012;302(6):H1285–H1293. doi: 10.1152/ajpheart.00340.2011. http://dx.doi.org/10.1152/ajpheart.00340.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82(1):131–185. doi: 10.1152/physrev.00021.2001. http://dx.doi.org/10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 13.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87(1):60–65. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- 14.Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, Harder DR. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol. 1998;507(Pt 3):771–781. doi: 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harder DR, Gebremedhin D, Narayanan J, Jefcoat C, Falck JR, Campbell WB, Roman R. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol. 1994;266(Pt 2):H2098–H2107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- 16.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem. 1997;272(43):27345–27352. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- 17.Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;29(1):229–234. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- 18.Harder DR, Roman RJ, Gebremedhin D. Molecular mechanisms controlling nutritive blood flow: role of cytochrome P450 enzymes. Acta Physiol Scand. 2000;168(4):543–549. doi: 10.1046/j.1365-201x.2000.00707.x. http://dx.doi.org/10.1046/j.1365-201x.2000.00707.x. [DOI] [PubMed] [Google Scholar]
- 19.Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke. 1997;28(5):1066–1072. doi: 10.1161/01.str.28.5.1066. [DOI] [PubMed] [Google Scholar]
- 20.Harder DR, Roman RJ, Gebremedhin D, Birks EK, Lange AR. A common pathway for regulation of nutritive blood flow to the brain: arterial muscle membrane potential and cytochrome P450 metabolites. Acta Physiol Scand. 1998;164(4):527–532. doi: 10.1111/j.1365-201x.1998.tb10702.x. [DOI] [PubMed] [Google Scholar]
- 21.Gebremedhin D, Ma YH, Imig JD, Harder DR, Roman RJ. Role of cytochrome P-450 in elevating renal vascular tone in spontaneously hypertensive rats. J Vasc Res. 1993;30(1):53–60. doi: 10.1159/000158975. [DOI] [PubMed] [Google Scholar]
- 22.Harder DR, Zhang C, Gebremedhin D. Astrocytes function in matching blood flow to metabolic activity. News Physiol Sci. 2002;17:27–31. doi: 10.1152/physiologyonline.2002.17.1.27. [DOI] [PubMed] [Google Scholar]
- 23.Dabertrand F, Nelson MT, Brayden JE. Ryanodine receptors, calcium signaling, and regulation of vascular tone in the cerebral parenchymal microcirculation. Microcirculation. 2013;20(4):307–316. doi: 10.1111/micc.12027. http://dx.doi.org/10.1111/micc.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filosa JA, Iddings JA. Astrocyte regulation of cerebral vascular tone. Am J Physiol Heart Circ Physiol. 2013;305(5):H609–H619. doi: 10.1152/ajpheart.00359.2013. http://dx.doi.org/10.1152/ajpheart.00359.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol. 2006;100(1):307–317. doi: 10.1152/japplphysiol.00938.2005. http://dx.doi.org/10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaura K, Gebremedhin D, Zhang C, Narayanan J, Hoefert K, Jacobs ER, Koehler RC, Harder DR. Contribution of epoxyeicosatrienoic acids to the hypoxia-induced activation of Ca2+ -activated K+ channel current in cultured rat hippocampal astrocytes. Neuroscience. 2006;143(3):703–716. doi: 10.1016/j.neuroscience.2006.08.021. http://dx.doi.org/10.1016/j.neuroscience.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Nithipatikom K, Grall AJ, Holmes BB, Harder DR, Falck JR, Campbell WB. Liquid chromatographic–electrospray ionization–mass spectrometric analysis of cytochrome P450 metabolites of arachidonic acid. Anal Biochem. 2001;298(2):327–336. doi: 10.1006/abio.2001.5395. http://dx.doi.org/10.1006/abio.2001.5395. [DOI] [PubMed] [Google Scholar]
- 28.Hoopes SL, Garcia V, Edin ML, Schwartzman ML, Zeldin DC. Vascular actions of 20-HETE. Prostaglandins Other Lipid Mediat. 2015;120:9–16. doi: 10.1016/j.prostaglandins.2015.03.002. http://dx.doi.org/10.1016/j.prostaglandins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller DN, Schmidt C, Barbosa-Sicard E, Wellner M, Gross V, Hercule H, Markovic M, Honeck H, Luft FC, Schunck WH. Mouse Cyp4a isoforms: enzymatic properties, gender-strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J. 2007;403(1):109–118. doi: 10.1042/BJ20061328. http://dx.doi.org/10.1042/BJ20061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, Henriksen FH, Salomonsson M, Morita H, Kawarabayashi Y, Mori M, Mori Y, Ito Y. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res. 2009;104(12):1399–1409. doi: 10.1161/CIRCRESAHA.108.193227. http://dx.doi.org/10.1161/CIRCRESAHA.108.193227. [DOI] [PubMed] [Google Scholar]
- 31.Wen H, Ostman J, Bubb KJ, Panayiotou C, Priestley JV, Baker MD, Ahluwalia A. 20-Hydroxyeicosatetraenoic acid (20-HETE) is a novel activator of transient receptor potential vanilloid 1 (TRPV1) channel. J Biol Chem. 2012;287(17):13868–13876. doi: 10.1074/jbc.M111.334896. http://dx.doi.org/10.1074/jbc.M111.334896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431(7005):195–199. doi: 10.1038/nature02827. http://dx.doi.org/10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 33.Bleicher KB, Pippert TR, Glaab WE, Skopek TR, Sina JF, Umbenhauer DR. Use of real-time gene-specific polymerase chain reaction to measure RNA expression of three family members of rat cytochrome P450 4A. J Biochem Mol Toxicol. 2001;15(3):133–142. doi: 10.1002/jbt.10. [DOI] [PubMed] [Google Scholar]
- 34.Marji JS, Wang MH, Laniado-Schwartzman M. Cytochrome P-450 4A isoform expression and 20-HETE synthesis in renal preglomerular arteries. Am J Physiol Renal Physiol. 2002;283(1):F60–F67. doi: 10.1152/ajprenal.00265.2001. http://dx.doi.org/10.1152/ajprenal.00265.2001. [DOI] [PubMed] [Google Scholar]
- 35.Wang MH, Wang J, Chang HH, Zand BA, Jiang M, Nasjletti A, Laniado-Schwartzman M. Regulation of renal CYP4A expression and 20-HETE synthesis by nitric oxide in pregnant rats. Am J Physiol Renal Physiol. 2003;285(2):F295–302. doi: 10.1152/ajprenal.00065.2003. http://dx.doi.org/10.1152/ajprenal.00065.2003. [DOI] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. http://dx.doi.org/10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Ma YH, Gebremedhin D, Schwartzman ML, Falck JR, Clark JE, Masters BS, Harder DR, Roman RJ. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res. 1993;72(1):126–136. doi: 10.1161/01.res.72.1.126. [DOI] [PubMed] [Google Scholar]
- 38.Gebremedhin D, Yamaura K, Zhang C, Bylund J, Koehler RC, Harder DR. Metabotropic glutamate receptor activation enhances the activities of two types of Ca2+-activated k+ channels in rat hippocampal astrocytes. J Neurosci. 2003;23(5):1678–1687. doi: 10.1523/JNEUROSCI.23-05-01678.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Maier KG, Roman RJ, De La Cruz L, Zhu J, Henderson L, Lombard JH. Expression of cytochrome P450-4A isoforms in the rat cremaster muscle microcirculation. Microcirculation. 2004;11(1):89–96. doi: 10.1080/10739680490266225. [DOI] [PubMed] [Google Scholar]
- 41.Gomes FC, Paulin D, Moura Neto V. Glial fibrillary acidic protein (GFAP): modulation by growth factors and its implication in astrocyte differentiation. Braz J Med Biol Res. 1999;32(5):619–631. doi: 10.1590/s0100-879x1999000500016. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Li C, Gebremedhin D, Hwang SH, Hammock BD, Falck JR, Roman RJ, Harder DR, Koehler RC. Epoxyeicosatrienoic acid-dependent cerebral vasodilation evoked by metabotropic glutamate receptor activation in vivo. Am J Physiol Heart Circ Physiol. 2011;301(2):H373–H381. doi: 10.1152/ajpheart.00745.2010. http://dx.doi.org/10.1152/ajpheart.00745.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gebremedhin D, Gopalakrishnan S, Harder DR. Endogenous events modulating myogenic regulation of cerebrovascular function. Curr Vasc Pharmacol. 2014;12(6):810–817. doi: 10.2174/15701611113116660153. [DOI] [PubMed] [Google Scholar]
- 44.Kehl F, Cambj-Sapunar L, Maier KG, Miyata N, Kametani S, Okamoto H, Hudetz AG, Schulte ML, Zagorac D, Harder DR, Roman RJ. 20-HETE contributes to the acute fall in cerebral blood flow after subarachnoid hemorrhage in the rat. Am J Physiol Heart Circ Physiol. 2002;282(4):H1556–65. doi: 10.1152/ajpheart.00924.2001. http://dx.doi.org/10.1152/ajpheart.00924.2001. [DOI] [PubMed] [Google Scholar]
- 45.Roman RJ, Renic M, Dunn KM, Takeuchi K, Hacein-Bey L. Evidence that 20-HETE contributes to the development of acute and delayed cerebral vasospasm. Neurol Res. 2006;28(7):738–749. doi: 10.1179/016164106X152016. http://dx.doi.org/10.1179/016164106x52016. [DOI] [PubMed] [Google Scholar]
- 46.Hacein-Bey L, Harder DR, Meier HT, Varelas PN, Miyata N, Lauer KK, Cusick JF, Roman RJ. Reversal of delayed vasospasm by TS-011 in the dual hemorrhage dog model of subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2006;27(6):1350–1354. [PMC free article] [PubMed] [Google Scholar]
- 47.Poloyac SM, Reynolds RB, Yonas H, Kerr ME. Identification, quantification of the hydroxyeicosatetraenoic acids 20-HETE, 12-HETE, in the cerebrospinal fluid after subarachnoid hemorrhage. J Neurosci Methods. 2005;144(2):257–263. doi: 10.1016/j.jneumeth.2004.11.015. http://dx.doi.org/10.1016/j.jneumeth.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Zeng Q, Han Y, Bao Y, Li W, Li X, Shen X, Wang X, Yao F, O’Rourke ST, Sun C. 20-HETE increases NADPH oxidase-derived ROS production and stimulates the L-type Ca2+ channel via a PKC-dependent mechanism in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2010;299(4):H1109–17. doi: 10.1152/ajpheart.00067.2010. http://dx.doi.org/10.1152/ajpheart.00067.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cambj-Sapunar L, Yu M, Harder DR, Roman RJ. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke. 2003;34(5):1269–1275. doi: 10.1161/01.STR.0000065829.45234.69. http://dx.doi.org/10.1161/01.STR.0000065829.45234.69. [DOI] [PubMed] [Google Scholar]
- 50.Takeuchi K, Miyata N, Renic M, Harder DR, Roman RJ. Hemoglobin NO, and 20-HETE interactions in mediating cerebral vasoconstriction following SAH. Am J Physiol Regul Integr Comp Physiol. 2006;290(1):R84–R89. doi: 10.1152/ajpregu.00445.2005. http://dx.doi.org/10.1152/ajpregu.00445.2005. [DOI] [PubMed] [Google Scholar]
- 51.Omura T, Tanaka Y, Miyata N, Koizumi C, Sakurai T, Fukasawa M, Hachiuma K, Minagawa T, Susumu T, Yoshida S, Nakaike S, Okuyama S, Harder DR, Roman RJ. Effect of a new inhibitor of the synthesis of 20-HETE on cerebral ischemia reperfusion injury. Stroke. 2006;37(5):1307–1313. doi: 10.1161/01.STR.0000217398.37075.07. http://dx.doi.org/10.1161/01.STR.0000217398.37075.07. [DOI] [PubMed] [Google Scholar]
- 52.Koide M, Bonev AD, Nelson MT, Wellman GC. Subarachnoid blood converts neurally evoked vasodilation to vasoconstriction in rat brain cortex. Acta Neurochir Suppl. 2013;115:167–171. doi: 10.1007/978-3-7091-1192-5_32. http://dx.doi.org/10.1007/978-3-7091-1192-5_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dabertrand F, Hannah RM, Pearson JM, Hill-Eubanks DC, Brayden JE, Nelson MT. Prostaglandin E2, a postulated astrocyte-derived neurovascular coupling agent, constricts rather than dilates parenchymal arterioles. J Cereb Blood Flow Metab. 2013;33(4):479–482. doi: 10.1038/jcbfm.2013.9. http://dx.doi.org/10.1038/jcbfm.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9(11):1397–1403. doi: 10.1038/nn1779. http://dx.doi.org/10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 55.Kim DH, Puri N, Sodhi K, Falck JR, Abraham NG, Shapiro J, Schwartzman ML. Cyclooxygenase-2 dependent metabolism of 20-HETE increases adiposity and adipocyte enlargement in mesenchymal stem cell-derived adipocytes. J Lipid Res. 2013;54(3):786–793. doi: 10.1194/jlr.M033894. http://dx.doi.org/10.1194/jlr.M033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basarsky TA, Duffy SN, Andrew RD, MacVicar BA. Imaging spreading depression and associated intracellular calcium waves in brain slices. J Neurosci. 1998;18(18):7189–7199. doi: 10.1523/JNEUROSCI.18-18-07189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winship IR, Plaa N, Murphy TH. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci. 2007;27(23):6268–6272. doi: 10.1523/JNEUROSCI.4801-06.2007. http://dx.doi.org/10.1523/JNEUROSCI.4801-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yagodin S, Holtzclaw LA, Russell JT. Subcellular calcium oscillators and calcium influx support agonist-induced calcium waves in cultured astrocytes. Mol Cell Biochem. 1995;149–150:137–144. doi: 10.1007/BF01076572. [DOI] [PubMed] [Google Scholar]