Abstract
Triiodothyronine (T3) is an important modulator of cardiac metabolism and function, often through modulation of gene expression. The cardiomyocyte circadian clock is a transcriptionally-based molecular mechanism capable of regulating cardiac processes, in part by modulating responsiveness of the heart to extra-cardiac stimuli/stresses in a time-of-day- (TOD) dependent manner. Although TOD-dependent oscillations in circulating levels of T3 (and its intermediates) have been established, whether oscillations in T3 sensitivity in the heart occur is unknown. To investigate the latter possibility, euthyroid male Wistar rats were treated with vehicle or T3 at distinct times of the day, after which induction of known T3 target genes were assessed in the heart (4-h later). The expression of mRNA was assessed by Real-Time qPCR. Here, we report greater T3 induction of transcript levels at the end of the dark phase. Surprisingly, use of cardiomyocyte-specific clock mutant (CCM) mice revealed that TOD-dependent oscillations in T3 sensitivity were independent of this cell autonomous mechanism. Investigation of genes encoding for proteins that affect T3 sensitivity revealed that Dio1, Dio2, and Thrb1 exhibited TOD-dependent variations in the heart, while Thra1 and Thra2 did not. Of these, Dio1 and Thrb1 were increased in the heart at the end of the dark phase. Interestingly, we observed that T3 acutely altered the expression of core clock components (e.g., Bmal1) in the rat heart. To investigate this further, rats were injected with a single dose of T3, after which expression of clock genes were interrogated at 3-h intervals over the subsequent 24h-period. These studies revealed robust effects of T3 on oscillations of both core clock components and clock-controlled genes. In summary, the current study exposed time-of-day-dependent rhythms in cardiac T3 sensitivity, and that T3 alters the circadian clock in the heart.
Keywords: T3, circadian clocks, heart, metabolism, gene expression
INTRODUCTION
The American Thyroid Association estimates that about 20 million Americans have some form of thyroid disease, and that 12 percent of the American population will develop a thyroid disorder at one point in their life (ATA). In fact thyroid dysfunction is the 2nd most prevalent endocrinopathy in the world. Cardiovascular disease (CVD) is a major cause of morbidity and mortality in the United States (Mozaffarian et al., 2015). Such observations highlight a need to better understand the mechanisms of thyroid hormones (THs) action on cardiovascular-relevant tissues/organs, including the heart.
In the heart, T3 acts as an important regulator of cardiac function and cardiovascular hemodynamic. This includes effects on heart rate, metabolism, translation/growth, and transcription. Indeed, most of THs actions occurs by classical transcriptional actions modulating gene expression. These actions are mediated through interaction of T3 with its nuclear receptors (THRs) associated to thyroid responsive elements (TRE) present in target genes (Yen, 2001). One of T3’s most characterized effects in the ventricle is the regulation of cardiac myosin isoforms composition, increasing MYH6 and concomitantly reducing MYH7 content (Dillmann, 1996; Morkin et al., 1983). T3 also regulates the expression and activity of SERCA2, phospholamban, HCN2 and β adrenergic receptors, which together likely contribute towards perturbations in heart rate and contractility (Biondi et al., 2002; Dillmann, 2002; Nadal-Ginard & Mahdavi, 1993; Nunes et al., 1985; Rohrer & Dillmann, 1988). In metabolic terms, T3 induces the expression of myoglobin in the heart (Giannocco et al., 2004), the expression of solute carrier family 2 (facilitated glucose transporter) member 4 (Slc2a4), as well as glycogen synthesis in cultured cardiomyocytes (Gosteli-Peter et al., 1996). Hearts of hypothyroid animals also present alterations in lactate and free fatty acid metabolism, as well as carnitine palmitoyltransferase 1 (CPT1) and pyruvate dehydrogenase kinase 2 (PDK2) protein levels (Hyyti et al., 2006).
When considering hormone action on target tissues, two major components should be addressed: 1) the level of the hormone; and 2) target tissue sensitivity. The synthesis of T3 and its intermediates is regulated by the activity of the hypothalamus-pituitary-thyroid axis. Hypothalamic thyrotropin-releasing hormone (TRH) stimulates the synthesis and secretion of thyroid-stimulating hormone (TSH) by thyrotrophs in the pituitary gland. In the thyrocytes, TSH promotes the synthesis of THs (Zoeller et al., 2007), which in turn, reduce TRH and TSH synthesis and secretion in the hypothalamus and pituitary, respectively, (Bargi-Souza et al., 2013; Guissouma et al., 2002) regulating their own expression. The thyroid gland secretes T4 and T3, as well as 3,3′-diiodothyronine (T2) and 3,3′,5′-triiodothyronine (reverse T3 - rT3). Although T4 is the major product of thyroid gland, T3 is the most active form, being derived locally from T4 conversion by deiodinases 1 and 2 (DIO1 and DIO2, respectively) in peripheral tissues (Leonard et al., 1983; Visser et al., 1982). Target tissue sensitivity to T3 is therefore influenced by local levels of DIO1/2, membrane receptors (alphaVbeta3 integrin) (Cody et al., 2007) and transporters (MCT8, MCT10 and OATP1) (Visser et al., 2011), as well as expression of THR isoforms (Yen, 2001).
Interestingly, a number of studies have demonstrated circadian and seasonal oscillations in T3 serum concentration from euthyroid male rats, reaching higher levels during the sleep phase (Ahlersova et al., 1997; Guo et al., 2015; Jordan et al., 1980). DIO2 presents a circadian variation in its activity in different regions of the rat brain. Furthermore, it was observed that the concentration of T3 in the hypothalamus is ten-fold higher during long days associated with an increase of DIO2 in this photoperiod (Yasuo et al., 2006). Such observations raise the possibility that T3 availability within tissues is under circadian control. However, whether this is true in the heart is currently unknown. The purpose of the present study was to interrogate the hypothesis that the heart exhibits a TOD-dependent oscillation in T3 responsiveness, at a transcriptional level.
MATERIALS AND METHODS
Animals
Rat
Male Wistar rats weighing 150–180 g were obtained from the Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil. The animals were kept on a 12 h/12 h light/dark (LD) cycle (light phase: lights on at 06:00 am = Zeitgeber Time – ZT 0; dark phase: red filter Kodak 1A, 0.5 to 1 lux), in a temperature-controlled room and had access to food and water ad libitum. Ethics approval was granted by the Committee of Ethics in Animal Experimentation of the Institute of Biomedical Sciences, University of São Paulo.
Mice
The cardiomyocyte-specific clock mutant (CCM) mouse was utilized to investigate whether the cardiomyocyte circadian clock effects T3 responsiveness. Wild-type (WT) and CCM (MYH6-dnCLOCK+/-) mice on the FVB/N background were described previously (Bray et al., 2008). Male mice were housed at the Animal Resource Program at the University of Alabama at Birmingham (UAB), under temperature-, humidity-, and light-controlled conditions. A strict 12 h/12 h light dark cycle regime was enforced (lights on at 6 am; Zeitgeber Time zero [ZT 0]). The animals received food and water ad libitum. The animal experimental procedure was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Experimental designs
Three different experimental protocols were performed in this study. First, rats were euthanized throughout ZTs (figure 3) or received a single intraperitoneal injection (i.p.) of vehicle (saline)/T3 [12.5 μg/100 g], exactly 4 h before heart isolation (figures 1 and 4); therefore the hearts were collected in ZTs (0/24, 3, 6, 9, 12, 15, 18, 21). Under this protocol it was possible to investigate time-of-day- (TOD) dependent oscillations in the heart or even in responsiveness to T3. In the second protocol, WT and CCM mice received a single i.p. injection of Vehicle/T3 [0.19 μg/g] exactly 4 h prior to heart isolation; injections were performed at ZT2 and ZT14 (figure 2). This protocol allowed assessment of the impact of cardiomyocyte circadian clock disruption on T3 responsiveness of the heart. In the third protocol, rats received a single IP injection of vehicle/T3 [12.5 μg/100 g] at ZT14, followed by heart isolation exactly 4, 7, 10, 13, 16, 19, 22, or 25 hours later (figure 5). This protocol allowed investigation of the effects of T3 administration on circadian clock function.
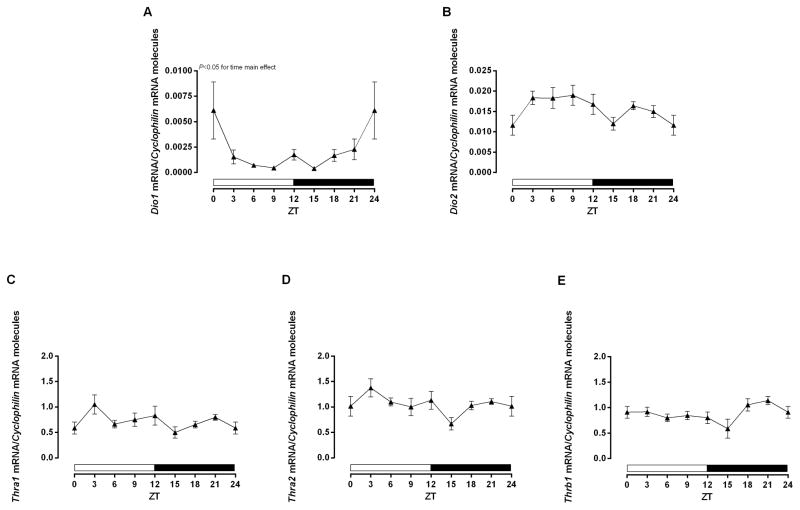
Figure 3.
Time-of-day-dependent mRNA expression in the heart. The rats were euthanized 4 h before each ZT. The ZT 24 was double plotted as ZT 0. A–E) Expression of Dio1, Dio2, Thra1, Thra2, and Thrb1, respectively, normalized by Cyclophilin mRNA molecules and presented as absolute values of means ± SEM. One-way ANOVA, P<0.05 for time main effect in A. n=5/ZT/group. ZT=Zeitgeber Time.
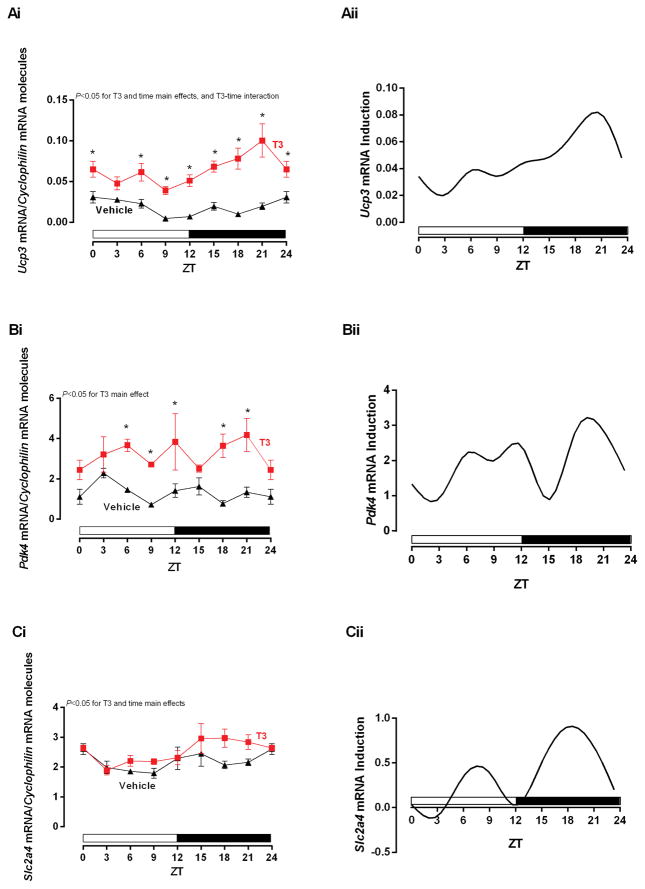
Figure 1.
Time-of-day-dependent mRNA expression in the heart challenged by T3. The rats received Vehicle (Saline) or T3 (i.p. [12.5 μg/100 g]) injections 4 h before each ZT. The ZT 24 was double plotted as ZT 0. A–C) i. T3 effects in mRNA expressions of Ucp3, Pdk4, and Slc2a4, respectively, normalized by Cyclophilin mRNA molecules and presented as absolute values of means ± SEM. ii. Induction analysis of mRNA expressions, calculated by the difference between T3 and Vehicle values. Two-way ANOVA, P<0.05 for T3 in A–C, time main effect in A and C, and T3-time interaction in A. Bonferroni’s post hoc test, *P<0.05 for pair-wise comparisons vs respective ZTs. n=5/ZT/group. ZT=Zeitgeber Time.
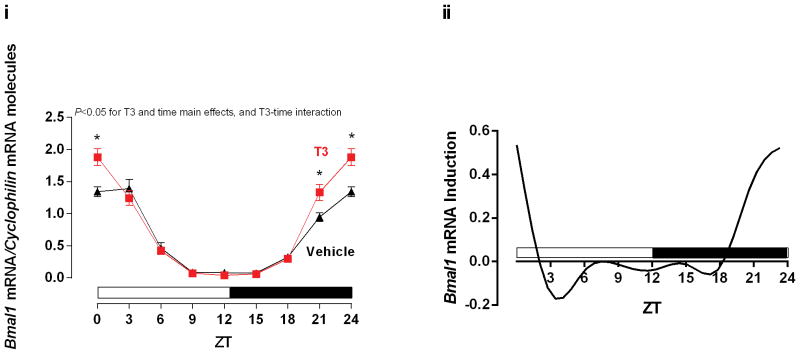
Figure 4.
Time-of-day-dependent expression of Bmal1. The rats received Vehicle (Saline) or T3 (i.p. [12.5 μg/100 g]) injections 4 h before each ZT. The ZT 24 was double plotted as ZT 0. i. T3 effects in Bmal1 mRNA expression, normalized by Cyclophilin mRNA molecules and presented as absolute values of means ± SEM. ii. Induction analysis of Bmal1 mRNA expressions, calculated by the difference between T3 and Vehicle values. Two-way ANOVA, P<0.05 for T3 and time main effect, and T3-time interaction (i). Bonferroni’s post hoc test, *P<0.05 for pair-wise comparisons vs respective ZTs. n=5/ZT/group. ZT=Zeitgeber Time.
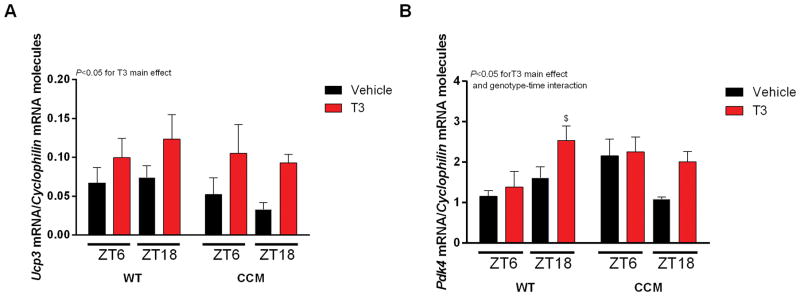
Figure 2.
Acute effects of T3 single injection in CCM mice. Mice received a single injection of Vehicle (Saline) or T3 (i.p. [0.19 μg/g]) 4 h prior to the harvest of the hearts, which occurred at ZTs 6 and 18, respectively. A–B) Ucp3 and Pdk4, respectively, normalized by Cyclophilin mRNA molecules and presented as absolute values of means ± SEM. Three-way ANOVA, P<0.05 for T3 main effects in A and B, and genotype-time interaction for B. Bonferroni’s post hoc test, $P<0.05 vs ZT6 WT Vehicle. n=6/ZT/group. ZT=Zeitgeber Time. Cardiomyocyte-specific clock mutant (CCM) mice. Wild-type (WT) mice.
Figure 5.
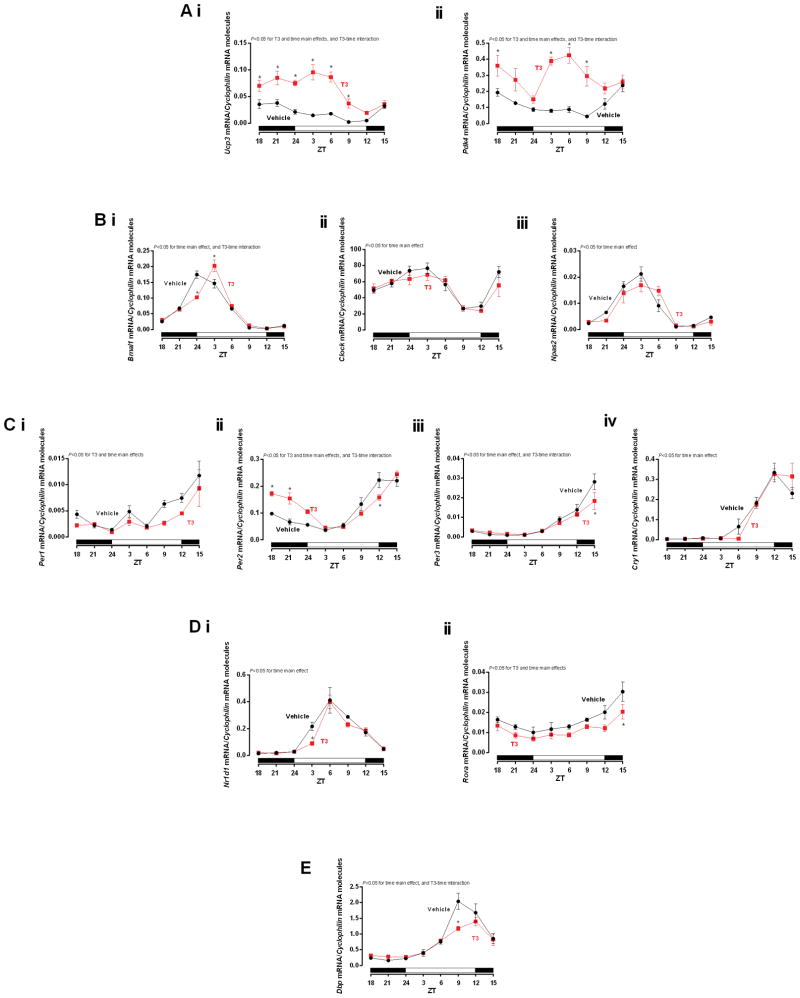
Single injection effect of T3 in mRNA expression in the heart. The rats received a single injection of Vehicle (Saline) or T3 (i.p. [12.5 μg/100 g]) at ZT 14 and the hearts were collected starting from ZT 18 and so on. A) Ucp3, Pdk4, B) Bmal1, Clock, Npas2, C) Per1, Per2, Per3, Cry1, D) Nr1d1, Rora, E) Dbp, mRNA expression in the heart. The gene expressions were normalized by Cyclophilin mRNA molecules and presented as absolute values of means ± SEM. Two-way ANOVA P<0.05 for T3 and time main effects for Ai–ii, Ci–ii, Dii, T3-time interaction for Ai–ii, Bi, Cii–iii, E, and time main effect for Bi–iii, Ciii–iv, Di, and E. Bonferroni’s post hoc test, *P<0.05 for pair-wise comparisons vs respective ZTs. n=5/ZT/group. ZT=Zeitgeber Time.
Quantitative RT-PCR
Total RNA was extracted from hearts using standard procedures (Chomczynski & Sacchi, 1987). Candidate gene expression analysis was performed by quantitative RT-PCR, using previously described methods (Gibson et al., 1996; Heid et al., 1996). Quantitative RT-PCR was performed using specific RNA standards and TaqMan assays. Oligonucleotides sequences are reported previously (rat-Bmal1, Clock, Per1,2,3, Cry1 and Dbp (Young et al., 2001b), Slc2a4, Pdk4 and Ucp3 (Young et al., 2001a), Rora (Peliciari-Garcia et al., 2011); mice-Pdk4 and Ucp3 (Tsai et al., 2010)) or were newly designed for each gene from rat sequences available in GenBank; primer and probe sequences for newly designed assays are listed in Table 1. All quantitative RT-PCR data are presented as the number of transcripts/constitutive gene molecules. Cyclophilin (Ppia) was used as internal control (i.e., constitutive gene).
Table 1.
Primer and probe sequences used in real-time RT-PCR.
| Gene/GenBank# | Primer/probe | Sequences |
|---|---|---|
|
Dio1 NM_021653.3 |
Forward | 5′-CAGCTGACTTCCTCATCATTTACATT-3′ |
| Reverse | 5′–TTCGGTGCTGCCTGATGTC–3′ | |
| Probe | 5′-FAM-TCACGCCACAGATGGATGGGCTTTT-TAMRA-3′ | |
|
Dio2 NM_031720.3 |
Forward | 5′–ACGCTGTGTCTGGAACAGCTT–3′ |
| Reverse | 5′–ATTGGACACGTGCACCACACT–3′ | |
| Probe | 5′6–FAM–ACAGGTTAAATTGGGTGAAGATGCTCCCA–TAMRA–3′ | |
|
Npas2 NM_001108214.2 |
Forward | 5′–AAGCCTTCATTCCTCAGTAACGA–3′ |
| Reverse | 5′–GTCACAACAATGACGAAGCCA–3′ | |
| Probe | 5′6–FAM–TCACCCAGCTGATGTTGGAGGCGT–TAMRA–3′ | |
|
Nr1d1 NM_145775.1 |
Forward | 5′–AGGTGACCCTGCTTAAGGCTG–3′ |
| Reverse | 5′–ACTGTCTGGTCCTTCACGTTGA–3′ | |
| Probe | 5′6–FAM–CAAAGCGCACCATCAGCACCTCAAA–TAMRA–3′ | |
|
Thra1 NM_001017960.1 |
Forward | 5′–CAAGGTGGAGTGTGGGTCAGA–3′ |
| Reverse | 5′–ATGCTGCTTTTCAGGGGACAT–3′ | |
| Probe | 5′6–FAM–AGGAGAACAGTGCCAGGTCACCAGATG–TAMRA–3′ | |
|
Thra2 NM_001017960.1 |
Forward | 5′–CGTCAACCACCGCAAACAC–3′ |
| Reverse | 5′–ATGCGGAGGTCAGTCACCTT–3′ | |
| Probe | 5′6–FAM–CATTCCGCACTTCTGGCCCAAGC–TAMRA–3′ | |
|
Thrb1 NM_012672.3 |
Forward | 5′–ACCAGAGTGGTGGATTTCGC–3′ |
| Reverse | 5′–CCATGCAGCAGCCTTTGA–3′ | |
| Probe | 5′6–FAM–AAGTTGCCCATGTTTTGTGAGCTGCC–TAMRA–3′ |
Statistical analysis
The data are plotted as the means ± SEM. One, two or three-way ANOVA was applied appropriately to evaluate the influence of variables such as time, treatment, and/or genotype, followed by Bonferroni’s post-hoc analyses for pair-wise comparisons, using GraphPad Prism (GraphPad Software version 6.01, San Diego, CA, USA) and Statgraphics Centurion (Statgraphics Centurion version 17.1.06, Warrenton, VA, USA). A second mathematical and statistical procedure was applied to study the presence of a daily 24 h rhythm (Nelson et al., 1979). The test consisted of adjusting a cosine curve to the real 24 h time series (Cosinor method). The theoretical cosine curve fit was applied to each temporal series using the least-square calculation. It was possible to estimate the goodness of fit of the data using the F statistics. The null hypothesis tested was that of zero amplitude, that is, no rhythmicity at the assumed frequency (24 h). We considered a periodic fit significant if the P value was <0.05. In addition, for each temporal data series, the acrophase, mesor and amplitude of the adjusted curve were calculated (Cornelissen et al., 1980). When appropriate, Student’s t test was applied to these parameters. The null hypothesis of no treatment effect was rejected at P<0.05.
RESULTS
In order to determine whether circadian clock mRNA expression exhibits a TOD dependence to T3 in the heart, acute administration of T3 was performed at distinct times of the day, after which, hearts were isolated exactly 4h later (i.e., first protocol); expression of T3 target genes (Ucp3, Pdk4, Slc2a4) were subsequently measured. As expected, T3 induced all target genes in the heart (i.e., T3 main effect). TOD also had a significant effect on Ucp3 and Slc2a4 expression (i.e., time main effect). Importantly, Ucp3 exhibited a significant T3-time interaction, with greater induction of this gene at between ZT18-ZT24. Induction of Pdk4 and Slc2a4 also tended to be greater at ZT18-ZT21 (although a significant T3-time interaction was not observed) (Figure 1A–C i and ii, respectively). Collectively, these data suggest that transcript levels modulation in the heart by T3 is highest at the end of the dark phase.
Next, CCM mice were utilized to evaluate whether TOD-dependent oscillations in T3 responsiveness were mediated by the cardiomyocyte circadian clock. Accordingly, WT and CCM mice were challenged with T3 during the light (ZT2) and dark (ZT14) phases, followed by assessment of T3 responsive genes in the heart 4h later (i.e., ZT6 and ZT18; second protocol). Consistent with our rat studies (Figure 1), T3 administration induced both Ucp3 and Pdk4 in the mouse heart (i.e., T3 main effect); however, T3 did not acutely induce Slc2a4 in the mouse heart (data not shown). Although a significant genotype-time interaction was observed for Pdk4, no T3-time-geneotype interactions were observed. Moreover, T3 appeared to induce Pdk4 and Ucp3 to a greater extent at ZT18 (versus ZT6) in both WT and CCM hearts (Figure 2A–B). Collectively, these data suggest that the TOD-dependence of T3 responsiveness of the heart is not mediated by the cardiomyocyte circadian clock.
In an attempt to provide insight with regards to the mechanisms mediating TOD-dependent oscillations in the heart to T3, we next investigated various isoforms of Dio (1 and 2) and Thr (a1, a2, and b1). Somewhat surprisingly, cosinor analysis indicated that none of the genes investigated exhibited a significant oscillation (with a periodicity of 24 hours). However, one-way ANOVA revealed that Dio1 exhibited a significant time main effect, while Dio2 and Thrb1 tended to exhibit TOD-dependent variations (P=0.07, time main effect for both Dio2 and Thrb1). When gene expression from Dio1 and Thrb1 data were compared during periods of low (average of ZT6, ZT9, and ZT12) versus high (average of ZT18, ZT21, and ZT24) mRNA expression were found to be 3.43- and 1.3- fold higher (respectively for those genes) at the end of the dark phase (Figure 3A–E).
Data presented so far suggest that T3 sensitivity in the heart is increased at the end of the dark phase independent of the cardiomyocyte circadian clock, which is associated with increased expression of Dio1 and Thrb1 at this time. Over the course of these studies, we also investigated the effects of T3 administration on expression of the circadian clock component, Bmal1. Interestingly, we found that T3 significantly induced Bmal1 expression in the heart at ZT21 and ZT24, a time at which T3 sensitivity is increased (Figure 4 i and ii). This observation raised the possibility that T3 acts as a zeitgeber (i.e., entrainment factor) for the clock in the heart. In an attempt to investigate this hypothesis further, rats were injected with either saline or T3 at ZT14, followed by heart isolation at 3-h intervals over the subsequent 24-h period (i.e., protocol 3).
Consistent with its known actions, T3 induced both Ucp3 and Pdk4 in the heart, which returned to baseline values 24-h post T3 challenge, also presenting T3-time interactions (Figure 5A i and ii). Interestingly, two-way ANOVA revealed that T3 significantly altered TOD-dependent expression of the clock component Bmal1, but not Clock and Npas2 (Figure 5B i–iii). In the inhibitory loop of the clock machinery, T3 affected Per1, Per2, and presented T3-time interactions for Per2 and Per3. Cry1 was not changed by T3 challenge (Figure 5C i–iv). Other important modulators of the core loop, Nr1d1 and Rora were investigated. Both genes presented time main effect, but only Rora showed a T3 main effect (Figure 5D i and ii). The clock-controlled gene Dbp was also investigated as a validation of the clockwork machinery. Dbp presented time and T3-time interaction. (Figure 5E). Cosinor analysis revealed that T3 administration significantly phase-shifted Per2 acrophase (2.9-h delay), and decreased Rora mesor. Unlike Per2 phase-shift, TOD-dependent variations in expression of all circadian clock components that fit a 24-h cosine curve (i.e., Bmal1, Rora, Nr1d1, Cry1, and Npas2) exhibited non-significant phase delays following T3 administration (between 0.4-h to 1.0-h). In addition, T3 tended to decrease Dbp amplitude (P=0.0569) (Table 2). Collectively, these data suggest that T3 affects in part the expression of circadian clock genes in the heart, associated with attenuation of circadian clock output, as Ucp3 and Pdk4.
Table 2.
Cosinor analysis (plotted as means ± SEM) of core clock components and CCG in the rat heart challenged by single injection of T3 (periodicity of 24 h period).
| Vehicle | T3 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mesor | Amplitude | Acrophase (ZT) | Mesor | Amplitude | Acrophase (ZT) | |
| Bmal1 | 0.06±0.01 | 0.08±0.01 | 19.06±0.41 | 0.06±0.01 | 0.08±0.02 | 19.52±0.58 |
| Per1 | - | - | - | - | - | - |
| Per2 | 0.11±0.01 | 0.09±0.02 | 7.21±0.41 | 0.13±0.01 | 0.09±0.01 | 10.13±0.31* |
| Per3 | - | - | - | 0.61±0.13 | 0.68±0.19 | 7.17±1.04 |
| Rora | 0.0163±0.0014 | 0.0073±0.002 | 7.55±1.03 | 0.0115±0.001* | 0.0047±0.0014 | 8.02±1.06 |
| Dbp | 0.79±0.12 | 0.86±0.16 | 4.15±0.44 | 0.68±0.05 | 0.55±0.07@ | 4.35±0.30 |
| Ucp3 | 0.02±0.001 | 0.02±0.001 | 13.51±0.58 | 0.06±0.001* | 0.03±0.001 | 18.44±0.51* |
| Pdk4 | 0.12±0.01 | 0.07±0.02 | 10.50±1.03 | - | - | - |
| Nr1d1 | 0.15±0.02 | 0.18±0.03 | 1.07±0.36 | 0.13±0.03 | 0.16±0.04 | 1.47±0.58 |
| Cry1 | 0.10±0.02 | 0.15±0.03 | 11.49±0.50 | 0.11±0.03 | 0.17±0.04 | 12.33±1.00 |
| Npas2 | 0.01±0.001 | 0.01±0.001 | 19.42±1.00 | 0.01±0.001 | 0.01±0.001 | 20.42±0.55 |
| Clock | - | - | - | - | - | - |
Student’s t test, one-tailed, unpaired.
P<0.05 vs respective Vehicles;
P=0.0569 vs respective Vehicle.
Lost or no rhythmicity (−).
DISCUSSION
The primary aim of this study was to investigate whether the heart modulates TOD-dependent rhythms in T3 sensitivity. We observed increased sensitivity of the rodent (rat and mouse) heart to T3 during the last 6 hours of the active/dark phase (i.e., ZT18-ZT24), which appeared to be independent of the cardiomyocyte circadian clock. Interrogation of possible mediators of T3 sensitivity oscillations in the heart revealed increased Dio1 and Thrb1 expression in the heart between ZT18-ZT24. Following the initial observation that T3 induces Bmal1 in the heart at the end of the active/dark phase, a secondary aim investigated whether T3 acts as a zeitgeber for the clock in the heart. These studies highlighted that T3 affects the phase, mesor, and amplitude of distinct clock gene expression oscillations in the heart. Collectively, the current study unveiled a complex interplay between T3, TOD, and the circadian clock in the heart.
When considering the effect of an extrinsic factor on a cell/organ, two main parameters should be considered: 1) the level of the stimulus; and 2) sensitivity of the target tissue to the stimulus. With regards to chronobiology, multiple examples exist wherein both these parameters exhibit TOD-dependent fluctuations. For example, insulin levels increase postprandially, while insulin sensitivity is increased soon after the onset of the active phase in both rodents (i.e. in the evening) and humans (i.e. in the morning) (la Fleur et al., 2001; Lee et al., 1992; Whichelow et al., 1974). In certain cases, changes in target tissue sensitivity can play a dominant role. This increases, decreased hepatic insulin sensitivity driving gluconeogenesis prior to waking (resulting in the Dawn Phenomenon) (Bolli et al., 1984; Bolli & Gerich, 1984; Carroll & Schade, 2005; Schmidt et al., 1984), as well as TOD-dependent oscillations in renal ACTH sensitivity, which drives oscillations in cortisol secretion (Dickmeis, 2009).
The heart exhibits marked fluctuations in its environment on a daily basis, in association with sleep/wake and fasting/feeding cycles. Oscillations in various stimuli/stresses are met with profound perturbations in cardiac function, including heart rate and cardiac output (Durgan & Young, 2010). However, TOD-dependent rhythms in cardiac processes likely result not only from fluctuations in extrinsic (e.g, neurohumoral factors) influences, but also in part by changes in the responsiveness/sensitivity of the myocardium to these extrinsic factors. Indeed, the heart has been shown to exhibit dramatic oscillations in its responsiveness to both physiologic stimuli (e.g., b-adrenergic stimulation, fatty acids) (Durgan et al., 2005; Durgan et al., 2006) and pathologic stresses (e.g., hypertrophic signals (Durgan et al., 2011), ischemia/reperfusion (Durgan et al., 2010)).
Despite knowledge that circulating T3 levels exhibit a diurnal variation, and that this extrinsic factor impacts cardiac processes (e.g., metabolism, heart rate, contractility), no studies have previously investigated whether responsiveness of the heart to T3 is TOD-dependent. The current study revealed increased transcript levels responsiveness of the rodent heart to T3 at the end of the dark/active period. Somewhat surprisingly, these rhythms in T3 responsiveness are antiphase to known rhythms in circulating T3 levels, which peak during the light/sleep period in rodents (Guo et al., 2015). We speculate that antiphase rhythms between stimulus and sensitivity may minimize responsiveness of the heart to T3 during the sleep phase, thus preventing adverse remodeling of the heart associated with hyperthyroidism.
An important question relates to the mechanism(s) mediating TOD-dependent oscillations in the heart to T3. One candidate mechanism is the cardiomyocyte circadian clock. Circadian clocks are cell autonomous mechanisms that allow cells/organs to anticipate stimuli/stresses that fluctuate on a daily basis in a predictable manner (Edery, 2000). One manner with which circadian clocks achieve this goal is through modulating sensitivity of target tissues to neurohumoral factors in a TOD-dependent manner. We therefore hypothesized that the cardiomyocyte circadian clock anticipates increased T3 levels during the sleep phase by decreasing responsiveness of the heart to this endocrine factor at this time. However, rhythms in cardiac T3 responsiveness persist in mice wherein the cardiomyocyte circadian clock is genetically disrupted (i.e., CCM mice), suggesting that this mechanism did not play a dominant role. The tissue specific expression of thyroid hormone receptors have been reported previously (Margolis, 2008). We therefore decided to investigate whether specific Thr isoforms oscillate in a TOD-dependent manner in the heart. Similarly, we also investigated whether distinct deiodinase isoforms oscillate in the heart, which could also contribute to fluctuations in T3 sensitivity. This analysis revealed increased expression of Dio1 and Thrb1 in the heart during the end of the active period, which correlates with increased T3 availability/sensitivity at this time. It is noteworthy that we have previously reported that transcripts responsiveness of the heart to fatty acids (FA) is also increased in the heart during the dark phase (Durgan et al., 2006). Given similarities in T3 and FA responsiveness rhythms, as well as overlap between the mechanisms by which these circulating factors elicit transcriptional control, the possibility arises that common mechanisms contribute. Possibilities include heterodimerization partners (e.g., RXR) (Hollenberg et al., 1995), co-activators/repressors (e.g., NCor1) (Alenghat et al., 2008; Mullur et al., 2014), and chromatin remodeling (e.g., acetylation) (Grimaldi et al., 2007).
Although the cardiomyocyte circadian clock did not appear to mediate TOD-dependent oscillations in responsiveness of the heart to T3, we decided to investigate whether T3 could influence expression of circadian clock components in the heart. In order to maintain its selective advantage, it is important for circadian clocks to remain synchronized with the environment. Signals that reset the timing of the clock mechanism are termed zeitgebers (time-keepers). The central clock within the suprachiasmatic nucleus is reset by light/photic signals via the retino-hypothalamic tract, while peripheral clocks are reset by neurohumoral factors (Hirota & Fukada, 2004). Despite knowledge that T3 exhibits a TOD-dependent oscillation, and that T3 affects target tissues in a transcriptional manner, whether T3 acts as a zeitgeber is currently known. The present study revealed that T3 affects the mesor, amplitude, and phase of various circadian clock components in the heart. With regards to phase, T3 significantly phase delayed Per2 oscillations, altered Rora mesor, and amplitude of Dbp. These observations lead us to speculate that T3 is a potential zeitgeber for the clock in the heart.
In summary, the present study revealed TOD-dependent oscillations in T3 sensitivity in the heart are independent of the cardiomyocyte circadian clock. In addition, evidence is provided in support of the hypothesis that T3 is a zeitgeber for the clock in the heart. Additional studies are required to elucidate fully the mechanisms underlying both observations.
Acknowledgments
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; RAP-G), Conselho Nacional de Pesquisa e Desenvolvimento (CNPq; RAP-G), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, MTN #2013/05629-4), and by the National Heart, Lung, and Blood Institute (HL106199, HL074259, HL123574, HL122975; MEY). We would like to thank Maximiliano H. Grenett for technical assistance.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors report no conflicts of interest.
References
- Ahlersova E, Ahlers I, Kassayova M, Smajda B. Circadian oscillations of serum thyroid hormones in the laboratory rat: the effect of photoperiods. Physiol Res. 1997;46:443–449. [PubMed] [Google Scholar]
- Alenghat T, Meyers K, Mullican SE, Leitner K, Adeniji-Adele A, Avila J, Bucan M, Ahima RS, Kaestner KH, Lazar MA. Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature. 2008;456:997–1000. doi: 10.1038/nature07541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATA. American Thyroid Association ATA. General Information/Press Room. http://www.thyroid.org/media-main/about-hypothyroidism/
- Bargi-Souza P, Romano RM, de Salgado RM, Goulart-Silva F, Brunetto EL, Zorn TM, Nunes MT. Triiodothyronine rapidly alters the TSH content and the secretory granules distribution in male rat thyrotrophs by a cytoskeleton rearrangement-independent mechanism. Endocrinology. 2013;154:4908–4918. doi: 10.1210/en.2013-1508. [DOI] [PubMed] [Google Scholar]
- Biondi B, Palmieri EA, Lombardi G, Fazio S. Effects of thyroid hormone on cardiac function: the relative importance of heart rate, loading conditions, and myocardial contractility in the regulation of cardiac performance in human hyperthyroidism. J Clin Endocrinol Metab. 2002;87:968–974. doi: 10.1210/jcem.87.3.8302. [DOI] [PubMed] [Google Scholar]
- Bolli GB, De Feo P, De Cosmo S, Perriello G, Ventura MM, Calcinaro F, Lolli C, Campbell P, Brunetti P, Gerich JE. Demonstration of a dawn phenomenon in normal human volunteers. Diabetes. 1984;33:1150–1153. doi: 10.2337/diab.33.12.1150. [DOI] [PubMed] [Google Scholar]
- Bolli GB, Gerich JE. The “dawn phenomenon”--a common occurrence in both non-insulin-dependent and insulin-dependent diabetes mellitus. N Engl J Med. 1984;310:746–750. doi: 10.1056/NEJM198403223101203. [DOI] [PubMed] [Google Scholar]
- Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai JY, Bugger H, Zhang D, Rohrwasser A, Rennison JH, Dyck JR, Litwin SE, Hardin PE, Chow CW, Chandler MP, Abel ED, Young ME. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1036–1047. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- Carroll MF, Schade DS. The dawn phenomenon revisited: implications for diabetes therapy. Endocr Pract. 2005;11:55–64. doi: 10.4158/EP.11.1.55. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cody V, Davis PJ, Davis FB. Molecular modeling of the thyroid hormone interactions with alpha v beta 3 integrin. Steroids. 2007;72:165–170. doi: 10.1016/j.steroids.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Cornelissen G, Halberg F, Stebbings J, Halberg E, Carandente F, Hsi B. Chronobiometry with pocket calculators and computer systems. Ric Clin Lab. 1980;10:333–385. doi: 10.1007/BF02905347. [DOI] [PubMed] [Google Scholar]
- Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200:3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- Dillmann WH. Editorial: thyroid hormone action and cardiac contractility - a complex affair. Endocrinology. 1996;137:799–801. doi: 10.1210/endo.137.3.8603587. [DOI] [PubMed] [Google Scholar]
- Dillmann WH. Cellular action of thyroid hormone on the heart. Thyroid : official journal of the American Thyroid Association. 2002;12:447–452. doi: 10.1089/105072502760143809. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Hotze MA, Tomlin TM, Egbejimi O, Graveleau C, Abel ED, Shaw CA, Bray MS, Hardin PE, Young ME. The intrinsic circadian clock within the cardiomyocyte. Am J Physiol Heart Circ Physiol. 2005;289:H1530–1541. doi: 10.1152/ajpheart.00406.2005. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, Young ME. Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ Res. 2010;106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgan DJ, Trexler NA, Egbejimi O, McElfresh TA, Suk HY, Petterson LE, Shaw CA, Hardin PE, Bray MS, Chandler MP, Chow CW, Young ME. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. The Journal of biological chemistry. 2006;281:24254–24269. doi: 10.1074/jbc.M601704200. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Tsai JY, Grenett MH, Pat BM, Ratcliffe WF, Villegas-Montoya C, Garvey ME, Nagendran J, Dyck JR, Bray MS, Gamble KL, Gimble JM, Young ME. Evidence suggesting that the cardiomyocyte circadian clock modulates responsiveness of the heart to hypertrophic stimuli in mice. Chronobiol Int. 2011;28:187–203. doi: 10.3109/07420528.2010.550406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgan DJ, Young ME. The cardiomyocyte circadian clock: emerging roles in health and disease. Circulation research. 2010;106:647–658. doi: 10.1161/CIRCRESAHA.109.209957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edery I. Circadian rhythms in a nutshell. Physiol Genomics. 2000;3:59–74. doi: 10.1152/physiolgenomics.2000.3.2.59. [DOI] [PubMed] [Google Scholar]
- Giannocco G, DosSantos RA, Nunes MT. Thyroid hormone stimulates myoglobin gene expression in rat cardiac muscle. Molecular and cellular endocrinology. 2004;226:19–26. doi: 10.1016/j.mce.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Gibson UE, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- Gosteli-Peter MA, Schmid C, Zapf J. Triiodothyronine increases glucose transporter isotype 4 mRNA expression, glucose transport, and glycogen synthesis in adult rat cardiomyocytes in long-term culture. Biochemical and biophysical research communications. 1996;221:521–524. doi: 10.1006/bbrc.1996.0629. [DOI] [PubMed] [Google Scholar]
- Grimaldi B, Nakahata Y, Sahar S, Kaluzova M, Gauthier D, Pham K, Patel N, Hirayama J, Sassone-Corsi P. Chromatin remodeling and circadian control: master regulator CLOCK is an enzyme. Cold Spring Harb Symp Quant Biol. 2007;72:105–112. doi: 10.1101/sqb.2007.72.049. [DOI] [PubMed] [Google Scholar]
- Guissouma H, Dupre SM, Becker N, Jeannin E, Seugnet I, Desvergne B, Demeneix BA. Feedback on hypothalamic TRH transcription is dependent on thyroid hormone receptor N terminus. Mol Endocrinol. 2002;16:1652–1666. doi: 10.1210/mend.16.7.0868. [DOI] [PubMed] [Google Scholar]
- Guo TY, Liu LJ, Xu LZ, Zhang JC, Li SX, Chen C, He LG, Chen YM, Yang HD, Lu L, Hashimoto K. Alterations of the daily rhythms of HPT axis induced by chronic unpredicted mild stress in rats. Endocrine. 2015;48:637–643. doi: 10.1007/s12020-014-0314-y. [DOI] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- Hirota T, Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zoolog Sci. 2004;21:359–368. doi: 10.2108/zsj.21.359. [DOI] [PubMed] [Google Scholar]
- Hollenberg AN, Monden T, Wondisford FE. Ligand-independent and -dependent functions of thyroid hormone receptor isoforms depend upon their distinct amino termini. The Journal of biological chemistry. 1995;270:14274–14280. doi: 10.1074/jbc.270.24.14274. [DOI] [PubMed] [Google Scholar]
- Hyyti OM, Ning XH, Buroker NE, Ge M, Portman MA. Thyroid hormone controls myocardial substrate metabolism through nuclear receptor-mediated and rapid posttranscriptional mechanisms. American journal of physiology. Endocrinology and metabolism. 2006;290:E372–379. doi: 10.1152/ajpendo.00288.2005. [DOI] [PubMed] [Google Scholar]
- Jordan D, Rousset B, Perrin F, Fournier M, Orgiazzi J. Evidence for circadian variations in serum thyrotropin, 3,5,3′-triiodothyronine, and thyroxine in the rat. Endocrinology. 1980;107:1245–1248. doi: 10.1210/endo-107-4-1245. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, Buijs RM. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes. 2001;50:1237–1243. doi: 10.2337/diabetes.50.6.1237. [DOI] [PubMed] [Google Scholar]
- Lee A, Ader M, Bray GA, Bergman RN. Diurnal variation in glucose tolerance. Cyclic suppression of insulin action and insulin secretion in normal-weight, but not obese, subjects. Diabetes. 1992;41:750–759. doi: 10.2337/diab.41.6.750. [DOI] [PubMed] [Google Scholar]
- Leonard JL, Mellen SA, Larsen PR. Thyroxine 5′-deiodinase activity in brown adipose tissue. Endocrinology. 1983;112:1153–1155. doi: 10.1210/endo-112-3-1153. [DOI] [PubMed] [Google Scholar]
- Margolis RN. The Nuclear Receptor Signaling Atlas: catalyzing understanding of thyroid hormone signaling and metabolic control. Thyroid : official journal of the American Thyroid Association. 2008;18:113–122. doi: 10.1089/thy.2007.0247. [DOI] [PubMed] [Google Scholar]
- Morkin E, Flink IL, Goldman S. Biochemical and physiologic effects of thyroid hormone on cardiac performance. Prog Cardiovasc Dis. 1983;25:435–464. doi: 10.1016/0033-0620(83)90004-x. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiological reviews. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal-Ginard B, Mahdavi V. Basic mechanisms of cardiac gene expression. Eur Heart J. 1993;14(Suppl J):2–11. [PubMed] [Google Scholar]
- Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6:305–323. [PubMed] [Google Scholar]
- Nunes MT, Bianco AC, Migala A, Agostini B, Hasselbach W. Thyroxine induced transformation in sarcoplasmic reticulum of rabbit soleus and psoas muscles. Z Naturforsch C. 1985;40:726–734. doi: 10.1515/znc-1985-9-1025. [DOI] [PubMed] [Google Scholar]
- Peliciari-Garcia RA, Zanquetta MM, Andrade-Silva J, Gomes DA, Barreto-Chaves ML, Cipolla-Neto J. Expression of circadian clock and melatonin receptors within cultured rat cardiomyocytes. Chronobiol Int. 2011;28:21–30. doi: 10.3109/07420528.2010.525675. [DOI] [PubMed] [Google Scholar]
- Rohrer D, Dillmann WH. Thyroid hormone markedly increases the mRNA coding for sarcoplasmic reticulum Ca2+-ATPase in the rat heart. The Journal of biological chemistry. 1988;263:6941–6944. [PubMed] [Google Scholar]
- Schmidt MI, Lin QX, Gwynne JT, Jacobs S. Fasting early morning rise in peripheral insulin: evidence of the dawn phenomenon in nondiabetes. Diabetes Care. 1984;7:32–35. doi: 10.2337/diacare.7.1.32. [DOI] [PubMed] [Google Scholar]
- Tsai JY, Kienesberger PC, Pulinilkunnil T, Sailors MH, Durgan DJ, Villegas-Montoya C, Jahoor A, Gonzalez R, Garvey ME, Boland B, Blasier Z, McElfresh TA, Nannegari V, Chow CW, Heird WC, Chandler MP, Dyck JR, Bray MS, Young ME. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. The Journal of biological chemistry. 2010;285:2918–2929. doi: 10.1074/jbc.M109.077800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser TJ, Leonard JL, Kaplan MM, Larsen PR. Kinetic evidence suggesting two mechanisms for iodothyronine 5′-deiodination in rat cerebral cortex. Proc Natl Acad Sci U S A. 1982;79:5080–5084. doi: 10.1073/pnas.79.16.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser WE, Friesema EC, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol. 2011;25:1–14. doi: 10.1210/me.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whichelow MJ, Sturge RA, Keen H, Jarrett RJ, Stimmler L, Grainger S. Diurnal variation in response to intravenous glucose. Br Med J. 1974;1:488–491. doi: 10.1136/bmj.1.5906.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuo S, Watanabe M, Iigo M, Yamamura T, Nakao N, Takagi T, Ebihara S, Yoshimura T. Molecular mechanism of photoperiodic time measurement in the brain of Japanese quail. Chronobiol Int. 2006;23:307–315. doi: 10.1080/07420520500521913. [DOI] [PubMed] [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiological reviews. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- Young ME, Razeghi P, Cedars AM, Guthrie PH, Taegtmeyer H. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circulation research. 2001a;89:1199–1208. doi: 10.1161/hh2401.100741. [DOI] [PubMed] [Google Scholar]
- Young ME, Razeghi P, Taegtmeyer H. Clock genes in the heart: characterization and attenuation with hypertrophy. Circulation research. 2001b;88:1142–1150. doi: 10.1161/hh1101.091190. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Tan SW, Tyl RW. General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit Rev Toxicol. 2007;37:11–53. doi: 10.1080/10408440601123446. [DOI] [PubMed] [Google Scholar]