Abstract
Fertility depends on the correct maturation and function of approximately 800 gonadotropin-releasing hormone (GnRH) neurons in the brain. GnRH neurons are at the apex of the hypothalamic-pituitary-gonadal axis that regulates fertility. In adulthood, GnRH neurons are scattered throughout the anterior hypothalamic area and project to the median eminence, where GnRH is released into the portal vasculature to stimulate release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary. LH and FSH then regulate gonadal steroidogenesis and gametogenesis. Absence of GnRH neurons or inappropriate GnRH release leads to infertility. Despite the critical role of GnRH neurons in fertility, we still have a limited understanding of the genes responsible for proper GnRH neuron development and function in adulthood. GnRH neurons originate in the olfactory placode then migrate into the brain. Homeodomain transcription factors expressed within GnRH neurons or along their migratory path are candidate genes for inherited infertility. Using a combined in vitro and in vivo approach, we have identified Ventral Anterior Homeobox 1 (Vax1) as a novel homeodomain transcription factor responsible for GnRH neuron maturation and fertility. GnRH neuron counts in Vax1 knock-out embryos revealed Vax1 to be required for the presence of GnRH-expressing cells at embryonic day 17.5 (E17.5), but not at E13.5. To localize the effects of Vax1 on fertility, we generated Vax1flox mice and crossed them with Gnrhcre mice to specifically delete Vax1 within GnRH neurons. GnRH staining in Vax1flox/flox:GnRHcre mice show a total absence of GnRH expression in the adult. We performed lineage tracing in Vax1flox/flox:GnRHcre:RosaLacZ mice which proved GnRH neurons to be alive, but incapable of expressing GnRH. The absence of GnRH leads to delayed puberty, hypogonadism and complete infertility in both sexes. Finally, using the immortalized model GnRH neuron cell lines, GN11 and GT1-7, we show that VAX1 is a direct regulator of Gnrh1 transcription by binding key ATTA sites within the Gnrh1 promoter. This study identifies VAX1 as a key transcription factor regulating GnRH expression and establishes VAX1 as a novel candidate gene implicated in heritable infertility.
Keywords: Inherited infertility, GnRH neuron, VAX1, homeodomain transcription factor, hypogonadism, development
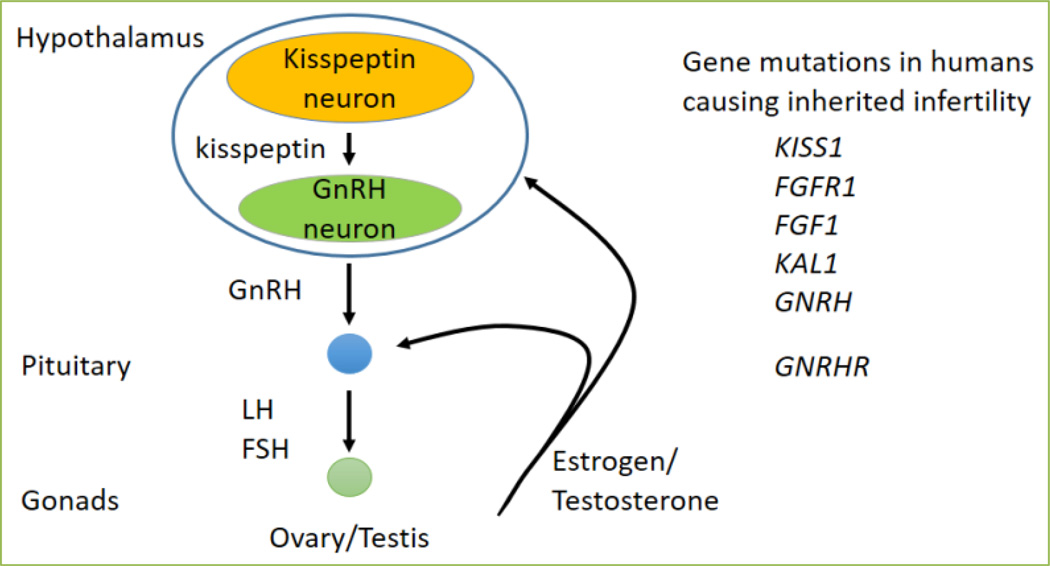
Infertility classified as idiopathic hypogonadotropic hypogonadism (IHH) is characterized by delayed or absent sexual maturation, and low gonadotropin and sex steroid levels due to hypothalamic-pituitary-gonadal (HPG) axis deficiency (Figure 1) [1, 2]. Due to the complexity of fertility regulation by the HPG axis, most cases of inherited infertility still have unknown genetic origins (Figure 1). Most genetic mutations known to cause IHH are autosomal recessive or dominant, however, it is becoming increasingly clear that a number of the unidentified genetic causes of IHH result from mutations in at least two distinct genes (complex heterozygosity). Despite the difficulty in detecting polygenic IHH, haploinsufficiencies adversely affecting fertility have been reported in both rodents and humans [3–7].
Figure 1. Mutations of genes in the hypothalamic-pituitary-gonadal axis cause inherited infertility.
The hypothalamic-pituitary-gonadal axis is controlled by kisspeptin input on to GnRH neurons. Pulsatile release of GnRH triggers LH and FSH release from the pituitary, which in turn stimulate the gonads to release sex steroids. Testosterone and estrogen (in the male and female, respectively), feedback to the hypothalamic kisspeptin neurons and gonadotropes in the pituitary. Mutations in key genes for GnRH or kisspeptin neuron function, or responsiveness of pituitary gonadotropes to GnRH, cause infertility.
Gonadotropin-releasing hormone (GnRH) neurons are localized at the apex of the HPG axis (Figure 1) and originate outside the brain in the olfactory placode. In the mouse, these GnRH neurons arise at embryonic day 10.5 (E10.5), then migrate through the cribriform plate, reaching their final destination in the anterior hypothalamic area between E15 to E18, when approximately 800 GnRH neurons are found in the brain. Abnormal GnRH neuron maturation, migration, or GnRH secretion results in failures of puberty, fertility, and reproductive function. GnRH neuron maturation is key in maintaining fertility. Thus, to identify novel genes important for GnRH neuron development, we compared gene expression levels in two immortalized GnRH cell lines: the immature, migratory GnRH cell line (GN11), and the mature, post migratory, GnRH secretory cell line (GT1-7) [8, 9]. The migration of GnRH neurons is principally restricted to the ventral forebrain, where homeodomain transcription factors expressed ventrally between E10 and E18 are involved in the correct maturation and migration of these neurons [6, 10–12]. Comparison of RNA sequencing data from GN11 and GT1-7 identified one such gene, Ventral anterior homeobox 1 (Vax1). Vax1 is differentially expressed between GN11 and GT1-7, and presents with a developmental expression profile overlapping with the area and timing of GnRH neuron migration as determined by comparing Vax1 and Gnrh1 expression patterns in the developing mouse brain on www.brain-map.org. VAX1 is a homeodomain transcription factor critical for embryonic development and essential for the formation of the eye, ventral forebrain and palate [13–15]. In the adult mouse, Vax1 is expressed at all levels of the reproductive axis: GnRH neurons, the testis, and the pituitary, but is absent in the pituitary gonadotropes and ovaries [16]. We first determined if Vax1 was involved in GnRH neuron development. We collected Vax1 wildtype, heterozygote and knock-out embryos at two developmental time points: E13.5, when most GnRH neurons are localized in the olfactory placode, and are starting to migrate toward the cribriform plate, and at E17.5, when most GnRH neurons have completed their migration to the hypothalamus. At E13.5, there were normal numbers of GnRH neurons in Vax1 knock-out mice. In stark contrast, at E17.5, ~50% of GnRH neurons were detected in the Vax1 heterozygote embryos, and none in the knock-out [17]. Thus, VAX1 is not required for generation of GnRH neurons, but instead for their maturation. As Vax1 knock-out is perinatal lethal [15], and we observed a dosage effect of Vax1 on GnRH neuron numbers, we investigated the impact on fertility in Vax1 heterozygote mice. In agreement with what was found in E17.5 Vax1 heterozygote embryos, adult Vax1 heterozygote mice of both sexes had approximately 60% fewer GnRH-expressing neurons than control littermates. A fertility study of Vax1 heterozygote males and females determined that both sexes were subfertile, Vax1 heterozygote females had smaller and fewer litters than controls, whereas Vax1 heterozygote males fathered smaller litters. The subfertility of female Vax1 heterozygote mice was associated with a slight increase in circulating LH and estrogen levels, which was accompanied by prolonged and irregular estrous cycles. However, as Vax1 was not expressed in the ovary or the pituitary gonadotropes, the pituitary cell population releasing FSH and LH (Figure 1), we concluded that female subfertility originated at the level of the GnRH neuron [16]. In contrast, the sub-fertility of the Vax1 heterozygote male, which was caused by an 80% reduction in the motile sperm population, could not be fully accounted for by the reduction in GnRH neurons as these mice were capable of maintaining normal LH, FSH, and testosterone levels. This suggests a combined effect of Vax1 in GnRH neuron development and an unknown role in the testis leading to sub-fertility in Vax1 heterozygote males [16].
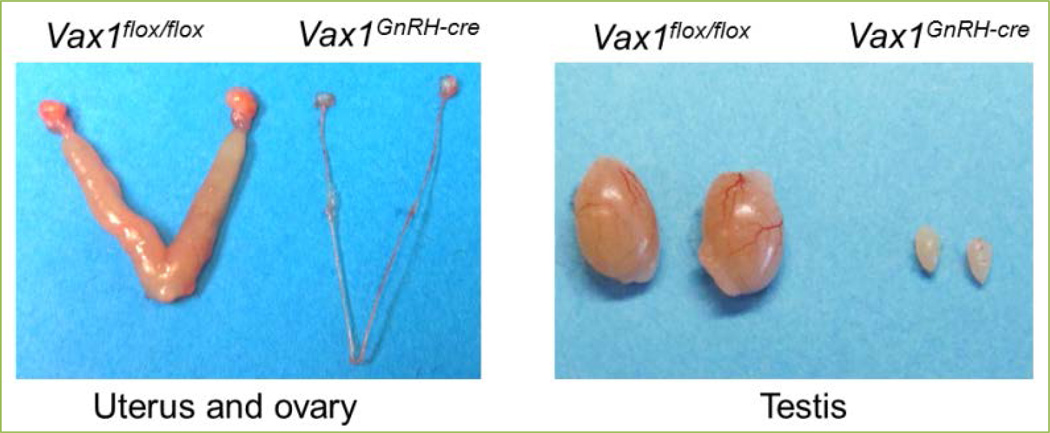
To determine the contribution of VAX1 to GnRH neurons specifically, we generated a Vax1flox mouse and crossed it with a GnRHcre mouse to generate a conditional knock-out Vax1GnRH-cre mouse [18]. Vax1GnRH-cre mice appear healthy and are indistinguishable from control littermates. Remarkably, adult Vax1GnRH-cre mice have no GnRH-expressing cells as determined by GnRH immunohistochemistry, leading to extremely low circulating FSH and LH levels. In the female Vax1GnRH-cre mouse, this resulted in delayed vaginal opening, an external marker of pubertal onset, hypogonadism (Figure 2), absence of mature ovarian follicles, and complete infertility. The low LH and FSH levels, in combination with estrogen levels below assay detection limits, correlated with an incapacity of Vax1GnRH-cre mice to progress through the estrous cycle, as evaluated by vaginal smears, and resulted in females being in permanent diestrus. In line with this, male Vax1GnRH-cre mice also presented with low LH and FSH levels, two hormones required for pubertal onset and normal testicular function. Indeed, Vax1GnRH-cre males had delayed pubertal onset as determined by preputial separation, a micropenis, were hypogonadal (Figure 2) with immature testes which were azoospermic, leading to complete infertility. To confirm that this infertility was due to absence of GnRH expression, and not due to an incapacity of the pituitary to release LH in response to GnRH, we performed a GnRH challenge. Indeed, an intra-peritoneal (ip) injection of GnRH resulted in a fold increase of LH release in both male and female Vax1GnRH-cre mice comparable to controls. In contrast, ip injection of the GnRH neuron activator kisspeptin (Figure 1), only allowed increased LH release in controls, and not in Vax1GnRH-cre mice. This localizes the origin of infertility of Vax1GnRH-cre mice at the level of the GnRH neuron, and excludes a contribution of the pituitary in their infertility. Evaluation of heterozygote Vax1GnRH-cre (Vax1flox/+:GnRHcre) recapitulated most of the subfertility phenotype of the full body Vax1 heterozygote mouse, but not all, indicating that Vax1 has a role in fertility maintenance outside of the GnRH neuron. To determine the destiny of GnRH neurons in Vax1GnRH-cre mice, we performed lineage tracing of GnRH neurons using Vax1GnRH-cre:RosaLacZ+ mice. This approach allows “Cre” to delete a Flox-Stop to activate LacZ [19] and thus marks all GnRHcre expressing cells with LacZ permanently regardless of ongoing GnRH gene expression. The specific expression of LacZ in GnRH neurons allowed us to determine whether GnRH neurons were alive without expressing GnRH. In this scenario, LacZ staining would be detected, while GnRH staining would be absent. Lineage tracing showed comparable localization and numbers of LacZ expressing cells in both control and Vax1GnRH-cre:RosaLacZ+ mice, proving that GnRH neurons in Vax1GnRH-cre mice stop expressing GnRH but survive. Thus, VAX1 is critical in maintaining GnRH expression after E13.5.
Figure 2. Deletion of Vax1 from GnRH neurons leads to hypogonadism.
Vax1GnRH-cre mice have no GnRH expression, leading to female (left) and male (right) hypogonadism.
To determine if the effect of VAX1 on GnRH expression was direct, we next asked if VAX1 could directly regulate the Gnrh1 promoter. To answer this, we used the two model GnRH cell lines, GN11 and GT1-7 cells. Transient transfections of GN11 and GT1-7 cells with various constructs of the Gnrh1 promoter driving the expression of a luciferase reporter, allowed us to identify four conserved ATTA sites in the Gnrh1 promoter potentially regulated by VAX1. To prove VAX1 directly interacted with the identified ATTA sites of the Gnrh1 promoter, we performed electrophoretic mobility-shift assays to show direct DNA-protein interactions. Indeed, VAX1 was able to directly bind the identified ATTA sites of the Gnrh1 promoter. In contrast to what we expected, our data suggested that, in GT1-7 cells, VAX1 was a repressor of Gnrh1 transcription. To explain these findings, we hypothesized that VAX1 was a weak activator that could compete for binding to the identified ATTA sites with other homeodomain transcription factors that were stronger activators of Gnrh1 transcription. One such transcription factor is SIX6 [10]. First, we asked if VAX1 was able to act as an activator of an ATTA-multimer in GT1-7 cells, which indeed it was. Thus, VAX1 can increase transcription in the context of GT1-7 cells. As SIX6 is a strong activator of the Gnrh1 promoter, replacing SIX6 with VAX1, a weak activator, would, in our experimental setting, show as a reduction in transcription levels. By cotransfecting various concentrations of VAX1 and SIX6 into GT1-7 cells, along with the Gnrh1 promoter driving a luciferase reporter, we determined a complex competition between SIX6 and VAX1. Depending on the specific concentrations of these transcription factors, different levels of transcription were revealed. To our satisfaction, we found that VAX1 can compete with SIX6 for binding to the Gnrh1 promoter, which to some extent can explain the absence of GnRH expression in Vax1GnRH-cre mice.
In summary, we have identified Vax1 as a key transcription factor involved in maintaining GnRH expression after E13.5. Expression of GnRH is Vax1 dose sensitive, and Vax1 haploinsufficiency leads to subfertility. Thus, Vax1 is a novel candidate gene for polygenic IHH. We show that the role of Vax1 within the GnRH neuron is to maintain GnRH expression through a direct effect on the Gnrh1 promoter. Absence of Vax1 from GnRH neurons abolishes GnRH expression and leads to complete infertility and hypogonadism.
Acknowledgments
We thank Crystal Trang, Ping Gong, Ikuo Kimura, and Erica C. Pandolfi for their contributions to the original work. H.M.H. was partially supported by NIH K99 HD084759. Research was supported by National Institutes of Health (NIH) Grants R01 DK044838, R01 HD072754, R01 HD082567 (to P.L.M.). It was also supported by National Institute of Child Health and Human Development/NIH through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to P.L.M.). P.L.M. was also partially supported by P30 DK063491, P30 CA023100, and P42 ES101337. The embryonic stem cells used to generate the Vax1 knock-out first mouse, giving rise to the Vax1flox mouse, used for this research project was generated by the trans-NIH Knock-Out Mouse Project (KOMP) and obtained from the KOMP Repository (www.komp.org). NIH grants to Velocigene at Regeneron Inc (U01HG004085) and the CSD Consortium (U01HG004080) funded the generation of gene-targeted ES cells for 8500 genes in the KOMP Program and archived and distributed by the KOMP Repository at UC Davis and CHORI (U42RR024244).
Abbreviations
- Vax1
Ventral Anterior Homeobox 1 gene
- GnRH
Gonadotropin-Releasing Hormone
- LH
luteinizing hormone
- FSH
follicle-stimulating hormone
- E
embryonic day
- IHH
idiopathic hypogonadotropic hypogonadism
- HPG
hypothalamic-pituitary-gonadal
- ip
intra-peritoneal
Footnotes
Conflicting interests
The authors have declared that no conflict of interests exist.
Author contributions
H.M.H. and P.L.M. wrote the manuscript.
References
- 1.Balasubramanian R, Dwyer A, Seminara SB, Pitteloud N, Kaiser UB, Crowley WF., Jr Human GnRH deficiency: a unique disease model to unravel the ontogeny of GnRH neurons. Neuroendocrinology. 2010;92:81–99. doi: 10.1159/000314193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianco SD, Kaiser UB. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol. 2009;5:569–576. doi: 10.1038/nrendo.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, et al. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest. 2007;117:457–463. doi: 10.1172/JCI29884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamou MI, Cox KH, Crowley WF. Discovering Genes Essential to the Hypothalamic Regulation of Human Reproduction Using a Human Disease Model: Adjusting to Life in the "-Omics" Era. Endocr Rev. 2015;36:603–621. doi: 10.1210/er.2015-1045. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Kim HG, Herrick SR, Lemyre E, Kishikawa S, Salisz JA, Seminara S, et al. Hypogonadotropic hypogonadism and cleft lip and palate caused by a balanced translocation producing haploinsufficiency for FGFR1. J Med Genet. 2005;42:666–672. doi: 10.1136/jmg.2004.026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larder R, Kimura I, Meadows J, Clark DD, Mayo S, Mellon PL. Gene dosage of Otx2 is important for fertility in male mice. Mol Cell Endocrinol. 2013;377:16–22. doi: 10.1016/j.mce.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tata B, Huijbregts L, Jacquier S, Csaba Z, Genin E, Meyer V, et al. Haploinsufficiency of Dmxl2, encoding a synaptic protein, causes infertility associated with a loss of GnRH neurons in mouse. PLoS Biol. 2014;12:e1001952. doi: 10.1371/journal.pbio.1001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wierman ME, Kiseljak-Vassiliades K, Tobet S. Gonadotropin-releasing hormone (GnRH) neuron migration: initiation, maintenance and cessation as critical steps to ensure normal reproductive function. Front Neuroendocrinol. 2011;32:43–52. doi: 10.1016/j.yfrne.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- 10.Larder R, Clark DD, Miller NL, Mellon PL. Hypothalamic dysregulation and infertility in mice lacking the homeodomain protein Six6. J Neurosci. 2011;31:426–438. doi: 10.1523/JNEUROSCI.1688-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Givens ML, Rave-Harel N, Goonewardena VD, Kurotani R, Berdy SE, Swan CH, et al. Developmental regulation of gonadotropin-releasing hormone gene expression by the MSX and DLX homeodomain protein families. J Biol Chemi. 2005;280:19156–19165. doi: 10.1074/jbc.M502004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaczok D, DiVall S, Matsuo I, Wondisford FE, Wolfe AM, Radovick S. Deletion of Otx2 in GnRH neurons results in a mouse model of hypogonadotropic hypogonadism. Mol Endocrinol. 2011;25:833–846. doi: 10.1210/me.2010-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taglialatela P, Soria JM, Caironi V, Moiana A, Bertuzzi S. Compromised generation of GABAergic interneurons in the brains of Vax1−/− mice. Development. 2004;131:4239–4249. doi: 10.1242/dev.01299. [DOI] [PubMed] [Google Scholar]
- 14.Hallonet M, Hollemann T, Pieler T, Gruss P. Vax1, a novel homeobox-containing gene, directs development of the basal forebrain and visual system. Genes Dev. 1999;13:3106–3114. doi: 10.1101/gad.13.23.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertuzzi S, Hindges R, Mui SH, O'Leary DD, Lemke G. The homeodomain protein vax1 is required for axon guidance and major tract formation in the developing forebrain. Genes Dev. 1999;13:3092–3105. doi: 10.1101/gad.13.23.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann HM, Tamrazian A, Xie H, Perez-Millan MI, Kauffman AS, Mellon PL. Heterozygous deletion of ventral anterior homeobox (Vax1) causes subfertility in mice. Endocrinology. 2014;155:4043–4053. doi: 10.1210/en.2014-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann HM, Trang C, Gong P, Kimura I, Pandolfi EC, Mellon PL. Deletion of Vax1 from GnRH neurons abolishes GnRH expression and leads to hypogonadism and infertility. J Neurosci. 2016;36:3506–3518. doi: 10.1523/JNEUROSCI.2723-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfe A, Divall S, Singh SP, Nikrodhanond AA, Baria AT, Le WW, et al. Temporal and spatial regulation of CRE recombinase expression in gonadotrophin-releasing hormone neurones in the mouse. J Neuroendocrinol. 2008;20:909–916. doi: 10.1111/j.1365-2826.2008.01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]