Abstract
Objectives
To evaluate the reliability and factorial validity of the four-item Morisky Green Levine Medication Adherence Scale (MGLS) among Atherosclerosis Risk in Communities (ARIC) Study participants.
Methods
We used cross-sectional data from ARIC Study Visit 5 to assess the measurement properties of MGLS. We measured the internal consistency using Cronbach’s Alpha, where α>.70 is considered reliable for group-level measurement, the response frequency, and inter-item correlation. Factor analysis of MGLS and five other adherence items in the survey was conducted using a polychoric correlation matrix to examine the dimensionality that underlies the MGLS. A vanishing tetrad test (VTT) was conducted to assess conformity with an effect indicator model.
Results
Among ARIC Visit 5 participants, 6,261 (96%) responded to the MGLS and other questions related to medication adherence in the survey (mean age 76±5, 59% women). The Cronbach’s alpha for MGLS was 0.47. The inter-item correlations ranged from 0.11 to 0.26. In the factor analysis of medication adherence survey questions a three-factor solution was used. One factor captured the extent of nonadherence while other factors focused on reasons for nonadherence. The MGLS items spread out across the factors that reflect the extent as well as the reasons of non-adherence. The VTT results indicated that MGLS consists of items other than effect indicators (p<0.0001).
Conclusions
The low reliability together with the factor analyses findings implies that MGLS may reflect causes as well as the extent of medication adherence. The findings suggest that the MGLS as presently used, lacks consistency in an elderly population.
Keywords: Medication adherence, Effect indicator, Causal indicator, Reliability, Factorial validity
Introduction
Medication adherence, which is defined as “the extent to which a person’s behavior with regard to taking medication corresponds with agreed recommendations from a health care provider” [1] has been the subject of extensive research for the past several decades. [2,3] The World Health Organization (WHO) estimated that approximately 50% of patients with chronic diseases are adherent to their medications in developed countries. [4] Studies have shown that medication non-adherence is associated with worse clinical outcomes, [5,6] including higher hospitalization rates, [7] greater risk of preventable medication-related hospital admissions, [8] and a higher mortality rate. [9]
Marcum and colleagues suggested that medication non-adherence should be viewed as a diagnosable and treatable medical condition. [10] In this framework, treating medication non-adherence requires establishing an accurate assessment of adherence and determining the causal factors associated with adherence. However, medication non-adherence remains unidentified in a significant portion of patients across various healthcare settings. [10]
Different methods of measuring non-adherence exist that are applicable across various clinical and research settings. Direct measures such as tracing the parent medication or its metabolites in the body are accurate but are associated with higher cost and less applicability in research and clinical practice settings. Indirect methods focus on measuring medication adherence as a behavior and include approaches ranging from electronic medication monitoring and pill count to pharmacy refills and self-report measures. [11] Several advantages are associated with self-report measures of medication adherence, including the ease of implementation in different settings and the ability to identify reasons underlying the medication adherence behavior. Hence, self-report measures are commonly used to measure medication adherence. However, self-report measures of medication adherence have been widely criticized for their less than desirable measurement characteristics [12] and tendency to overestimate medication adherence by 10–20% compared to other methods. [13,14,15,16]
One of the most commonly used self-reported measure of medication non-adherence is the four-item Morisky Green Levine Medication Adherence Scale (MGLS). [17,18,19] Although MGLS is widely used for measuring the extent of medication non-adherence, Voils and colleagues argue that MGLS contains the items that identify respondents’ reasons for non-adherence. [11] Therefore, conceptually MGLS cannot be appropriately classified as a measure of extent of medication non-adherence (i.e., an effect indicator model) and more appropriately falls into the category of measures of causes of medication non-adherence (i.e., a causal indicator model). [11] These two models have divergent implications for measure validation. [11] High inter-item correlation and internal consistency are required for a measure in an effect indicator model, while in a causal indicator model the items ideally capture different and perhaps unrelated aspects of the construct. Therefore, high internal consistency and inter-item correlations are not expected for causal indicator models. [11] Since MGLS has been commonly used as an effect indicator model, we evaluate MGLS within this framework to see if practical data supports the use of MGLS as a measure of the extent of medication adherence. This study aims to evaluate the reliability and factorial validity of the MGLS as a measure of medication non-adherence in an elderly cohort from four US communities.
Methods
Data
We use the cross-sectional Visit 5 data from the Atherosclerosis Risk in Communities (ARIC) Study. The ARIC Study is a prospective cohort study investigating the etiology of atherosclerotic disease in a middle-aged, predominantly biracial population. Details regarding the ARIC study have been previously published. [20] Briefly, in 1987–1989, 15,792 men and women aged 45–64 attended the baseline clinic examination (Visit 1). Participants were invited to three subsequent visits at approximately three-year intervals followed by Visit 5 in 2011–2013. Among 10,749 participants who were eligible for Visit 5, 706 participants died before Visit 5 was completed and 3,505 people refused or were unable to participate [21]. Among the remaining 6,538 participants, the data for 6,261 participants (96%) who completed all questions regarding medication adherence were analyzed. Participants who reported no medication consumption at Visit 5 were also excluded from the analyses. Racial composition is highly linked to geographic site in the ARIC cohort, with only African-Americans enrolled in Jackson, and extremely few African-Americans in the Minneapolis and Washington county sites or participants of other race at any site. We therefore included only the majority race at each site except for Forsyth County (which has a mix of whites and African-Americans).
Measures
In this study, we used the four-item Morisky Medication Adherence Scale (MGLS), which includes four questions with yes/no response options. The MGLS results in a score ranging from 0 to 4, and the developers suggested three levels of medication adherence based on this score: high, medium and low adherence with 0, 1–2, and 3–4 points, respectively. [19] A dichotomous definition of adherence based on MGLS is also commonly used with 0 points indicating perfect adherence and 1+ points indicating some level of non-adherence. [11]
In addition to MGLS, other measures of medication adherence used in the Visit 5 survey include: three questions asking about the frequency of non-adherence with a four-week recall period; a single question directly asking the percent of the time that a participant was fully adherent in the past four weeks; and for cohort participants who reported less than 100% adherence in the past four weeks, self-reported reasons for medication non-adherence (including 11 potential reasons for non-adherence) were asked (Table 1).
Table 1.
Descriptive statistics of questions indicating medication adherence in the ARIC Visit-5 exam survey (n=6,261)
| Measures
| |||||
|---|---|---|---|---|---|
| MGLS | Mean (SD)/Percent | ||||
| 1. | Ever forget to take medicines | 33.25% | |||
| 2. | Ever careless about taking medicines | 9.94% | |||
| 3. | Stop taking medicines when feeling better | 4.89% | |||
| 4. | Stop taking medicines if you feel worse | 4.86% | |||
| Frequency of non-adherence in past 4 weeks | Never | Rarely | Sometimes | Often | |
| 5. | Frequency of stretching medicines in the past 4 weeks | 94.30% | 3.31% | 1.90% | 0.49% |
| 6. | Frequency of running out of medicines in the past 4 weeks | 90.09% | 7.45% | 2.28% | 0.18% |
| 7. | Frequency of missed taking of medicines in the past 4 weeks | 63.35% | 29.02% | 6.67% | 0. 96% |
| Direct measure of the extent of adherence | |||||
| 8. | Percent of the time you have taken your medications in the past 4 weeks | 96.8 (9.5) | |||
| Self-reported reasons for medication non-adherence (n=2116) | |||||
| 9. | Medication not taken on time because could not afford | 1.47% | |||
| 10. | Medication not taken on time because lack of transportation | 0.28% | |||
| 11. | Medication not taken on time because poor memory* | 72.83% | |||
| 12. | Medication not taken on time because ran out of it* | 7.37% | |||
| 13. | Medication not taken on time because confusing directions | 0.33% | |||
| 14. | Medication not taken on time because felt better* | 2.88% | |||
| 15. | Medication not taken on time because felt worse* | 1.32% | |||
| 16. | Medication not taken on time because too complicated | 0.33% | |||
| 17. | Medication not taken on time because scared of side effects | 1.75% | |||
| 18. | Medication not taken on time because no belief in effectiveness | 1.37% | |||
| 19. | Medication not taken on time because other reason | 19.38% | |||
MGLS: Morisky Green Levine Medication Adherence Scale.
Four sets of items found to be redundant based on both wording and factor analysis results: Items 11 and 1, 12 and 6, 14 and 3, and 15 and 4.
Statistical Analysis
We assess the psychometric properties (reliability, and congruent and factorial validity) of the MGLS as an effect indicator model for measuring the extent of medication non-adherence. All the analyses were conducted using STATA 13. College Station, TX.
Reliability (internal consistency)
Reliability refers to the degree to which an instrument is free from measurement error. Internal consistency measures whether items that propose to measure the same general construct produce similar scores. In this study we conducted item analyses including response frequencies and Spearman’s inter-item correlations to assess the reliability of MGLS. Internal consistency was assessed using Cronbach’s Coefficient Alpha, where α>.70 is considered reliable for group-level measurement. [22].
Assessing the validity of MGLS
The magnitude of association between the MGLS dichotomous score and a single-item measure directly reflecting the extent of medication adherence (i.e. adherent 100% of the time vs. any level of non-adherence) was estimated to test the scale congruent validity as a measure of the extent of adherence. We selected the MGLS dichotomous score primarily because of the distribution of the scores i.e., only 2.3% of study participants had 3 or 4 points indicating low adherence.
Factor analysis was used to examine the dimensionality of the MGLS. The standard methods of performing factor analysis use a Pearson correlations matrix to estimate the factor patterns and hence assume that the variables are on a normal interval scale. Since MGLS items have a binary response option, we conducted a factor analysis using a polychoric correlation matrix to adjust for the binary nature of the data. The polychoric correlation is a measure of association for ordinal variables, which assumes an underlying joint continuous distribution. [23]
We also conducted a factor analysis of all the questions related to medication adherence in the survey except for the redundant items (e.g. medication not taken on time because poor memory) and rare items (e.g. medication not taken on time because too complicated) to identify how the number of underlying factors and MGLS items factor loading compare to other items in the survey (Table 1). To obtain a simple factor structure and improve the interpretability of the factors, unrotated solutions were compared to the rotated factor solutions. Oblique rotation (promax rotation) was used to account for the potential correlation of factors and improve the interpretability of the results. In order for an item to be considered a relevant contributor to a factor, the factor loading associated with that item (i.e. the correlation of an item with a factor) has to be > 0.40. [19]
We conducted the vanishing tetrad test (VTT) to examine whether the MGLS conforms to an effect indicator model or a causal indicator model. The VTT compares the number of vanishing tetrads among items under different assumptions about the items being effect or causal indicators. [24] The VTT directly determines whether a possible indicator item should be treated as a cause or an effect of the latent construct of interest.
Results
Cohort Characteristics
The total number of observations in the study was 6,261. Mean age of the participants was 76 years (standard deviation 5.25 years) and 59% of participants were female. This study included Caucasian and African American races with 77% and 23%, respectively. Among the participants 23% resided in Forsyth County, North Carolina, 21% in Jackson, Mississippi, 29% in Minneapolis, Minnesota and 27% in Washington, Maryland. Among participants, 85% had high school or above education and 66% were married. The participants took on average 9.4 (standard deviation 5) different medications including prescription and over-the-counter medications as well as supplements (Table 2). The most commonly taken prescription medications in the sample at Visit 5 were antihypertensive drugs (68.5%), drugs for high blood cholesterol (54.2%), blood thinning drugs (29.9%), and medications for high blood sugar or diabetes (21.7%).
Table 2.
Demographic characteristics of the cohort (n=6,261)
| Mean (SD) or Percent | |
|---|---|
| Age in years | 76 (5) |
| Sex, male | 41% |
| Education less than high school | 15% |
| Married | 66% |
| ARIC site and race | |
| Forsyth, Caucasian | 21% |
| Forsyth, African American | 2% |
| Jackson, African American | 21% |
| Minneapolis, Caucasian | 29% |
| Washington, Caucasian | 27% |
| No. of medications | 9.4 (4.9) |
On the basis of the MGLS, 60.36% of the participants indicated full medication adherence while 39.64% reported some level of non-adherence (37.2% intermediate adherence and 2.43% low adherence). The majority of participants (66.2%) indicated that they took their medications 100% of the time during the past four weeks (Table 1).
Measures of reliability
The Spearman inter-item correlation ranged from 0.11 to 0.26 (0.18 on average) that indicates a low association among items (Table 3). In the study sample the standardized Cronbach’s coefficient alpha for MGLS was 0.47 (Table 4).
Table 3.
The Spearman inter-item correlation matrix of the MGLS
| Morisky items | Forgetfulness | Carelessness | Stop when well | Stop when worse |
|---|---|---|---|---|
| Forgetfulness | 1.0000 | |||
| Carelessness | 0.2616 | 1.0000 | ||
| Stop when well | 0.1093 | 0.2076 | 1.0000 | |
| Stop when worse | 0.1068 | 0.1486 | 0.2457 | 1.0000 |
MGLS: Morisky Green Levine Medication Adherence Scale.
Table 4.
Reliability of MGLS using standardized coefficient alpha (n=6,261)
| Item | Item-rest correlation* | Alpha |
|---|---|---|
| Ever forget to take medicines | 0.23 | 0.43 |
| Ever careless about taking medicines | 0.31 | 0.35 |
| Stop taking medicines when feeling better | 0.28 | 0.38 |
| Stop taking medicines if you feel worse | 0.25 | 0.42 |
| Test Scale | 0.47 |
MGLS: Morisky Green Levine Medication Adherence Scale.
Calculated using the correlation between each item and the overall score of the measure excluding that item.
Congruent and Factorial Validity
A binary measure of medication non-adherence derived from MGLS and a single-item indicating the extent of medication adherence (100% of the time medications were taken in the past 4 weeks vs. less than 100%) results in the Spearman correlation of 0.6 (results not shown).
Because the MGLS only includes four items, it is difficult to identify more than one factor when recommendations suggest three or more items are needed to form a factor. Factor analysis of the MGLS suggests one large factor exists accounting for 45% of the total variation in the scale. The results of the second exploratory factor analysis, which include the MGLS items as well as all other relevant items that assessed medication adherence, are presented in Table 5. Based on the Scree Plot presented in Figure 1, a dominant factor exists with the possibility of two minor factors. Relevant items (i.e. items with factor loading > 0.4) for the first factor include “ever forget to take medicines” and “ever careless about taking medicines” from the MGLS and “frequency of missed taking medicine” and “percent of the time medication was taken” from the other adherence items in the survey. This factor reflects the extent of medication adherence but includes forgetfulness, the most prevalent reason for non-adherence. The second factor includes two relevant items from MGLS: “stop taking medicines when feeling better” and “stop taking medicines if you feel worse”. Two relevant items that load on the third factor include “frequency of running out of medicines” and “frequency of stretching medications”. A sensitivity analysis where the redundant items (Table 1) were included in the factor analysis indicated that the redundant items loaded on the same factors as their corresponding items that were kept in the analysis.
Table 5.
Polychoric factor analysis of MGLS and other items indicating the extent of adherence (n=6,261)
| Questionnaire item | Factor1 | Factor2 | Factor3 | Uniqueness |
|---|---|---|---|---|
| Ever forget to take medicines | 0.91* | −0.10 | −0.02 | 0.30 |
| Ever careless about taking medicines | 0.40* | 0.38 | −0.02 | 0.52 |
| Stop taking medicines when feeling better | −0.05 | 0.75* | 0.05 | 0.45 |
| Stop taking medicines if you feel worse | −0.05 | 0.67* | 0.01 | 0.58 |
| Frequency of stretching medicines in past 4 weeks | 0.06 | 0.08 | 0.62* | 0.51 |
| Frequency of running out of medicines in past 4 weeks | 0.07 | −0.01 | 0.64* | 0.54 |
| Frequency of missed taking of medicines in past 4 weeks | 0.84* | 0.01 | 0.10 | 0.18 |
| Percent of the time medication was taken in past 4 weeks | −0.40* | −0.16 | 0.03 | 0.75 |
MGLS: Morisky Green Levine Medication Adherence Scale.
Factor loading greater than 0.4.
Figure 1.
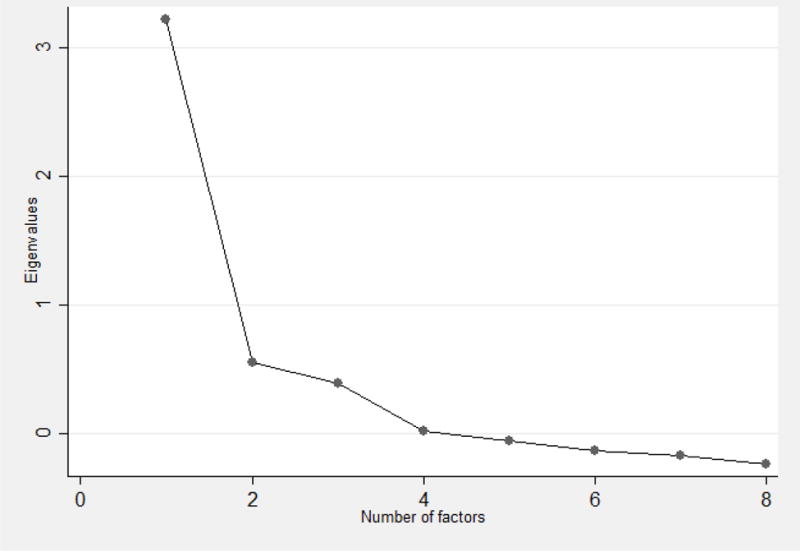
Scree plot indicating the Eigenvalues after factor analysis of adherence-related questions in the survey
Vanishing Tetrad Test
Running VTT on MGLS results in two non-redundant vanishing tetrads (NRVTs). The associated p-value was highly significant (p<0.0001), indicating poor model fit. The finding suggests that one or more of the two NRVTs do not vanish as expected assuming the effect indicator structure of the model. Therefore, the all effect indicator model assumption for MGLS is not consistent with the data.
Discussion
The ARIC cohort provides a large sample of MGLS responses in the elderly population that allows for assessment of psychometric properties of MGLS. This analysis can therefore inform our understanding of the MGLS as a measure of medication adherence. The MGLS has the advantage of being a brief measure of medication adherence that is widely used both in research and medical practice capacities. [19]
Numerous studies have reported the internal consistency reliability of the MGLS, showing mixed results ranging from low (0.32) to high (0.86) values of Cronbach’s alpha. However, the majority of studies including the original MGLS study reported MGLS’ Cronbach’s alpha reliability to be below the acceptable value of 0.7 for group level measurement. [25,26,27,28,29,30,31,32] Our findings also suggest that the Morisky scale, as currently used, lacks consistency when used in an elderly population. In particular, MGLS as a single measure of medication adherence did not show an acceptable level of reliability (alpha=0.47, compared to 0.7 and 0.9 as the standard level for group- and individual-level measurement) [22].
As with the findings of internal consistency estimations of the MGLS, several studies examined the dimentioanlity of the scale using principal components analysis. [30] The majority of these studies including the original MGLS paper suggested a single factor solution, [18,29,33,34] while Toll and colleagues principal component analysis found a two-factor solution: (1) unintentional nonadherence; (2) purposeful nonadherence” [30] In our study, when combined with other items on non-adherence, the MGLS items loaded on different meaningful factors. There appears to be a dominant factor that captures extent of non-adherence. This factor included the item of frequency of missed medicines and forgetting medicines, which is the most prevalent reason for people missing medications; thus the forgetting item is correlated with the extent item. More minor factors included reasons such as not taking medicines due to health reasons (feeling better or feeling worse) and stretching or running out of medication. We also note low correlations among the items in MGLS (correlations range from 0.11 to 0.26). In addition, the results of the VTT indicated that an effect indicator model for the MGLS in not consistent with the data. Our results appear consistent with the findings of Voils et al. 2011, suggesting the need to differentiate extent versus reasons for non-adherence. [11]
In 2003, the World Health Organization claimed that improved adherence interventions might have far greater impact on population health than any improvement in specific medical treatments. [23] Design and delivery of effective adherence interventions require valid and reliable information about individuals’ medication-taking behavior across clinical and research settings. [11] Although measures with lower administrative and respondent burden are easier to implement and therefore preferred [35] the selected measure should reflect the type of information that is required to modify the medication-taking behavior (e.g. the extent of or reasons for non-adherence). Many researchers have tried to measure self-reported medication non-adherence, which has resulted in several self-report instruments. [36] Some measures contain only effect indicators or only causal indicators, and some measures such as MGLS contain a combination of effect and causal indicators items without being able to identify the unique information provided by each. [11] Given the importance and uniqueness of such information, it is imperative for a medication adherence self-report instrument to reliably capture both the extent and reasons of non-adherence. To our knowledge, the Voils’ scale is the only medication adherence questionnaire that purposively measures both extent and reasons for medication adherence. [36]
In 2008, the eight-item Morisky Medication Adherence Scale (MMAS-8) was developed from the original four-item MGLS. [37] The MMAS-8 identifies more reasons for medication nonadherence and has better internal consistency with Cronbach’s alpha value of 0.83. [31, 36] Nevertheless, like the MGLS, MMAS-8 has disadvantages such as capturing only a few reasons or factors associated with non-adherence, thus providing limited information to develop interventions targeting non-adherence. [11,17,31] The MGLS is sometimes viewed and used as a screener for nonadherence, where any “yes” response is taken as a positive screen for nonadherence. MGLS can be used as a screening and monitoring tools to identify those patients who might have medication adherence problems. [31]
This study is subject to a few limitations. The sample included only those participants who were willing to participate in Visit 5 and responded to the medication adherence questions; these participants may be healthier and thus more adherent than the general population. Therefore, the full range of medication adherence that is theoretically possible from the MGLS may not have been obtained in the ARIC study. In addition, the cross-sectional nature of data does not provide the opportunity to assess other measurement properties such as test-retest reliability, known group validity and responsiveness of the measure to changes in medication adherence over time. The MGLS was asked in ARIC with regard to all medications that the cohort participants were taking. Asking about all medications versus those for a specific condition may also explain some of the differences in the distribution of the reasons for non-adherence seen in this study, which can potentially affect the generalizability of the results. Similarly, the exclusion of the redundant and rarely endorsed items can limit the generalizability of the results. In addition, the list of reasons for non-adherence included in the ARIC study medication adherence questionnaire is not comprehensive and excludes some of the commonly reported reasons seen in other studies, for example, lack of insurance.
Conclusion
This assessment showed that the MGLS lacks consistency when used in an elderly population. These findings support the Voils recommendation that medication adherence should be conceptualized as both the extent of adherence and reason for adherence. [11] Beyond psychometric reasons, distinguishing between these two factors has clinical relevance. The measure of extent provides a metric for distinguishing among different levels of medication adherence and allows clinicians and researchers to determine if interventions (e.g. pill boxes) may improve extent of adherence. The reasons are descriptive in nature (e.g. whether the patient is forgetful, does not like the side effects of treatment, or cannot afford the medication) and provide valuable insights into what may cause suboptimal adherence. Knowing the extent of and the reasons for non-adherence may help clinicians and researchers identify appropriate interventions for the right context.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions.
References
- 1.World Health Organization; Sabete E, editor. Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003. [Google Scholar]
- 2.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218–24. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 3.Vermeire E, Hearnshaw H, van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331–42. doi: 10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization; Sabete E, editor. Adherence to Long-Term Therapies: Policy for Action Meeting Report. Geneva: World Health Organization; 2001. [Google Scholar]
- 5.Rozenfeld Y, Hunt JS, Plauschinat C, Wong KS. Oral antidiabetic medication adherence and glycemic control in managed care. Am J Manag Care. 2008;14:71–5. [PubMed] [Google Scholar]
- 6.Schectman J, Nadkarni M, Voss J. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care. 2002;25:1015–21. doi: 10.2337/diacare.25.6.1015. [DOI] [PubMed] [Google Scholar]
- 7.Lau D, Nau DP. Oral antihyperglycemic medication non-adherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care. 2004;27:2149–53. doi: 10.2337/diacare.27.9.2149. [DOI] [PubMed] [Google Scholar]
- 8.Howard RL, Avery AJ, Slavenburg S, et al. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol. 2007;63:136–47. doi: 10.1111/j.1365-2125.2006.02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho P, Rumsfeld J, Masoudi F, et al. Effect of medication non-adherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166:1836. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 10.Marcum ZA, Sevick MA, Handler SM. Medication non-adherence: a diagnosable and treatable medical condition. JAMA. 2013;309:2105–6. doi: 10.1001/jama.2013.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voils CI, Hoyle RH, Thorpe CT, Maciejewski ML, Yancy WS., Jr Improving the measurement of self-reported medication non-adherence. Journal of clinical epidemiology. 2011;64:250–4. doi: 10.1016/j.jclinepi.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bangsberg D. Monitoring adherence to HIV antiretroviral therapy in routine clinical practice: the past, the present, and the future. AIDS Behav. 2006;10:249–51. doi: 10.1007/s10461-006-9121-7. [DOI] [PubMed] [Google Scholar]
- 13.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 14.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10:227–45. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med. 2008;31:213–24. doi: 10.1007/s10865-007-9147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosworth HB. Medication treatment adherence. In: Bosworth HB, Oddone E, Weinberger M, editors. Patient treatment adherence. Mahwah, NJ: Lawrence Erlbaum Associates; 2006. pp. 147–94. [Google Scholar]
- 17.Unni EJ, Farris KB. Development of a new scale to measure self-reported medication nonadherence. Research in Social and Administrative Pharmacy. 2009 Oct 9; doi: 10.1016/j.sapharm.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Koschack J, Marx G, Schnakenberg J, Kochen MM, Himmel W. Comparison of two self-rating instruments for medication adherence assessment in hypertension revealed insufficient psychometric properties. Journal of Clinical Epidemiology. 2010;63:299–306. doi: 10.1016/j.jclinepi.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 20.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American Journal of Epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 21.The Atherosclerosis Risk in Communities (ARIC) Study. Description of ARIC Visit 5 NCS Stage 1. Available from: https://www2.cscc.unc.edu/aric/system/files/Description%20of%20V5%20stage%201%20140304.pdf [Accessed September 1, 2015]
- 22.Nunnally JC, Bernstein IH. Psychometric Theory. 3rd. McGraw-Hill; New York: [Google Scholar]
- 23.Ekström J. A generalized definition of the polychoric correlation coefficient. Department of Statistics, UCLA; 2011. Oct 25, [Google Scholar]
- 24.Bollen KA, Lennox RD, Dahly DL. Practical application of the vanishing tetrad test for causal indicator measurement models: An example from health-related quality of life. Statistics in medicine. 2009;28:1524–36. doi: 10.1002/sim.3560. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Escamilla B, Franco-Trigo L, Moullin JC, Martínez-Martínez F, García-Corpas JP. Identification of validated questionnaires to measure adherence to pharmacological antihypertensive treatments. Patient preference and adherence. 2015;9:569. doi: 10.2147/PPA.S76139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben AJ, Neumann CR, Mengue SS. The Brief Medication Questionnaire and Morisky-Green test to evaluate medication adherence. Rev Saude Publica. 2012;46:279–289. doi: 10.1590/s0034-89102012005000013. [DOI] [PubMed] [Google Scholar]
- 27.Shalansky SJ, Levy AR, Ignaszewski AP. Self-reported Morisky score for identifying nonadherence with cardiovascular medications. Annals of Pharmacotherapy. 2004;38:1363–8. doi: 10.1345/aph.1E071. [DOI] [PubMed] [Google Scholar]
- 28.AlGhurair SA, Hughes CA, Simpson SH, Guirguis LM. A Systematic Review of Patient Self-Reported Barriers of Adherence to Antihypertensive Medications Using the World Health Organization Multidimensional Adherence Model. The Journal of Clinical Hypertension. 2012;14:877–86. doi: 10.1111/j.1751-7176.2012.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Lee J, Toh MP, Tang WE, Ko Y. Validity and reliability of a self-reported measure of medication adherence in patients with Type 2 diabetes mellitus in Singapore. Diabetic Medicine. 2012;29:e338–44. doi: 10.1111/j.1464-5491.2012.03733.x. [DOI] [PubMed] [Google Scholar]
- 30.Toll BA, McKee SA, Martin DJ, Jatlow P, O’Malley SS. Factor structure and validity of the Medication Adherence Questionnaire (MAQ) with cigarette smokers trying to quit. Nicotine & tobacco research. 2007;9:597–605. doi: 10.1080/14622200701239662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan X, Patel I, Chang J. Review of the four item Morisky Medication Adherence Scale (MMAS-4) and eight item Morisky Medication Adherence Scale (MMAS-8) [Google Scholar]
- 32.Pratt RJ, Robinson N, Loveday HP, Pellowe CM, Franks PJ, Hankins M, Loveday C. Adherence to antiretroviral therapy: Appropriate use of self-reporting in clinical practice. HIV Clinical Trials. 2001;2:146–159. doi: 10.1310/89E2-XNJL-W107-R2GL. [DOI] [PubMed] [Google Scholar]
- 33.Fialko L, Garety PA, Kuipers E, Dunn G, Bebbington PE, Fowler D, Freeman D. A large-scale validation study of the Medication Adherence Rating Scale (MARS) Schizophrenia research. 2008;100:53–9. doi: 10.1016/j.schres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 34.Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophrenia research. 2000;42:241–7. doi: 10.1016/s0920-9964(99)00130-9. [DOI] [PubMed] [Google Scholar]
- 35.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clinic Proceedings. 2011;86:304–14. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voils CI, Maciejewski ML, Hoyle RH, Reeve BB, Gallagher P, Bryson CL, Yancy WS., Jr Initial validation of a self-report measure of the extent of and reasons for medication non-adherence. Medical care. 2012;50:1013. doi: 10.1097/MLR.0b013e318269e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


