Abstract
Highly active antiretroviral treatment (HAART) has considerably increased the life expectancy of patients infected with HIV. Coronary artery disease is a leading cause of mortality in patients infected with HIV. This is primarily attributed to their increased survival, HAART-induced metabolic derangements, and to HIV itself. The pathophysiology of atherosclerosis in HIV is both multifactorial and complex – involving direct endothelial injury and dysfunction, hypercoagulability, and a significant contribution from traditional cardiac risk factors. The advent of HAART has since heralded a remarkable improvement in outcomes, but at the expense of other unforeseen issues. It is thus of paramount importance to swiftly recognize and manage acute coronary syndromes in HIV-infected patients to attenuate adverse complications, which should translate into improved clinical outcomes.
Keywords: AIDS, acute coronary syndromes, coronary artery disease, HIV, highly active antiretroviral treatment
Introduction
Highly active antiretroviral treatment (HAART) has considerably increased the life expectancy of patients infected with HIV. Coronary artery disease (CAD) with associated acute coronary syndromes (ACS) is now a leading cause of death in patients with HIV. This is primarily attributed to their increased survival, HAART-induced metabolic derangements, and to HIV itself 1. The pathophysiology of atherosclerosis in HIV is both multifactorial and complex – involving direct endothelial injury and dysfunction, hypercoagulability, and a significant contribution from traditional cardiac risk factors 2,3 (Fig. 1). The advent of HAART has since heralded a remarkable improvement in outcomes, but at the expense of other unforeseen issues. It is thus of paramount importance to swiftly recognize and manage ACS in HIV-infected patients to attenuate adverse complications, which should translate into improved clinical outcomes.
Fig. 1.
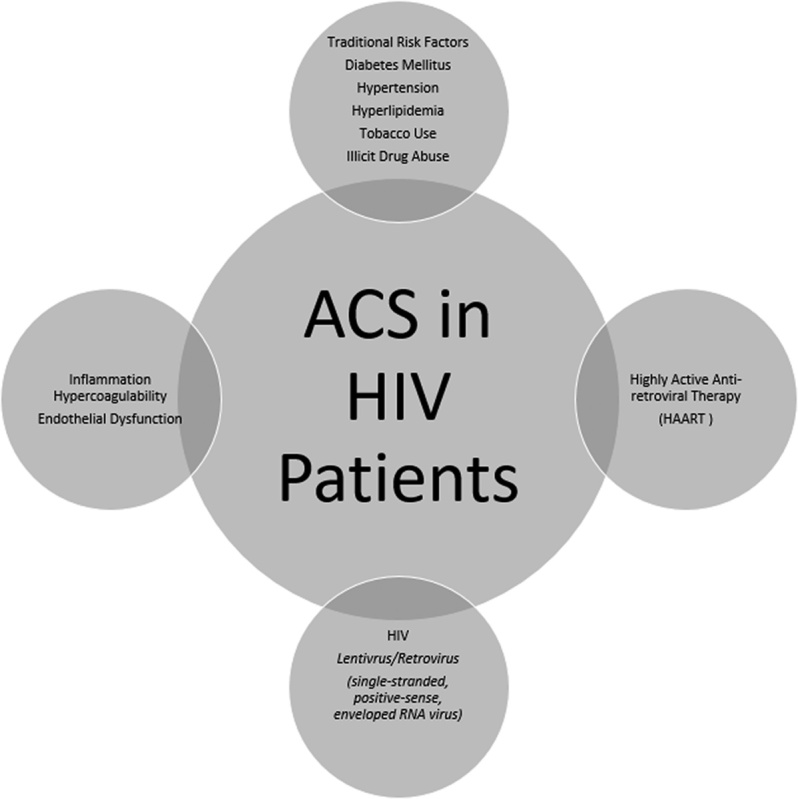
The pathophysiology of ACS in HIV-infected patients is both multifactorial and complex. ACS, acute coronary syndrome.
Epidemiology of acute coronary syndrome in HIV
The advent of HAART has significantly improved the survival of patients infected with HIV and this has resulted in more non-AIDS-related causes of death as opposed to AIDS-related causes of death 4. Of the non-AIDS-related causes of death, Bedimo et al. 4 noted that cardiovascular disease (CVD) accounts for 8–22% of deaths among HIV-infected patients and the percentage appears to be increasing in the aging HIV population. HIV infection portends an increased risk for CAD and ACS compared with the general population 5. Durand et al. 5 found an incidence rate of 3.88 per 1000 patient-years in HIV-positive patients compared with 2.21 per 1000 patient-years in HIV-negative patients.
Pathogenesis and pathophysiology
Traditional risk factors
Overall, HIV-infected patients tend to be hospitalized more frequently with CAD, as well as present with ACS 5. Expectedly, traditional cardiac risk factors are inextricably linked to ACS in these patients as they are for noninfected patients. There is generally a higher prevalence of diabetes mellitus (11.5 vs. 6.6%), hypertension (21.2 vs. 15.9%), and hyperlipidemia (23.3 vs. 17.6%) in HIV-infected patients compared with their uninfected counterparts. Impaired kidney function as reflected by an abnormal glomerular filtration rate of cystatin C also shows a robust association with increased cardiovascular events and mortality 6. HIV-infected patients have a higher rate of illicit substance abuse, which portends worse cardiovascular outcomes 7–12, specifically more so in the younger, male patient subgroup. Severino et al. 7 also determined an increased risk of ACS in HIV-infected patient population, independent of the aforementioned conventional risk factors, suggesting additional mechanistic effects.
Dyslipidemia
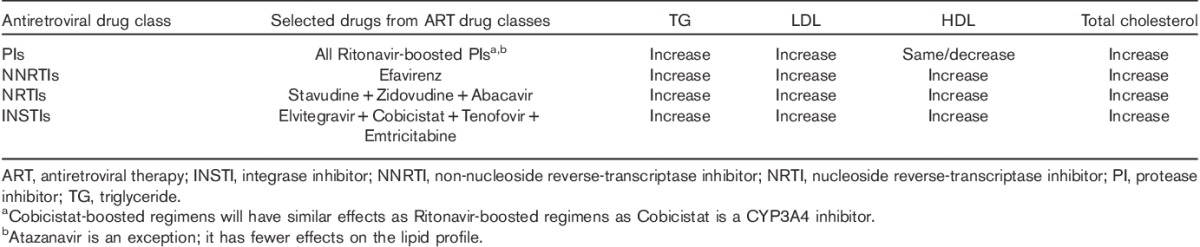
Several autopsy studies have shown evidence of premature CAD in HIV-infected patients, even before HAART initiation 8,9. HIV-infected patients manifest a complex dyslipidemic pattern – reduced total cholesterol, HDL, and apolipoprotein B. In addition, LDL clearance is decreased, which in turn leads to increased serum levels 9. Also, there is a direct correlation between hypertriglyceridemia and viremia. Atherosclerotic lesions in these patients show a mixed histologic pattern with features similar to both traditional CAD and transplant vasculopathy 10. HDL and apolipoprotein A1 may increase following HAART initiation depending on the baseline level of inflammation, which suggests that activation of inflammatory pathways contribute toward HIV-associated changes in HDL 11. Decreased HDL levels can independently identify HIV-infected patients at increased risk of CAD 13. Generally, integrase inhibitors, fusion inhibitors, and C-C chemokine receptor type 5 antagonists have little impact on the lipid profile. Non-nucleoside reverse-transcriptase inhibitors (NNRTIs) and nucleoside reverse-transcriptase inhibitors tend to have more variable effects on the lipid profile on the basis of the agent used (Table 1).
Table 1.
Lipid abnormalities that tend to be observed in HIV-infected patients on selected antiretroviral drugs
Inflammation
Inflammation is associated with endothelial dysfunction in HIV-infected patients. CAD can appear throughout the spectrum of HIV infection, ranging from clinical quiescence to advanced AIDS with opportunistic diseases. A study by Hsue et al. 12 reported increased carotid intima-media thickness and C-reactive protein levels despite HAART and degree of viremia, implying that chronic inflammation may play a pivotal role in premature CAD within this population. Another study with HIV-infected patients showed that elevated interleukin-6 and d-dimer levels are associated with both a higher risk of fatal CVD and death after a nonfatal CVD event 6 (odds ratio 2.8, P=0.03). The results from these studies suggest that these circulating molecules may in part explain this increased risk by contributing to both a pro-inflammatory and prothrombotic milieu. This inherent risk is almost equivalent to that of insulin-resistant obesity with the attendant sequelae of the accelerated diabetogenesis and elevated cardiac risk, even in treated HIV infection 14. In addition, a virtual histology-intravascular ultrasound analysis 7 showed a high prevalence of unstable plaque morphology rich in necrotic tissue. These plaques are in contrast to traditional CAD plaques as they tend to be less calcific, more necrotic, and with a thicker fibrous cap.
Hypercoagulability
HIV-associated thrombogenicity arises from both the plasmatic and the cellular coagulation pathways. HIV replication in itself can lead to a prothrombotic state, chiefly attributed to the upregulation of the extrinsic tissue factor coagulation pathway. There are also changes in serum factor VIII and antithrombin levels that suggest underlying hepatocyte dysfunction 15. Moreover, a high incidence of thromboembolic events and intraluminal demonstration of fresh thrombus has been reported, probably related to a highly thrombophilic state 16. A positive correlation has been found between thrombocytopenia and advancing HIV disease with sequelae of opportunistic diseases 17,18. There is increased platelet reactivity in HIV-infected patients that may increase the risk for thrombosis; however, the contribution of platelets in HIV-related procoagulant activity requires further study 19,20. With respect to HAART, interrupting therapy increases the risk of thrombocytopenia, but reinitiation typically reverses it.
Highly active antiretroviral treatment therapy and vascular disease
The literature is replete with conflicting studies suggesting a possible link between ACS and HAART. Several studies confirmed a statistically significant association 21, whereas others refuted this relationship 4,22. These studies have considerable heterogeneity with respect to the study design, population, and endpoint definitions 23. A pivotal study, Data Collection on Adverse Events of Anti-HIV Drugs study 23,24, indicated that the relative risk of myocardial infarction per year of protease inhibitor (PI) exposure was 1.16 (95% confidence interval: 1.10–1.23) adjusting for several parameters. In contrast, a recent study reported that HIV-infected patients who presented with an ACS had significantly lower viral loads and a higher cluster of differentiation 4 counts 22–24 than patients with HIV/AIDS-related cardiomyopathy, suggesting that HIV infection is not principally implicated in CAD. The Strategies for Management of Antiretroviral Therapy trial showed that the rate of major cardiovascular events was higher if HAART was interrupted, compared with continuous treatment, with a hazard ratio of 1.57 (95% confidence interval: 1.0–2.46, P=0.05) 25. This association between treatment interruption and coronary events does not appear to be related to the level of viremia 13,25. Moreover, treatment interruption may increase the risk of mortality, evidenced by increased inflammatory markers of interleukin-6 and d-dimer 26. Interruption does not favorably impact a patient’s lipid panel, with little or no effect on the total/HDL cholesterol ratio, LDL, and HDL. Lowering of lipid parameters after HAART interruption was not associated with a class of ART and may be linked to increased viral replication, inflammation, and coagulation 27.
HIV and vascular disease
As mentioned above, several studies suggest that HIV-infected patients are exposed to an increased risk of premature CAD, whereas others suggest differently. A recent meta-analysis 28 of 11 studies including more than 2000 HIV-infected patients presenting with ACS showed that the most common presentation was ST-segment elevation myocardial infarction 29. Coronary anatomy seems to be variable, with some studies showing a higher prevalence of single-vessel disease and others showing a higher prevalence of two-vessel and three-vessel disease than noninfected control participants. Traditional factors are the predominant determinants of risk. Higher levels of N-terminal prohormone of brain natriuretic peptide are associated with an increased risk of CVD in HIV-infected patients even after considering established CAD risk factors 30. ECG evidence of asymptomatic ischemic heart disease (IHD) was common and more so than a history of symptomatic IHD. No clear association was noted between HAART type or duration and asymptomatic IHD 31. It is unknown whether HIV-infected patients have a higher frequency of atypical presentations such as silent ischemia that can be seen in other chronic diseases, for example, diabetes mellitus and chronic kidney disease.
Management
Coronary artery disease and acute coronary syndromes
The early detection and treatment of comorbidities and modifiable risk factors through lifestyle changes such as smoking cessation, dietary changes, and exercise is likely to have a significant impact on cardiovascular risk in this population. Because HIV infection by itself and HAART likely increase the risk of plaque rupture and atherothrombosis 2,32, routine primary and secondary prevention should be considered in HIV-infected patients. However, as reported in some studies 2, LDL goals are less frequently achieved in HIV-infected patients during follow-up. Within the current armamentarium of cardiovascular medications and HAART, there are other important issues to consider when treating ACS in an HIV-infected patient (Fig. 2). The management of ACS in HIV patients is similar to its management in non-HIV patients (Table 2). This suggests that the coronary risk of HIV-infected patients is not fully addressed by conventional secondary prevention measures and that more aggressive preventive measures and/or specifically targeted treatments may be required to attenuate this risk 2. In attaining anti-ischemic effects, potent antithrombotic therapies can have devastating and catastrophic bleeding events in patients with advanced HIV/AIDS and opportunistic infections. These patients often have coagulopathies and thrombocytopenia amidst other intracranial and gastrointestinal pathology that make them susceptible to severe bleeding. Serious adverse events with drug–drug interactions must also be considered as many of these pharmacotherapies share a common pathway of metabolism (Table 2).
Fig. 2.
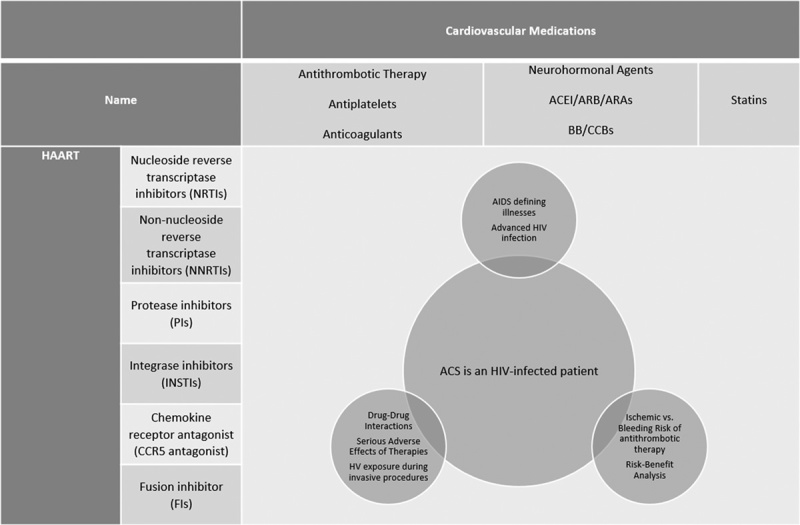
Clinical implications of treating ACS in an HIV-infected patient. ACE-I, angiotensin-converting enzyme inhibitor; ACS, acute coronary syndrome; ARA, aldosterone receptor antagonist; ARB, angiotensin receptor blocker; BB, β-blocker; CCB, calcium channel blocker; HAART, highly active antiretroviral treatment.
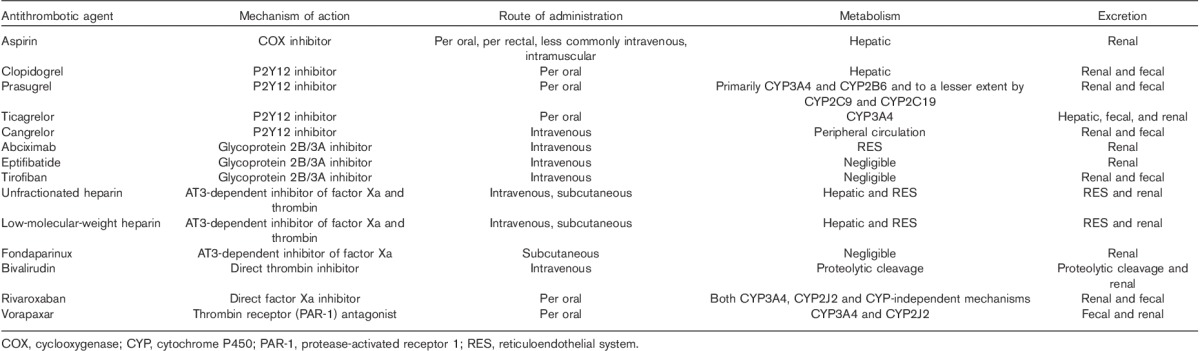
Table 2.
Characteristics of commonly used antithrombotic agents in acute coronary syndrome
Percutaneous coronary intervention and coronary artery bypass grafting
Percutaneous coronary intervention (PCI) in HIV-infected patients has been associated with a high incidence of nonfatal reinfarction, restenosis, and in-stent thrombosis 34. PCI with and without stenting as well as coronary artery bypass grafting seems to be a safe, effective, and feasible option in HIV patients, but it is associated with a higher incidence of repeat revascularization in the long term 2,29,35. Recurrent ACS and urgent PCI were more frequent in HIV-infected patients, with no difference in the rates of major adverse cardiovascular and cerebral events and clinical restenosis at the 1-year follow-up. Another major concern is occupational exposure to HIV during invasive procedures such as PCI and coronary artery bypass grafting and immediate availability of postexposure prophylaxis (PEP). In the event that an exposure occurs, there should be prompt reporting and management according to institutional protocols. This would involve administration of antiretroviral therapy, three or more drugs, ideally within hours of exposure. PEP becomes less effective more than 72 h after exposure. Regimens that are well tolerated and have the least side effects are used and healthcare workers should be counseled on adherence. PEP should be administered for 4 weeks and healthcare workers should have HIV testing performed at baseline, 6, 12 weeks, and 6 months. Testing can be concluded at 4 months if the fourth-generation HIV assay is performed. Counseling should also be a part of PEP treatment protocols 36.
Noninvasive testing
Noninvasive stress testing should be preceded by a prerequisite history, physical examination, 12-lead ECG, and an assessment of pretest probability of CAD 37. Routine evaluation of CAD in patients infected with HIV/AIDS should be guided by the established clinical practice guidelines and appropriateness criteria for test selection used in patients without HIV/AIDS 37,38. These recommendations are largely based on conventional populations and there is a paucity of data with respect to HIV-specific populations that call the performance of these noninvasive testing modalities into question.
Dyslipidemia
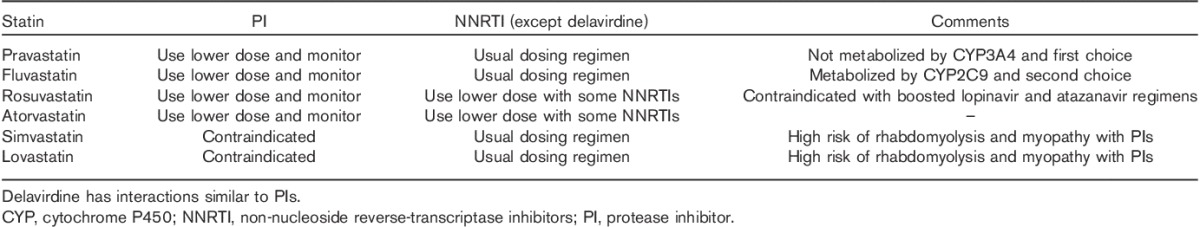
Specific guidelines for the evaluation and management of HAART-related HLD have been developed by the Infectious Disease Society of America, Adult AIDS Clinical Trials Group 39, and the European AIDS Clinical Society. These guidelines recommend estimation of Framingham-predicted 10-year cardiovascular risk 40. Currently, there is no difference in HLD goal treatment between HIV-infected and non-HIV-infected patients. In the choice of specific lipid-lowering therapy, it is critical to consider drug–drug interactions. In general, all PIs and delavirdine, an NNRTI, inhibit CYP3A4. Nevirapine and Efavirenz result in the induction of the enzyme. Therefore, the first-choice agents for lowering LDL are Pravastatin (not metabolized by CYP3A4), with Fluvastatin (metabolized CYP2C9) as the second choice. Rosuvastatin concentrations appear to be increased when used in combination with some NNRTIs; thus, in that setting, 10 mg should be considered the maximum safe dose 41,42. Similarly, Atorvastatin should be used at lower doses in HIV patients. Finally, during PI therapy, Simvastatin and Lovastatin are not recommended because of the high risk of rhabdomyolysis 42,43. Nucleoside reverse-transcriptase inhibitors, integrase inhibitors, and entry inhibitors do not have drug–drug interactions with statins. PIs and NNRTIs can have significant drug–drug interactions with statins and these need to be reviewed before prescribing statins for patients on these regimens 44. Cobicistat-boosted regimens will have similar effects as ritonavir-boosted regimens as cobicistat is a CYP3A4 inhibitor. Lack of data limits the precise estimation of benefits related to anti-inflammatory properties of statins. Clinicians can consider switching HAART in cases of dyslipidemia because of adverse drug effects (Table 3).
Table 3.
Use of statins in patients on antiretroviral therapy 33
Controversial issues and future research
Our understanding of CAD in HIV continues to evolve; however, there are still knowledge gaps and controversial issues that are yet to be resolved. These include noninvasive modalities and their application and performance in patients infected with HIV, clinical utility of novel biomarkers, comparing the clinical presentations of angina and silent ischemia, and determining long-term outcomes to enhance screening and management strategies. These will be addressed in several ongoing studies that will provide valuable information with respect to the interplay of these two disease processes. Firstly, there is a German HIV/HEART study, which will assess the incidence, prevalence, and clinical course of CVD in HIV-infected patients over a 10-year period. A French study comparing the rate of major adverse cardiovascular events in both HIV-positive and HIV-negative patients after an index ACS event over 3-year period is currently underway. There is also a Danish study evaluating the effect of omega-3-acid ethyl esters on lipid parameters and on function and stiffness on vasculature in HIV-infected patients on HAART. Finally, there are two American studies, one of which is utilizing cardiac computed tomography angiography to evaluate CAD in patients on long-term HAART whereas the other is comparing moderate-dose statin therapy with high-dose statin therapy in HIV-infected patients taking HAART who have CAD.
Conclusion
ACS represents a leading cause of mortality in patients infected with HIV. Despite treatment regimens involving novel and contemporary HAART, this remains a challenging issue. In addition, evolving procedural techniques and pharmacology may prove useful in attenuating many of the adverse complications that commonly arise in this population.
Acknowledgements
The authors thank David J. Schneider, MD, FACC of The University of Vermont Medical Center (UVMMC), Burlington, Vermont, USA, and The Cardiovascular Research Institute of Vermont (CVRI-VT), Burlington, Vermont, USA, for his valuable contribution in reviewing the manuscript.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Valmiki K. Seecheran, Stanley L. Giddings, and Naveen A. Seecheran contributed equally to the writing of this article.
References
- 1.Worm SW, de Wit S, Weber R, Sabin CA, Reiss P, El-Sadr W, et al. Diabetes mellitus, preexisting coronary heart disease, and the risk of subsequent coronary heart disease events in patients infected with human immunodeficiency virus: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D study). Circulation 2009; 119:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boccara F, Mary-Krause M, Teiger E, Lang S, Lim P, Wahbi K, et al. Acute coronary syndrome in human immunodeficiency virus-infected patients: characteristics and 1 year prognosis. Eur Heart J 2011; 32:41–50. [DOI] [PubMed] [Google Scholar]
- 3.Matetzky S, Domingo M, Kar S, Noc M, Shah PK, Kaul S, et al. Acute myocardial infarction in human immunodeficiency virus-infected patients. Arch Intern Med 2003; 163:457–460. [DOI] [PubMed] [Google Scholar]
- 4.Bedimo RJ, Westfall AO, Drechsler H, Vidiella G, Tebas P. Abacavir use and risk of acute myocardial infarction and cerebrovascular events in the highly active antiretroviral therapy era. Clin Infect Dis 2011; 53:84–91. [DOI] [PubMed] [Google Scholar]
- 5.Durand M, Madeleine D, Odile S, Jean-Guy B, Jacques L, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case–control study using Québec’s Public Health Insurance Database. J Acquir Immune Defic Syndr 2011; 57:245–253. [DOI] [PubMed] [Google Scholar]
- 6.Nordell AD, McKenna M, Borges AH, Duprez D, Neuhaus J, Neaton JD, et al. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc 2014; 3:e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Severino P, Paolo S, Simone C, Gennaro S, Gabriella D, Vincenzo V, et al. Pathophysiology of acute coronary syndrome in HIV positive patients: insight from virtual histology analysis. J Am Coll Cardiol 2015; 65:A43. [Google Scholar]
- 8.Tabib A, Greenland T, Mercier I, Loire R, Mornex JF. Coronary lesions in young HIV-positive subjects at necropsy. Lancet 1992; 340:730. [DOI] [PubMed] [Google Scholar]
- 9.Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA 2003; 289:2978–2982. [DOI] [PubMed] [Google Scholar]
- 10.Tabib A, Leroux C, Mornex JF, Loire R. Accelerated coronary atherosclerosis and arteriosclerosis in young human-immunodeficiency-virus-positive patients. Coron Artery Dis 2000; 11:41–46. [DOI] [PubMed] [Google Scholar]
- 11.Baker JV, Jacqueline N, Daniel D, Cooper DA, Jennifer H, Lewis K, et al. Inflammation predicts changes in high-density lipoprotein particles and apolipoprotein A1 following initiation of antiretroviral therapy. AIDS 2011; 25:2133–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS 2009; 23:1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips AN, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther 2008; 13:177–187. [DOI] [PubMed] [Google Scholar]
- 14.Samaras K, Gan SK, Peake PW, Carr A, Campbell LV. Proinflammatory markers, insulin sensitivity, and cardiometabolic risk factors in treated HIV infection. Obesity (Silver Spring) 2009; 17:53–59. [DOI] [PubMed] [Google Scholar]
- 15.Baker JV, Brummel-Ziedins K, Neuhaus J, Duprez D, Cummins N, Dalmau D, et al. HIV replication alters the composition of extrinsic pathway coagulation factors and increases thrombin generation. J Am Heart Assoc 2013; 2:e000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sliwa K, Carrington MJ, Becker A, Thienemann F, Ntsekhe M, Stewart S. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J 2011; 33:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blann AD, Seigneur M, Constans J, Pellegrin JL, Conri C. Soluble P-selectin, thrombocytopenia and von Willebrand factor in HIV infected patients. Thromb Haemost 1997; 77:1221–1222. [PubMed] [Google Scholar]
- 18.Seigneur M, Constans J, Blann A, Renard M, Pellegrin JL, Amiral J, et al. Soluble adhesion molecules and endothelial cell damage in HIV infected patients. Thromb Haemost 1997; 77:646–649. [PubMed] [Google Scholar]
- 19.Karmochkine M, Ankri A, Calvez V, Bonmarchant M, Coutellier A, Herson S. Plasma hypercoagulability is correlated to plasma HIV load. Thromb Haemost 1998; 80:208–209. [PubMed] [Google Scholar]
- 20.Zetterberg E, Neuhaus J, Baker JV, Somboonwit C, Llibre JM, Palfreeman A, et al. Platelet count kinetics following interruption of antiretroviral treatment. AIDS 2013; 27:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D:A:D Study Group, Sabin CA, Worm SW, Weber R, Reiss P, El-Sadr W, Dabis F. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet 2008; 371:1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biondi-Zoccai G, D’Ascenzo F, Modena MG. Novel insights on HIV/AIDS and cardiac disease: shedding light on the HAART of darkness. Eur Heart J 2011; 33:813–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribaudo HJ, Benson CA, Zheng Y, Koletar SL, Collier AC, Lok JJ, et al. No risk of myocardial infarction associated with initial antiretroviral treatment containing abacavir: short and long-term results from ACTG A5001/ALLRT. Clin Infect Dis 2011; 52:929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DAD Study Group, Friis-Møller N, Reiss P, Sabin CA, Weber R, Monforte AD, El-Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–1735. [DOI] [PubMed] [Google Scholar]
- 25.Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283–2296. [DOI] [PubMed] [Google Scholar]
- 26.Kuller LH, Tracy R, Belloso W, de Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lampe FC, Duprez DA, Kuller LH, Russell T, James O, Erik S, et al. Changes in lipids and lipoprotein particle concentrations after interruption of antiretroviral therapy. J Acquir Immune Defic Syndr 2010; 54:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Ascenzo F, Cerrato E, Biondi-Zoccai G, Moretti C, Omede P, Sciuto F, et al. Acute coronary syndromes in human immunodeficiency virus patients: a meta-analysis investigating adverse event rates and the role of antiretroviral therapy. Eur Heart J 2011; 33:875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boccara F, Franck B, Murielle MK, Emmanuel T, Sylvie L, Pascal L, et al. 012 Acute coronary syndrome in HIV-infected patients: characteristics and prognosis. Arch Cardiovasc Dis Suppl 2011; 3:4. [Google Scholar]
- 30.Duprez DA, Jacqueline N, Russell T, Kuller LH, Deeks SG, Chloe O, et al. N-terminal-proB-type natriuretic peptide predicts cardiovascular disease events in HIV-infected patients. AIDS 2011; 25:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr A, Grund B, Neuhaus J, El-Sadr WM, Grandits G, Gibert C, et al. Asymptomatic myocardial ischaemia in HIV-infected adults. AIDS 2008; 22:257–267. [DOI] [PubMed] [Google Scholar]
- 32.Oliviero U, Ugo O, Giovanni B, Valentina A, Maria F, Giorgio B, et al. Human immunodeficiency virus per se exerts atherogenic effects. Atherosclerosis 2009; 204:586–589. [DOI] [PubMed] [Google Scholar]
- 33.Coffey S. Interactions between ARVs and statin medications: recommendations for coadministration. Available at: http://hivinsite.ucsf.edu/InSite?page=md-rr-30. [Accessed 18 May 2016].
- 34.Mestres C. Long-term results after cardiac surgery in patients infected with the human immunodeficiency virus type-1 (HIV-1). Eur J Cardiothorac Surg 2003; 23:1007–1016. [DOI] [PubMed] [Google Scholar]
- 35.Boccara F, Ederhy S, Janower S, Benyounes N, Odi G, Cohen A. Clinical characteristics and mid-term prognosis of acute coronary syndrome in HIV-infected patients on antiretroviral therapy. HIV Med 2005; 6:240–244. [DOI] [PubMed] [Google Scholar]
- 36.Kuhar DT, Henderson DK, Struble KA, Heneine W, Thomas V, Cheever LW, et al. US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol 2013; 34:875–892. [DOI] [PubMed] [Google Scholar]
- 37.Gibbons RJ, Balady GJ, Timothy Bricker J, Chaitman BR, Fletcher GF, Froelicher VF, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. J Am Coll Cardiol 2002; 40:1531–1540. [DOI] [PubMed] [Google Scholar]
- 38.Brindis RG, Douglas PS, Hendel RC, Peterson ED, Wolk MJ, Allen JM, et al. ACCF/ASNC appropriateness criteria for single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI): a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group and the American Society of Nuclear Cardiology endorsed by the American Heart Association. J Am Coll Cardiol 2005; 46:1587–1605. [DOI] [PubMed] [Google Scholar]
- 39.Dube MP, Stein JH, Aberg JA, Fichtenbaum CJ, Gerber JG, Tashima KT, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: Recommendations of the HIV Medicine Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis 2003; 37:613–627. [DOI] [PubMed] [Google Scholar]
- 40.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421. [PubMed] [Google Scholar]
- 41.Van der Lee M, Sankatsing R, Schippers E, Vogel M, Fätkenheuer G, van der Ven A, et al. Pharmacokinetics and pharmacodynamics of combined use of lopinavir/ritonavir and rosuvastatin in HIV-infected patients. Antivir Ther 2007; 12:1127–1132. [PubMed] [Google Scholar]
- 42.Busti AJ, Bain AM, Hall RG, Bedimo RG, Leff RD, Claudia M, et al. Effects of atazanavir/ritonavir or fosamprenavir/ritonavir on the pharmacokinetics of rosuvastatin. J Cardiovasc Pharmacol 2008; 51:605–610. [DOI] [PubMed] [Google Scholar]
- 43.Hare CB, Vu MP, Grunfeld C, Lampiris HW. Simvastatin–nelfinavir interaction implicated in rhabdomyolysis and death. Clin Infect Dis 2002; 35:e111–e112. [DOI] [PubMed] [Google Scholar]
- 44.Feeney ER. HIV and HAART-associated dyslipidemia. Open Cardiovasc Med J 2011; 5:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]


