Abstract
A generation ago, the immunoglobulin intramolecular signaling, or allosteric, hypothesis was abandoned in favor of the associative hypothesis, which posited that Fc receptor crosslinking produced the increased affinity of antigen-antibody complexes. This essay argues that there is sufficient evidence to resuscitate the allosteric hypothesis, at least for some antibodies.
Keywords: antibody, immunoglobulin, intramolecular signaling, allosteric model, associative model
Antibody Signaling upon Antigen Binding
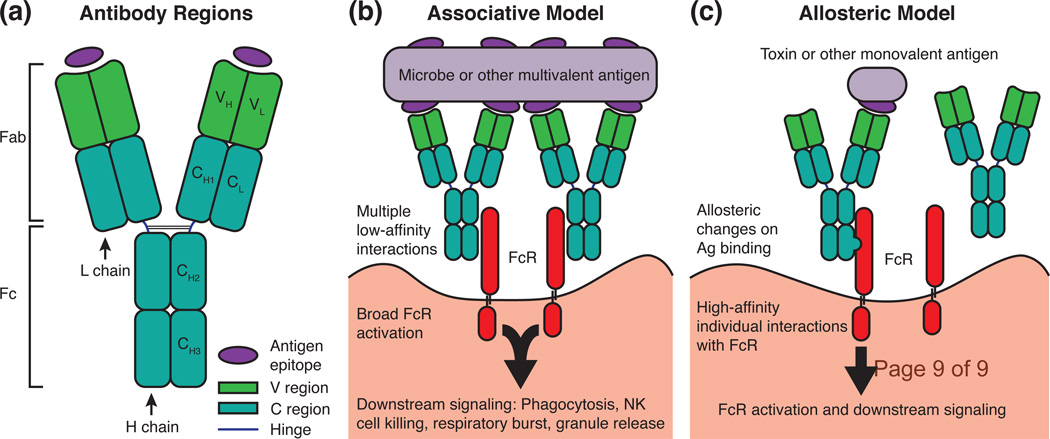
One of the most striking phenomena in immunology is antibody (Ab)-mediated opsonophagocytosis, which is a fundamental mechanism of host defense that removes microbes and foreign antigens. For opsonophagocytosis to occur, the antigen (Ag) and Ab complex must have higher affinity for Fc receptors (FcR) than Ab alone. Although this debate is now largely forgotten, the mechanism by which this occurs was once a major problem in immunology with two competing hypotheses: the allosteric model, in which immunoglobulin (Ig) intramolecular signaling leads to the transmission of conformational changes from the variable (V) region to the constant (C) region that increase affinity for FcR, or the associative model, in which the increased avidity of Ag-Ab complexes increases FcR activation through the engagement of multiple receptors (Figure 1). These two distinct signaling models were also debated to be responsible for Ab-mediated complement activation and B-cell receptor activation. By the late 1980s, the intramolecular signaling hypothesis had been largely discarded as a result of several negative studies finding no major Ab structural changes upon Ag binding as well as a plethora of evidence detailing the importance of Ab aggregation in signaling (reviewed in [1]). This forum essay makes the argument that the abandonment of the immunoglobulin intramolecular signaling hypothesis represents a case in science of a field prematurely dropping a problem.
Figure 1. Comparison of the Associative and Allosteric Models of Antibody Signaling.
(a) A simplified schematic of an antibody molecule is shown depicting the H and L chains along with the V and C regions. Immunoglobulins can be broken into two antigen-binding Fab fragments and one Fc fragment. Each Fab contains one combining site that interacts with a single antigen epitope. (b) The associative model of antibody signaling requires multivalent antigens that possess at least two epitopes. The proximity of many antigen (Ag)-bound immunoglobulins leads to many low-affinity interactions between antibody Fc regions and cellular Fc receptors (FcR), ultimately causing FcR activation and downstream signaling. (c) The allosteric, or intramolecular signaling, model can explain FcR activation by antibody bound to monovalent antigens. In this model, antigen binding at the V region causes conformational changes that propagate to the Fc, increasing affinity of the antibody for FcR. Note that the associative and allosteric models of antibody signaling are not mutually exclusive.
Intramolecular Signaling in Antibodies
Although the negative observations won the day, it is worthwhile to remember that there was a compelling body of information supporting the intramolecular signaling mechanism. While we only have space to cite a limited number of studies here, we are confident that this sample will make the point. In the 1970s, circular dichroism, sedimentation, hydrogen-exchange, and proteolysis experiments produced admittedly indirect evidence that was interpreted as indicative of Ig conformational changes upon Ag binding [2–4]. However, in 1976 crystallographic data and molecular modelling studies were used to propose an allosteric model whereby Ag binding triggered a conformational change in the V region that propagated to produce structural changes in the C region, ultimately increasing FcR affinity [5]. Furthermore, functional data showing complement activation by IgM binding to a monovalent Ag precluded the crosslinking explanation [6]. Since the 1980s, a stream of observations has continued to support the allosteric model. In 1992, a study reported that changes in the V region led to complement activation independently of differences in avidity and suggested V-region-mediated changes on C region structure [7]. In 1994, a crystallographic study provided evidence that Ag binding to the V region resulted in a large (19 Å) displacement of a glutamic acid residue in the hinge, illustrating propagation of conformational changes from V to C domains [8]. In 2003, a thermodynamic analysis showed that Ag binding inhibited the interaction of the C region with Ab-binding protein G and protein A [9]. In recent years, there have been several more studies providing additional evidence for V region effects on C region structure [10, 11]. In addition, some functional studies are difficult to understand in the context of the FcR receptor crosslinking hypothesis, such as the observation that a monoclonal IgG can drive the internalization of a toxin [12], since the monovalent Ag-Ab complex should not have been able to crosslink FcR.
In hindsight, it is difficult to understand how the set of negative studies finding no evidence that Ag binding affected C region structure was accepted in spite of a large body of data that argued otherwise. In fact, the acceptance of negative data over positive data is particularly mystifying since it is difficult to prove a negative and it is usually not possible to distinguish between a true negative result or a false negative finding due to experimental limitations. Although we cannot place ourselves in the zeitgeist of the time, we can imagine some explanations for this turn of events. First, a clean division between V and C region function could have been attractive on the heels of the elegant studies that revealed how V element recombination and somatic mutation solved the problem of Ab diversity. Second, dividing the Ig molecule into two non-interacting domains with well-defined functions fit the increasingly reductionist emphasis of the times. Third, crystallographic studies revealed that the hinge region between Fab and Fc regions was often disordered, arguing against a tight structural linkage that could easily transmit structural information. Fourth, there was an attractive alternative explanation in the form of crosslinking FcRs, an event that not only increased avidity and thus explained increased FcR affinity, but also triggered signaling, thus appealing to the nascent emphasis on cellular biology that began in the 1980s. Fifth, there was considerable direct experimental evidence for the associative hypothesis. However, the associative and allosteric hypothesis were not mutually exclusive, which meant it was not necessary to discard one in favor of the other. In addition to these scientific reasons, perhaps there were additional factors in the sociology of the field, unknown to us, whereby strong advocates of the associative model were able to drive that paradigm over the allosteric model.
While studying the inverse phenomenon, where V-region-identical Abs differing in isotype manifested differences in fine specificity and affinity for Ag, we have noted that not all Abs appear capable of transmitting structural information from the C region to the V region upon Ag binding [1]. In fact, V regions that have been reported to undergo C-region-mediated structural changes appear to be phylogenetically related suggesting that features in the primary structure of the V region or the emerging V-C structure confer permissiveness or non-permissiveness to allosteric changes. This, in turn, suggests a potential explanation for the inconsistent results obtained with various Abs studied in the 1970s and 1980s, based on the notion that allosteric changes upon Ag binding occurred with some V-C combinations and not others. In other words, it is possible that both the positive and negative studies were correct and that the differences simply reflected the Abs studied. If this reconciliation is true it would open new fertile pastures for Ig research. We note that there are still many unresolved questions about intramolecular signaling in Abs (Box 1) and that the mere presence of long-range conformational changes in Abs following Ag binding is not enough to prove a function for these changes. Future studies will need to examine the effects of conformational changes on downstream functions such as FcR and complement activation.
Box 1. Unanswered Questions About Intramolecular Signaling in Immunoglobulins.
While numerous studies have found conformational changes and functional effects consistent with the allosteric model [2–12], many unresolved questions remain. First, do the types and magnitudes of conformational changes following Ag binding depend on VH, CH, VL, or CL usage, and are there any discernable patterns? It is possible that certain germline V genes and certain Ab isotypes determine the extent of intramolecular signaling that occurs on Ag binding. Second, can the specific Ag influence allosteric effects that occur on the Ab? Third, do the downstream signaling pathways differ between the associative model and the allosteric model? This is conceivable if, for example, conformational changes increase or decrease affinity for specific FcR subtypes. Fourth, if the associative and allosteric mechanisms act in concert, can allosteric effects positively or negatively modulate receptor activation by aggregated Abs? Fifth, are there differences in intramolecular signaling in Abs for different recognition pathways (e.g. complement, FcR, B-cell receptors, and microbial Ab-binding proteins)? While none of these questions are trivial to answer, a better understanding of the role allosteric signaling plays in Ab function has the potential to transform our understanding of these complex and fascinating molecules.
Concluding Remarks
Medicine is now in the midst of a revolution in therapy caused by the introduction of dozens of mAbs to treat such diverse conditions as cancer, asthma, and inflammatory diseases. Understanding Ab structural constraints that allow information transfer from V to C and vice versa could find immediate clinical applications. For example, it may be desirable for some Abs to monovalent Ags to engage FcR upon Ag binding to increase efficacy. For others, however, avoiding FcR engagement could reduce toxicity. Hence, we believe that there is sufficient information available to revisit the immunoglobulin intramolecular signaling hypothesis and use newer methodologies to determine when and if it occurs and how it relates to the classical FcR crosslinking mechanisms.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Janda A, et al. Ig constant region effects on variable region structure and function. Front Microbiol. 2016;7:22. doi: 10.3389/fmicb.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warner C, et al. The detection of a conformational change in the antibody molecule upon interaction with hapten. Biochem Biophys Res Commun. 1970;38:125–128. doi: 10.1016/0006-291x(70)91093-4. [DOI] [PubMed] [Google Scholar]
- 3.Ashman RF, Metzger H. A search for conformational change on ligand binding in a human M macroglobulin. II. Susceptibility to proteolysis. Immunochemistry. 1971;8:643–656. doi: 10.1016/0019-2791(71)90204-7. [DOI] [PubMed] [Google Scholar]
- 4.Ashman RF, et al. A search for conformational change on ligand binding in a human M macroglobulin. I. Circular dichroism and hydrogen exchange. Immunochemistry. 1971;8:627–641. doi: 10.1016/0019-2791(71)90203-5. [DOI] [PubMed] [Google Scholar]
- 5.Huber R, et al. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976;264:415–420. doi: 10.1038/264415a0. [DOI] [PubMed] [Google Scholar]
- 6.Brown JC, Koshland ME. Evidence for a long-range conformational change induced by antigen binding to IgM antibody. Proc Natl Acad Sci U S A. 1977;74:5682–5686. doi: 10.1073/pnas.74.12.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horgan C, et al. Effect of H chain V region on complement activation by immobilized immune complexes. J Immunol. 1992;149:127–135. [PubMed] [Google Scholar]
- 8.Guddat LW, et al. Intramolecular signaling upon complexation. FASEB J. 1995;9:101–106. doi: 10.1096/fasebj.9.1.7821748. [DOI] [PubMed] [Google Scholar]
- 9.Oda M, et al. Evidence of allosteric conformational changes in the antibody constant region upon antigen binding. Int Immunol. 2003;15:417–426. doi: 10.1093/intimm/dxg036. [DOI] [PubMed] [Google Scholar]
- 10.Piekarska B, et al. The indirect generation of long-distance structural changes in antibodies upon their binding to antigen. Chem Biol Drug Des. 2006;68:276–283. doi: 10.1111/j.1747-0285.2006.00448.x. [DOI] [PubMed] [Google Scholar]
- 11.Sela-Culang I, et al. A systematic comparison of free and bound antibodies reveals binding-related conformational changes. J Immunol. 2012;189:4890–4899. doi: 10.4049/jimmunol.1201493. [DOI] [PubMed] [Google Scholar]
- 12.Abboud N, et al. A requirement for FcγR in antibody-mediated bacterial toxin neutralization. J Exp Med. 2010;207:2395–2405. doi: 10.1084/jem.20100995. [DOI] [PMC free article] [PubMed] [Google Scholar]