Abstract
Transcranial magnetic stimulation (TMS) over the opercular somatosensory region (OP), which includes the secondary somatosensory cortex and the insular cortex, suppresses pain sensation. However, whether transcranial direct current stimulation (tDCS) over the OP has a similar effect on pain sensation remains unknown. We examined whether pain sensation would be suppressed by tDCS over the OP. Our experiment with a triple-blind, sham-controlled, crossover design involved 12 healthy participants. Participants were asked to rate their subjective pain intensity during and after three types of bihemispheric tDCS: right anodal/left cathodal OP tDCS, left anodal/right cathodal OP tDCS (2 mA, 12 min), and sham tDCS (15 s). Pain stimuli were alternately applied to the dorsum of each index finger using intraepidermal electrical stimulation. We observed no significant effect of tDCS over the OP on the perception of experimentally induced pain. Subjective pain intensity did not differ significantly between the three tDCS conditions. The present null results have crucial implications for the selection of optimal stimulation regions and parameters for clinical pain treatment.
Keywords: neurorehabilitation, noninvasive brain stimulation, pain, secondary somatosensory cortex
Introduction
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage. Brain imaging studies have shown that noxious stimulation can activate the opercular somatosensory region (OP), which includes the secondary somatosensory cortex and the insular cortex 1. Patients with brain lesions in the OP show impaired pain sensation 2. Similarly, electrical stimulation mapping during brain surgery has indicated that the OP is a center for pain sensation 3. Thus, the OP is a critical brain area for pain sensation in humans.
Noninvasive brain stimulation methods such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) have been found to alleviate pain in human. Lockwood et al. 4 reported that TMS over the right OP impaired discrimination sensitivity for the intensity of pain stimuli. Moreover, TMS over the right OP reduced chronic visceral pain 5 and increased the pain threshold 6. However, in tDCS studies, the target regions for pain have been limited to the primary motor cortex 7–10, the primary somatosensory cortex 11, and the dorsolateral prefrontal cortex 8. Thus, it remains unknown whether tDCS over the OP has a similar effect with TMS on pain sensation. In the present study, using a triple-blind, sham-controlled design, we investigated whether tDCS over the bilateral OP would suppress pain sensation compared with a sham intervention.
From a clinical perspective, tDCS has some advantages over TMS because the tDCS device is portable, and the procedure is inexpensive, easy to apply, and safe to use in a clinical practice. If the present tDCS protocol targeting the OP shows promise in suppressing the magnitude of subjective pain sensation after noxious stimulation, then this new method may have potential as a clinical pain treatment.
Materials and methods
Participants
Twelve (seven men and five women, mean age 25.1±4.7 years, 11 right-handed and one left-handed) healthy participants took part in the present study. Handedness was assessed in accordance with the Edinburgh inventory 12. Participants had no neurological diseases, psychiatric disorders, chronic pain disorders, or family history of epilepsy. The mean educational level of the participants was at the graduate level (mean academic years; 19.1±4.7 years). Exclusion criteria were acute severe pain within the last 4 weeks, intake of analgesics within the last 24 h, and implanted electrical devices. All experimental procedures were approved by the Human Research Ethics Committee at the National Institute for Physiological Sciences in accordance with the Declaration of Helsinki, and all participants provided informed consent before participating in the study.
Experimental procedure
We used a triple-blind, sham-controlled, crossover trial design to test the analgesic effect of tDCS over the bilateral OP on experimentally induced pain sensations. Participants and two investigators (one experimenter and one statistician) were blinded to the conditions of the study. The participants underwent three tDCS sessions with different stimulation conditions: anodal tDCS over the left OP and cathodal tDCS over the right OP (L-A/R-C OP tDCS), anodal tDCS over the right OP and cathodal tDCS over the left OP (R-A/L-C OP tDCS), and sham tDCS. We hypothesized that, compared with a sham intervention, bihemispheric tDCS over the bilateral OP would suppress pain sensation on the side contralateral to the cathodal tDCS 13–16. To avoid carryover effects of the tDCS, the sessions were spaced at least 1 week apart. The order of the sessions was randomized across participants on the basis of a Latin square design. A schematic of the experimental procedure is shown in Fig. 1. At the beginning of the experiment, we determined the current intensities of different amounts of experimentally induced pain for each participant. As we had hypothesized that tDCS over the OP would suppress subjective pain intensity, we used a numerical rating scale (NRS) to identify current intensities corresponding with five levels of subjective pain intensity. These current intensities were used consistently throughout the experiment. As a baseline measurement, we applied a noxious pain stimulus alternately to the dorsal area of each index finger every 30 s for 5 min. For example, the noxious pain stimulus was applied to the left finger and then to the right finger 30 s later. This was followed by a 3-min rest period before commencing tDCS. Two minutes after tDCS onset, the same noxious pain stimulus was applied alternately to the dorsal area of each index finger every 30 s for 10 min. The tDCS was then discontinued and the participant continued to receive pain stimulation in each hand alternately every 30 s for 10 min. After each experimental period, the participants completed questionnaires measuring their subjective state during tDCS with respect to attention, fatigue, pain, sleepiness, and discomfort. The same instruction and experimental procedure were provided for each tDCS session because subjective feelings of pain can be easily influenced by instruction and/or context 17.
Fig. 1.
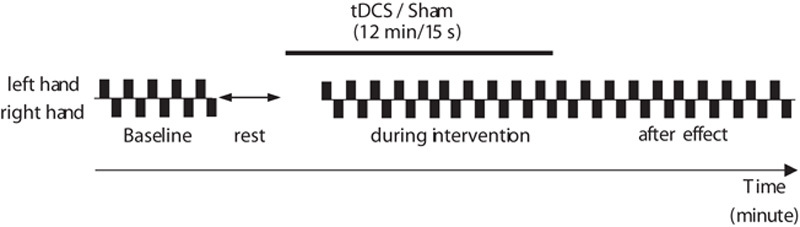
Experimental paradigm. The general experimental procedure. tDCS, transcranial direct current stimulation.
Transcranial direct current stimulation
We used the DC Stimulator Plus (NeuroConn, Ilmenau, Germany) to deliver direct current through two sponge surface electrodes (surface area: 5×5 cm2) soaked with sodium chloride. The centers of the stimulation electrodes were placed over the bilateral OP. The stimulation points were identified by anatomical brain images obtained using a 3T MRI scanner (Verio; Siemens Ltd, Erlangen, Germany). The stimulation area was localized using a frameless stereotaxic navigation system (Brainsight2; Rogue Research Inc., Montreal, Quebec, Canada). The OP region was defined as the cortical area adjacent to the junction of the rostral end of the postcentral gyrus and the sylvian fissure 4. We placed two electrodes over the bilateral OP. In the bihemispheric tDCS conditions, the current was ramped up over the first 15 s of stimulation to a maximum of 2 mA. This intensity was maintained for 690 s and then ramped down over the last 15 s (12 min in total). For the sham stimulation, we used the same procedure, although the constant current was delivered for only the first 15 s. This procedure enabled us to blind the investigator and participants to the experimental condition.
Intraepidermal electrical stimulation
We delivered the noxious pain stimulus using three pushpin-like stainless-steel concentric bipolar needle electrodes 18 and a portable peripheral nerve stimulation device (PNS-7000; Nihon Koden, Tokyo, Japan). The electrode consisted of an outer ring with a small diameter of 1.3 mm and an inner needle that protruded by 0.02 mm from the outer ring. The inner needle acted as the cathode and the outer ring acted as the anode. The electric pulse was a triangular wave with a rise and fall time of 0.5 ms, and the pulse train comprised 10 pulses with an interpulse interval 10 ms.
Pain measurement
We evaluated the magnitude of subjective pain sensation using the NRS, which is used widely in tDCS studies of pain 9–11. Participants were asked to rate their subjective pain intensity every 5 min (from noxious pain stimulus onset to offset) by verbal response using a scale that ranged from 0 (no pain) to 10 (worst imaginable pain).
Questionnaires
To assess the subjective state of the participants during tDCS, we asked them to complete a questionnaire that measured their level of attention, fatigue, pain, sleepiness, and discomfort levels at the end of each intervention. The questionnaire had a four-point scale [e.g. attention (1=no distraction; 4=highest distraction)].
Data analysis and statistical analysis
We averaged the mean NRS data for every 5-min period across the participants. The Shapiro–Wilk test indicated that the present data satisfied normality. To compare the baseline measurements, we used a one-way repeated-measures analysis of variance (ANOVA). The mean NRS data were subjected to a three-way repeated-measures ANOVA with three tDCS conditions (R-A/L-C OP tDCS, L-A/R-C OP tDCS, and sham tDCS), both hands (left and right), and four time points (every 5 min from noxious pain stimulus onset to offset) as within-participant factors. Greenhouse–Geisser adjustment for nonsphericity was applied whenever appropriate. The questionnaire data were analyzed using the Friedman test. Statistical significance was set at P value less than 0.05.
Results
All participants completed the three experimental conditions without any side effects. There were no significant differences between baseline measurements [Greenhouse–Geisser, F(2.2, 24.1)=3.07, P>0.05, ηp2=0.22, 1−β=0.56].
Subjective pain intensity
The three-way (tDCS conditions×hands×time) repeated-measures ANOVA showed no significant three-way interactions among tDCS condition, hand, and time point [F(6, 66)=0.926, P=0.482, ηp2=0.078, 1−β=0.34] and no main effects of tDCS condition [F(2, 22)=1.91, P=0.17, ηp2=0.15, 1−β=0.35] or hand [F(1, 11)=0.009, P=0.93, ηp2=0.001, 1−β=0.05]. In contrast, the main effect of time was significant [Greenhouse–Geisser, F(1.2, 13.19)=19.67, P<0.05, ηp2=0.641, 1−β=0.99] (Fig. 2a and b). We also found a significant interaction between hands and time [F(3, 33)=5.982, P<0.05, ηp2=0.352, 1−β=0.93], but no other significant two-way interactions (i.e. between tDCS condition vs. time, tDCS condition vs. hand). The simple main effect of hand on time was significantly different [right hand F(3, 9)=6.412, P<0.05, ηp2=0.681, 1−β=0.85, left hand F(3, 9)=8.180, P<0.05, ηp2=0.732, 1−β=0.93]. In contrast, the simple main effect of time on hand was not significant (P>0.05), indicating that the subjective pain intensity decreased with time in all three conditions in both hands. These results indicate that subjective pain intensity decreased with repetitive pain stimulation, irrespective of condition, which is known as habituation. Thus, we found no significant differences in pain sensation between the three tDCS conditions.
Fig. 2.
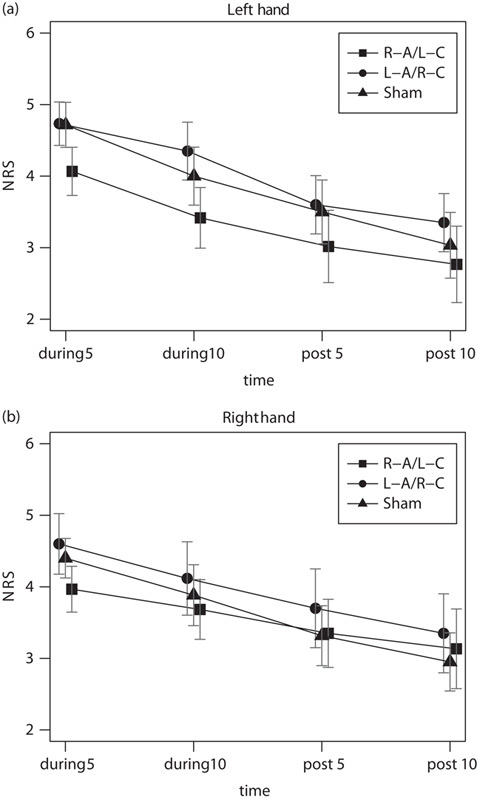
Changes in pain scores over time in the three tDCS conditions. (a) Mean scores on the numerical rating scale (NRS) for the left and (b) right hands are shown separately for the following time periods: pain stimulation onset to 5 min after onset (during 5), 5–10 min after pain stimulation onset (during 10), end of tDCS to 5 min after offset (post 5), and 5–10 min after tDCS offset (post 10). R-A/L-C, right anodal/left cathodal OP tDCS; L-A/R-C, left anodal/right cathodal OP tDCS. Each value includes the mean and SEM. Error bars indicate SEM. NRS, numerical rating scale; OP, opercular somatosensory region; tDCS, transcranial direct current stimulation.
Questionnaire scores
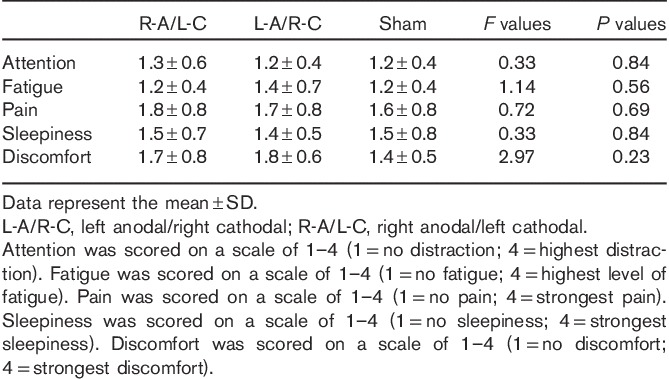
The questionnaire scores showed that the difference in the tDCS conditions did not significantly influence participant attention, fatigue, pain, sleepiness, or discomfort (Table 1).
Table 1.
Questionnaire scores
Discussion
We used a triple-blind, sham-controlled, crossover trial design to test the effects of bihemispheric tDCS over the bilateral OP on pain sensation. Contrary to our expectations, NRS scores did not differ significantly between the three tDCS conditions. Thus, our data do not support the analgesic effect of bihemispheric tDCS over the bilateral OP using the current parameters. We believe that it is important to report the null result to prevent unnecessary application to patients in clinical trials.
We used a triple-blind, sham-controlled, crossover trial design to exclude as many potential confounders as possible (e.g. experimenter expectations in terms of intervention outcome). In addition, we used a questionnaire to assess the subjective state of the participants during tDCS. According to the questionnaire scores, subjective state, such as changes in attention, fatigue, pain, sleepiness, and discomfort, did not differ significantly between tDCS conditions. No significant difference in subjective state indicates that participants did not notice which condition was real or sham. The relatively low scores of subjective pain and discomfort level suggest that participants’ emotional states during tDCS were stable and did not counteract the effect of tDCS. In sum, we believe that the present results include minimum experimental bias and the other confounding variables. To the best of our knowledge, the present study is the first to investigate the effects of tDCS over the OP on pain sensation in healthy adults using a triple-blind procedure. The effects of tDCS on pain sensation are controversial. As well as the present study, several studies have reported negative results previously 19–21.
Although the reasons for the negative results in the present study are unclear, we propose three possible explanations. The first relates to the stimulation intensity of the tDCS. We chose 2 mA tDCS because the OP region is located deeper than other target regions such as the hand primary motor cortex and primary somatosensory cortex. However, even the current intensity of 2 mA might not have been sufficient to modulate the deeper-located OP activity.
The second possibility is that our results were tied to the configuration of the electrode montage. Previous investigations of the analgesic effects of tDCS have used a unihemispheric tDCS montage 7–11, whereas the present study used a bihemispheric tDCS montage. The different configurations of the electrode may have induced different current directions 22. Therefore, it is possible that the present configuration of the electrode montage might induce the electric current into the OP region in an ineffective way to modulate pain sensation.
A third possibility is the large interindividual variability of responses to tDCS 23. Some computational studies have suggested that the effect of tDCS is influenced by individual anatomical differences such as skull thickness, cerebrospinal fluid thickness, and subcutaneous head fat 24,25. Thus, interindividual variability in the effects of tDCS because of individual anatomical differences might have contributed toward our negative results at a group level. In this context, the low statistical power associated with the small sample size may reduce a chance of detecting a true tDCS effect. Future studies with large sample sizes are needed to clarify this point.
Conclusion
We used a triple-blind, crossover, sham-controlled study design to test whether bihemispheric tDCS over the bilateral OP could suppress pain sensation. We did not observe any significant analgesic effects of bihemispheric tDCS over the bilateral OP with the stimulation parameters used in the study. Our findings have implications for the selection of optimal stimulation regions for potential clinical treatment.
Acknowledgements
This study was supported by funding from the Japanese Society for the Promotion of Science (JSPS) to ST (JSPS KAKENHI grant no. 16H03201 and 24680061). We are grateful to Dr Kakigi, Dr Sadato, and Dr Inui for their kind support for this study.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science 1991; 251:1355–1358. [DOI] [PubMed] [Google Scholar]
- 2.Greenspan JD, Lee RR, Lenz FA. Pain sensitivity alterations as a function of lesion location in the parasylvian cortex. Pain 1999; 81:273–282. [DOI] [PubMed] [Google Scholar]
- 3.Mazzola L, Isnard J, Peyron R, Mauguire F. Stimulation of the human cortex and the experience of pain: Wilder Penfield’s observations revisited. Brain 2012; 135:631–640. [DOI] [PubMed] [Google Scholar]
- 4.Lockwood PL, Iannetti GD, Haggard P. Transcranial magnetic stimulation over human secondary somatosensory cortex disrupts perception of pain intensity. Cortex 2013; 49:2201–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fregni F, DaSilva D, Potvin K, Ramos-Estebanez C, Cohen D, Pascual-Leone A, Freedman SD. Treatment of chronic visceral pain with brain stimulation. Ann Neurol 2005; 58:971–972. [DOI] [PubMed] [Google Scholar]
- 6.Valmunen T, Pertovaara A, Taiminen T, Virtanen A, Parkkola R, Jääskeläinen SK. Modulation of facial sensitivity by navigated rTMS in healthy subjects. Pain 2009; 142:149–158. [DOI] [PubMed] [Google Scholar]
- 7.Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 2006; 122:197–209. [DOI] [PubMed] [Google Scholar]
- 8.Boggio PS, Zaghi S, Lopes M, Fregni F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol 2008; 15:1124–1130. [DOI] [PubMed] [Google Scholar]
- 9.Terney D, Bergmann I, Poreisz C, Chaieb L, Boros K, Nitsche MA, et al. Pergolide increases the efficacy of cathodal direct current stimulation to reduce the amplitude of laser-evoked potentials in humans. J Pain Symptom Manage 2008; 36:79–91. [DOI] [PubMed] [Google Scholar]
- 10.Csifcsak G, Antal A, Hillers F, Levold M, Bachmann CG, Happe S, et al. Modulatory effects of transcranial direct current stimulation on laser-evoked potentials. Pain Med 2009; 10:122–132. [DOI] [PubMed] [Google Scholar]
- 11.Antal A, Brepohl N, Poreisz C, Boros K, Csifcsak G, Paulus W. Transcranial direct current stimulation over somatosensory cortex decreases experimentally induced acute pain perception. Clin J Pain 2008; 24:56–63. [DOI] [PubMed] [Google Scholar]
- 12.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9:97–113. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto S, Yamaguchi T, Otaka Y, Kondo K, Tanaka S. Dual-hemisphere transcranial direct current stimulation improves performance in a tactile spatial discrimination task. Clin Neurophysiol 2014; 125:1669–1674. [DOI] [PubMed] [Google Scholar]
- 14.Koyama S, Tanaka S, Tanabe S, Sadato N. Dual-hemisphere transcranial direct current stimulation over primary motor cortex enhances consolidation of a ballistic thumb movement. Neurosci Lett 2015; 588:49–53. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa K, Mochizuki H, Koyama S, Tanaka S, Sadato N, Kakigi R. A transcranial direct current stimulation over the sensorimotor cortex modulates the itch sensation induced by histamine. Clin Neurophysiol 2016; 127:827–832. [DOI] [PubMed] [Google Scholar]
- 16.Fujimoto S, Kon N, Otaka Y, Yamaguchi T, Nakayama T, Kondo K, et al. Transcranial direct current stimulation over the primary and secondary somatosensory cortices transiently improves tactile spatial discrimination in stroke patients. Fronti Neurosci 2016; 10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dar R, Ariely D, Frenk H. The effect of past-injury on pain threshold and tolerance. Pain 1995; 60:189–193. [DOI] [PubMed] [Google Scholar]
- 18.Inui K, Tran TD, Hoshiyama M, Kakigi R. Preferential stimulation of Adelta fibers by intra-epidermal needle electrode in humans. Pain 2002; 96:247–252. [DOI] [PubMed] [Google Scholar]
- 19.Jürgens TP, Schulte A, Klein T, May A. Transcranial direct current stimulation does neither modulate results of a quantitative sensory testing protocol nor ratings of suprathreshold heat stimuli in healthy volunteers. Eur J Pain 2012; 16:1251–1263. [DOI] [PubMed] [Google Scholar]
- 20.Luedtke K, May A, Jürgens TP. No effect of a single session of transcranial direct current stimulation on experimentally induced pain in patients with chronic low back pain – an exploratory study. PLoS One 2012; 7:e48857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ihle K, Rodriguez-Raecke R, Luedtke K, May A. tDCS modulates cortical nociceptive processing but has little to no impact on pain perception. Pain 2014; 155:2080–2087. [DOI] [PubMed] [Google Scholar]
- 22.Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, Bikson M. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol 2013; 591:2563–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiethoff S, Hamada M, Rothwell JC. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul 2014; 7:468–475. [DOI] [PubMed] [Google Scholar]
- 24.Laakso I, Tanaka S, Koyama S, De Santis V, Hirata A. Inter-subject variability in electric fields of motor cortical tDCS. Brain Stimul 2015; 8:906–913. [DOI] [PubMed] [Google Scholar]
- 25.Laakso I, Tanaka S, Mikkonen M, Koyama S, Sadato N, Hirata A. Electric fields of motor and frontal tDCS in a standard brain space: a computer simulation study. Neuroimage 2016; 137:140–151. [DOI] [PubMed] [Google Scholar]


