Abstract
The HetR protein has long been recognized as a key player in the regulation of heterocyst development. HetR is known to possess autoproteolytic and DNA-binding activities. During a search for mutants of Anabaena sp. PCC 7120 that can overcome heterocyst suppression caused by overexpression of the patS gene, which encodes a negative regulator of differentiation, a bypass mutant strain, S2-45, was isolated that produced a defective pattern (Pat phenotype) of irregularly spaced single and multiple contiguous heterocysts (Mch phenotype) in combined nitrogen-free medium. Analysis of the S2-45 mutant revealed a R223W mutation in HetR, and reconstruction in the wild-type background showed that this mutation was responsible for the Mch phenotype and resistance not only to overexpressed patS, but also to overexpressed hetN, another negative regulator of differentiation. Ectopic overexpression of the hetRR223W allele in the hetRR223W background resulted in a conditionally lethal (complete differentiation) phenotype. Analysis of the heterocyst pattern in the hetRR223W mutant revealed that heterocysts differentiate essentially randomly along filaments, indicating that this mutation results in an active protein that is insensitive to the major signals governing heterocyst pattern formation. These data provide genetic evidence that, apart from being an essential activator of differentiation, HetR plays a central role in the signaling pathway that controls the heterocyst pattern.
Keywords: patS, pattern formation, prokaryotic development, signal pathway
In Anabaena sp. strain PCC 7120, the first step in the establishment of aerobic diazotrophic growth is the formation of a pattern of cells that will differentiate into heterocysts. To understand how the heterocyst pattern is formed de novo upon nitrogen deprivation and maintained during continuous diazotrophic growth, it is crucial to identify several distinct groups of genes: those that positively regulate very early steps in development, the negative regulators of differentiation, and the components of signaling pathways that define when and where along the filament heterocysts should form.
Several genes have been shown to be involved in the early regulation of heterocyst development. The ntcA gene encodes a transcriptional regulator of the C-reactive protein family that controls a number of genes involved in nitrogen metabolism in cyanobacteria (1). Mutants lacking NtcA are pleiotropic and fail to show any signs of morphological differentiation upon nitrogen deprivation (2, 3). A mutant with a disrupted hanA gene encoding histone-like protein HU is also highly pleiotropic and is blocked in the earliest stage of heterocyst differentiation (4). Both ntcA and hanA are not heterocyst-specific genes, and there is no indication of their involvement in pattern formation.
The HetR protein has long been recognized as a major player in the regulation of heterocyst development (5, 6). Disruption of hetR by transposon insertion (6) and missense mutations S179N (5) and S152A (7) block the earliest stage of heterocyst differentiation without affecting growth on combined nitrogen. Overexpression of hetR in trans from its own promoters on a multicopy plasmid (5) or from the copper-inducible petE promoter (8) leads to constitutive production of supernumerary heterocysts. HetR is an unusual serine-type protease, and two serine residues, Ser-152 and Ser-179, are required for both autoproteolysis and heterocyst differentiation, the Ser-152 apparently being the active site of the protease (7, 9). It has been recently found that HetR dimer binds specific DNA fragments containing promoter regions of hetR, patS, and hepA genes, and that its DNA-binding activity is inhibited by a synthetic PatS pentapeptide in vitro (10).
Two negative regulators, patS and hetN, in multicopy cause suppression of heterocyst differentiation and are required for normal heterocyst pattern formation and maintenance, respectively. Deletion of patS, which encodes a small peptide, results in an aberrant pattern and formation of multiple contiguous heterocysts (Pat Mch phenotype) upon nitrogen step-down (11). The phenotype becomes far less pronounced during continuous diazotrophic growth (12). The hetN product shows high sequence similarity to ketoacyl reductases (13, 14). Overexpression of hetN prevents up-regulation and patterned expression of hetR after nitrogen step-down (15, 16). HetN protein is present in vegetative cells grown with combined nitrogen, but after nitrogen step-down, it is found only in mature heterocysts (16). Depletion of HetN by switching off a copper-regulated promoter driving a chromosomal copy of hetN does not alter the initial heterocyst pattern, but causes a Mch phenotype during subsequent rounds of heterocyst formation (15). Thus, PatS and HetN have different but complementary functions in establishment and maintenance of the heterocyst pattern.
Several other genes may be related to the mechanism of pattern formation. In Nostoc punctiforme, the HetF protein is essential for triggering heterocyst differentiation (17). Similar to a hetR mutant, a hetF null mutant shows no sign of morphological differentiation or cell-specific degradation of phycobiliproteins 24 h after nitrogen step-down. Unlike hetR, extracopy hetF supplied on a replicative plasmid does not cause constitutive heterocyst production in ammonium- or nitrate-containing media. However, an increase in the frequency of heterocysts and production of short clusters of contiguous heterocysts were observed upon nitrogen deprivation, resembling the effect of extracopy hetR (5, 18). The HetF protein apparently typifies a unique family of caspase-hemoglobinase fold-domain proteases (19). Inactivation of hetP, whose product shows no homology to known proteins, resulted in a Het– phenotype in Anabaena PCC 7120, whereas in multicopy, it caused production of Mch in combined nitrogen-free medium (20). PatA, a protein with a C-terminal domain similar to the CheY family of response regulators, is known to be essential for differentiation of intercalary, but not terminal, heterocysts (21). The patA mutation suppresses the Mch phenotype produced by extra copies of hetR (21) and confines hetR expression to terminal cells of a filament. It was postulated that PatA is required for the activation of HetR and increased expression of hetR in differentiating cells (8). PatB protein has ferredoxin-like domains near the N terminus and a DNA-binding domain near the C terminus, suggesting that PatB might be a redox-sensing transcription factor (22, 23). Its expression is induced 12 h after nitrogen step-down and is confined to heterocysts, and mutations in patB result in delayed heterocyst formation, poor or no diazotrophic growth (depending on the nature of mutation), and a gradually developing Mch phenotype, although initially formed heterocysts show a normal pattern (23).
Two more genes, patN and patU, were recently implicated in heterocyst pattern formation in Nostoc punctiforme (18). Both PatN and PatU have close orthologs in Anabaena PCC 7120, but show no similarity to other proteins. Mutations in patU result in strings of four to eight proheterocysts that ultimately resolve to single, double, or triple (Mch) heterocysts. A patN mutant yields a unique multiple singular heterocysts (Msh) phenotype, which is a high frequency of heterocysts located singly in the filaments.
The available genetic data indicate that aberrations in the heterocyst pattern can be obtained by altered expression or mutation of hetR, hetP, hetN, hetF, patS, patA, patB, patU, and hetN genes. In addition, high light intensity and certain chemicals (reviewed in ref. 18) cause production of Mch. However, no mutation producing random spacing of heterocysts has been reported. This led to a suggestion that the mechanism underlying the heterocyst pattern must either result from multiple redundant processes or be embedded in basic cellular functions and thus be immutable (18). Here, we present genetic evidence that a single mutation in hetR can produce a functional protein that is insensitive to the major signals governing heterocyst pattern formation.
Materials and Methods
Strains, Plasmids, and Growth Conditions. Anabaena sp. strain PCC 7120 and its derivatives were grown as described (24, 25). Mutant strains were grown in the presence of appropriate antibiotics at the following final concentrations: neomycin, 25 μg/ml for solid medium and 15 μg/ml for liquid medium; erythromycin, 5 μg/ml; spectinomycin, 5 μg/ml; streptomycin, 2.5 μg/ml. To induce heterocyst formation and diazotrophic growth, portions of early-growth-phase cultures were washed twice with BG-110 medium, which lacks combined nitrogen, and diluted 1:2 in BG-110. Scoring of the heterocyst pattern was performed as described (12). For heterocyst induction on agar plates, filaments from fresh streaks on BG-11 plates were patched on BG-110 plates and examined microscopically after 2–3 days of diazotrophic growth.
Microscopy. Bright-field and fluorescence microscopy were performed as described (12) except that an extra-long working-distance (ELWD) ×40 objective (M Plan 40, 0.5 ELWD, 210/0, Nikon) was used, permitting visualization of vegetative cells and heterocysts in Anabaena filaments and of gfp expression directly on the surface of agar plates.
DNA Manipulations and Plasmid Constructions. Total DNA from cyanobacterial strains was extracted by vortexing cells with glass beads in the presence of phenol (26). Recombinant DNA procedures and enzyme reactions were performed by standard techniques or according to recommendations of the supplier. The mutant hetR allele was amplified from genomic DNA of strain AMC1285 by PCR with primers AMO-550 5′-GCACGACTAGTAACAACACTGTTGC-3′ (an engineered SpeI site is italicized) and AMO-551 5′-CGCCGAGTCATTTGTCATCACTAGC-3′ using Pwo polymerase (Roche Applied Sciences). The amplified fragment was cut with SpeI and cloned into the XbaI site of pAM2178 (25) with hetR oriented parallel to lacZ, producing pAM3316, or into the XbaI site of pAM2179 (25) in the opposite orientation, producing pAM3317. These plasmids contain the hetR upstream region starting at –181, which is 2 nt 3′ of the first –184 transcription start site mapped by Buikema and Haselkorn (8).
The hetN gene was overexpressed from the copper-inducible petE promoter on shuttle plasmid pAM1537, which was constructed as follows. The hetN gene was subcloned as a 1.3-kb ClaI fragment from cosmid clone 3E10 (13) into the ClaI site of pIC20H (27), such that hetN is in the opposite orientation relative to lacZ, to make pAM1531. The petE promoter was isolated from pPet1 (8) as a 372-bp BamHI–EcoRI fragment and then cloned into the same sites of pAM1531 to make pAM1533. A BamHI–XhoI fragment from pAM1533 containing PpetE–hetN was then cloned into the BamHI–SalI sites of shuttle vector pJL-3 (28) to make pAM1537.
Shuttle plasmid pAM1830 contains a gfp reporter downstream of patS as a transcriptional fusion and was constructed as follows. The patS ORF and ≈650 bp of upstream region was generated as a 0.72-kb BamHI–SmaI fragment by PCR of template pAM1035 (11) with primers 5′-CGCTCTAGAACTAGTGGATC-3′ (Bluescript SK) and 5′-CGCCCGGGTCTATCTACCACTACCGC-3′ followed by restriction enzyme digestion. This fragment was inserted into XbaI (filled in with Klenow polymerase) and BamHI sites of pKEN2-GFPmut2 (29) to make pAM1863. A HindIII (filled in with Klenow polymerase)–SacI fragment containing patS–gfp from pAM1863 was then cloned into SacI–SmaI sites of shuttle vector pAM505 (11) to make pAM1830.
For construction of a PpetE–hetRR223W transcriptional fusion, the Spr/Smr Ω cassette from pAM684 (30) was inserted into the SmaI site of the pAM3317 polylinker downstream of and parallel to hetRR223W, and the HincII–SacI fragment with hetR–Ω was moved into the SmaI–SacI sites of pPet1 (8) downstream of the PpetE promoter. Then the PstI–SacI fragment containing PpetE–hetR-Ω was moved into the same sites of shuttle vector pAM504 (3), producing pAM3318.
Results
Identification of a hetR Mutation Responsible for Aberrant Heterocyst Pattern. In an approach to clarify the functions of regulatory genes and unravel interactions among regulatory proteins during heterocyst differentiation and pattern formation, we isolated extragenic suppressors of the negative regulator PatS. During a search for transposon Tn5-1058-derived (31) mutants of Anabaena PCC 7120 that can overcome heterocyst suppression by extracopy patS supplied on plasmid pAM1888 (Emr) (32), a bypass mutant strain, named S2-45, was isolated that produced long and irregularly spaced strings of multiple contiguous heterocysts in nitrogen-free medium (Fig. 1A). However, reconstruction of the transposon insertion in a wild-type background failed to reproduce the original phenotype, indicating the presence of a secondary mutation(s) in the original S2-45 bypass mutant.
Fig. 1.
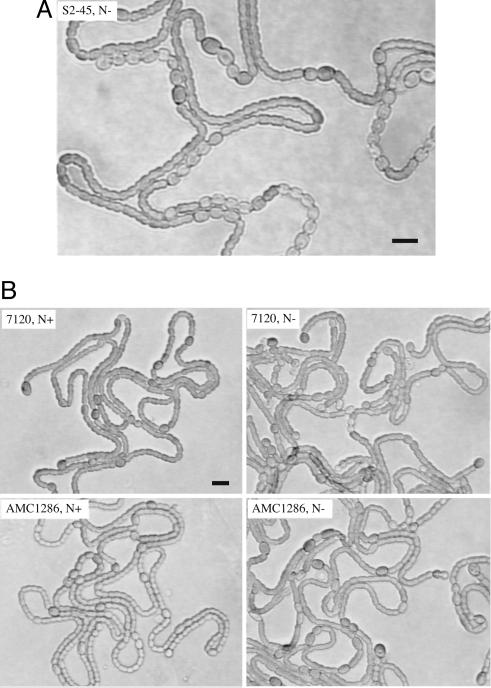
Pattern-formation defects of a patS-overexpression-bypass mutation complemented by hetR. (A) Original S2-45 mutant strain containing pAM1888 showing Pat Mch phenotype when grown on combined nitrogen-free BG-110 plate. Heterocysts are the larger cells with thicker cell envelopes; cyanophycin granules can be seen at the poles of some heterocysts. (B) Anabaena PCC 7120 and AMC1286 strains, both containing extracopy hetR supplied in trans on replicative plasmid pWB216S2.4, were grown on BG-11 (N+) or BG-110 (N–) plates. (Scale bars, 10 μm.)
To identify the mutation(s) responsible for the S2-45 (pAM1888) phenotype, the mutant strain was first cured of pAM1888 by repeated subculturing in liquid BG-110 medium without antibiotics and subsequent cloning. The cured strain, AMC1285, exhibited the original Pat Mch phenotype. To allow conjugative transfer of different pDU1-based plasmids carrying a Nmr marker, the Nmr Bmr Smr genes on Tn5-1058 in the S2-45 chromosome were replaced with a Ω Spr Smr cassette. The resulting strain, AMC1286, exhibited the original Pat Mch phenotype.
We were interested in testing how AMC1286 would respond to extracopy hetR, which in the wild type causes constitutive heterocyst formation in nitrogen-replete conditions and production of supernumerary heterocysts with aberrant pattern in medium lacking combined nitrogen (Fig. 1B). Introduction of pWB216S2.4 (Nmr) (5) carrying wild-type hetR into AMC1286 resulted in formation of constitutive heterocysts but with normalized pattern, approximately the same as the wild-type strain carrying the same plasmid (Fig. 1B). This apparent complementation of the Pat Mch phenotype hinted that a recessive mutation in hetR might be the cause of the original phenotype. Indeed, PCR amplification and sequencing of hetR from strain AMC1285 revealed a single C to T transition at position 667, producing a R223W mutation. To introduce this mutation into the wild-type background, the mutant hetRR223W lacking its upstream regulatory region was cloned into the suicide vector pAM2178, and the resulting pAM3316 was integrated into the wild-type hetR locus by homologous recombination, producing the merodiploid AMC1287. In this strain, the mutant copy of hetR is under its native regulation, whereas the wild-type copy lacks upstream promoter regions and is presumably silent. The reconstructed merodiploid AMC1287 exhibited the same Pat Mch phenotype as the original S2-45 mutant (for example, see Fig. 2).
Fig. 2.
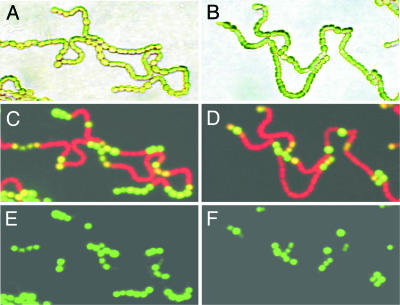
The R223W mutation in HetR does not inhibit patS expression upon nitrogen step-down. The AMC1287 merodiploid strain bearing pAM1951, which carries a partial patS ORF followed by gfp as a transcriptional reporter (A, C, and E), or pAM1830, which carries a complete patS gene followed by gfp as a transcriptional reporter (B, D, and F). Images were taken in visible light (A and B) and with fluorescein isothiocyanate-specific illumination (484 ± 8 nm) with (E and F) and without (C and D) GFP-specific emission (518 ± 13 nm) filter sets to cut out red chlorophyll fluorescence from vegetative cells. GFP fluorescence is confined to developing and mature heterocysts.
hetRR223W Mutation Overcomes patS- and hetN-Mediated Negative Regulation of Differentiation. Introduction of plasmids that overexpress patS did not suppress heterocyst formation in AMC1286 and reconstructed AMC1287 merodiploid strains, indicating that both the bypass and Pat Mch phenotypes were caused by the R223W mutation in HetR. The insensitivity to PatS inhibition could result from a lack of response to PatS-mediated signaling or from impaired regulation of patS expression. To discriminate between these possibilities, two plasmids were used, pAM1951 containing a gfp transcriptional reporter fused to an incomplete patS ORF (12) and pAM1830 with the same reporter fused 3′ of full-length, active patS. Both constructs contain the same ≈650-bp 5′ regulatory region.
In the wild-type strain grown diazotrophically, the gfp reporter on pAM1951 is expressed only in mature and developing heterocysts, and practically no fluorescence can be detected with the gfp reporter on pAM1830, because the overproduction of PatS effectively shuts off its own expression. When introduced into the AMC1287 merodiploid, gfp reporters on both pAM1951 and pAM1830 plasmids produced the same pattern of gfp expression confined to mature and developing heterocysts (Fig. 2). These data show that the mutant HetRR223W does not impair spatially localized patS transcription.
Consistent with the gfp reporter data, synthetic PatS-5 pentapeptide (RGSGR), which at 2 μM completely suppresses differentiation in the wild-type strain (11), did not suppress heterocyst production and did not normalize pattern in the original S2-45 mutant or AMC1287 merodiploid strains (data not shown). It is clear that insensitivity of the mutant HetRR223W to PatS inhibition results from altered interactions in a PatS signaling pathway.
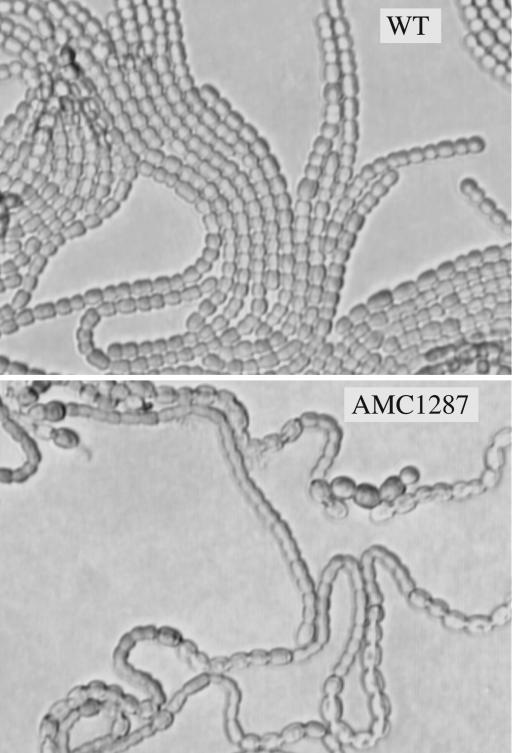
hetN overexpression prevents heterocyst formation in the wild-type strain (13, 14). However, hetN-overexpressing plasmid pAM1537 failed to suppress heterocyst formation in the reconstructed merodiploid strain AMC1287 on BG-110 plates, whereas it completely inhibited differentiation of the wild type (Fig. 3). Therefore, the mutant HetRR223W protein also has diminished sensitivity to inhibition by HetN.
Fig. 3.
Extracopy hetN fails to suppress heterocyst differentiation in a strain carrying the hetRR223W allele. Wild-type Anabaena PCC 7120 (A) and the reconstructed merodiploid AMC1287 (B) strains bearing plasmid pAM1537, which carries hetN under the control of the copper-inducible petE promoter, 2 days after transfer to combined nitrogen-free BG-110 plates. The copper concentration in standard BG-11 and BG-110 medium should produce approximately half-maximal expression from the petE promoter (8).
Ectopic Overexpression of the hetRR223W Allele Results in Complete Differentiation. Our initial attempts to reconstruct the hetRR223W mutation in the wild-type strain by single recombination with the promoterless mutant allele cloned into a suicide vector produced single recombinants with varying phenotypes, depending on the nature of the suicide vector used. On BG-110 medium, most initial colonies of pAM3316-derived single recombinants had a Pat Mch phenotype very similar to the original S2-45 mutant strain, whereas a few exhibited a wild-type phenotype or contained a mixture of Pat Mch, wild-type, and nearly completely differentiated filaments. When pAM3317 was used for reconstruction, which differs from pAM3316 only in the antibiotic-resistant cassette (Cmr Emr C.CE3 versus Spr/Smr Ω in the same location, see ref. 25) and orientation of the insert, the resultant single recombinants were either similar to the wild type or exhibited a conditionally lethal phenotype, differentiating completely upon transfer to combined nitrogen-free medium (Fig. 4). Heterocyst differentiation was not synchronous, and it took 2–3 days to get nearly 100% heterocysts. One such clone, AMC1288, was used to recover integrated plasmids that contained either the upstream or downstream copies of hetR, and DNA sequencing showed that both copies contained the R223W mutation. In another clone with a normal heterocyst pattern, both copies of hetR were found to be wild type. Thus, gene conversion leading to two copies of the same allele apparently occurred frequently. High frequency of putative gene conversion between chromosomal and replicative plasmid-borne hetR alleles has been observed (5). We suspect that ectopic vector-derived transcription of the downstream copy of hetRR223W, which lacks its native promoter region, results in the conditionally lethal phenotype. A similar phenotype with nearly 100% differentiation was observed when strain AMC1289 (see below), containing a hetRR223W allele expressed from the copper-inducible petE promoter on pAM3318, was incubated in BG-110 medium containing 2 μM copper. The wild-type strain containing this plasmid showed a Mch phenotype but contained strings of vegetative cells and could grow diazotrophically, which, together with our earlier complementation experiments, indicates that the wild-type and R223W hetR alleles show incomplete dominance.
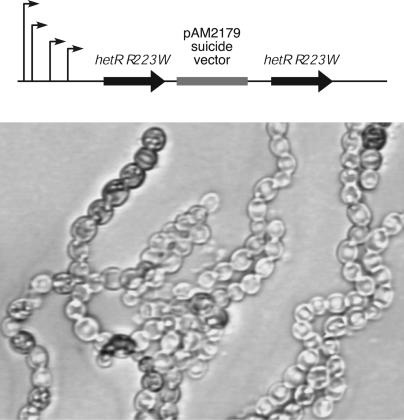
Fig. 4.
The strain AMC1288, which contains two copies of the hetRR223W allele, showed nearly complete differentiation of vegetative cells into heterocysts 72 h after nitrogen step-down. The map shows the positions of four putative hetR transcriptional start sites (8), as indicated by bent arrows. The downstream copy of hetRR223W lacks this 5′ regulatory region, but may be expressed from upstream vector sequences.
The conditionally lethal mutant AMC1288, which contains two copies of hetRR223W, was used to reconstruct a single-copy hetRR223W mutation through looping out of the suicide vector along with one copy of mutant hetR. After subculturing AMC1288 in nonselective conditions and streaking on a BG-110 plate without antibiotics, several green colonies appeared on the bleached background of completely differentiated filaments. All of these clones had lost the Emr Cmr markers of the suicide vector and exhibited the same Pat Mch phenotype as the original S2-45 mutant. One of these clones, designated AMC1289, was used for further phenotypic characterization.
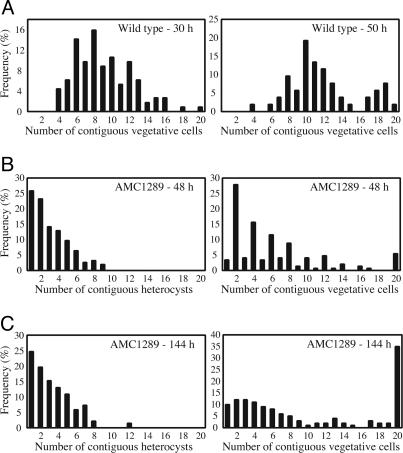
Initial Heterocyst Pattern in the hetRR223W Mutant Appears Nearly Random. A striking feature of hetRR223W mutants is the formation of irregularly spaced strings of contiguous heterocysts, a phenotype resembling the de novo heterocyst pattern of a patS mutant, but more extreme. Fig. 5A shows the heterocyst pattern in the wild type 30 and 50 h after nitrogen step-down. The frequency distribution of contiguous vegetative cells peaked at 8 cells at 30 h, and the peak shifted to 10 cells at 50 h; heterocyst frequencies were 10.4% and 7.9%, respectively. The heterocyst pattern in AMC1289 appears to be nearly random, showing a continuous decline in the frequency of contiguous vegetative cells (Fig. 5B). A similar distribution was observed for the number of contiguous heterocysts. The strong bias toward even-numbered intervals of vegetative cells is expected if, during formation of mature heterocysts, the nondifferentiating vegetative cells divide synchronously at least once before the time of observation. Heterocyst frequencies declined from 33.8% at 48 h to 20.5% at 144 h after removal of combined nitrogen, and there was a clear increase in the probability of longer distances between clusters of heterocysts after prolonged diazotrophic growth (144 h), although very short distances (two to four cells) still prevailed (Fig. 5C). The distribution of contiguous heterocysts did not change (Fig. 5C).
Fig. 5.
Heterocyst pattern formation and maintenance in the wild type (A) and AMC1289 hetRR223W mutant (B and C). Both strains were grown in BG-11 medium to an OD750 of 0.2 and induced to form heterocysts by washing and transfer to BG-110 medium. Vegetative cells and heterocysts were scored microscopically at indicated times after heterocyst induction. The wild type produced <5% heterocyst doublets and no higher numbers of contiguous heterocysts. Interval lengths ≥20 are shown as 20. The data shown are representative of two independent experiments.
Discussion
When grown without combined nitrogen, ≈8–10% of Anabaena PCC 7120 vegetative cells differentiate at semiregular intervals along a filament into highly specialized nitrogen-fixing heterocysts. A mechanism to establish control over heterocyst frequency and spacing is required to ensure the efficient supply of fixed nitrogen to vegetative cells and fixed carbon, as a source of energy and reductant, from vegetative cells to heterocysts. The molecular mechanisms accounting for such control are poorly understood. Among several known genes that influence the heterocyst pattern, the patS gene, which encodes a small peptide, is an essential negative regulator of pattern formation (11). To identify components of the PatS-signaling pathway, we used transposon mutagenesis to isolate bypass mutants of patS-overexpressing strains. However, this experimental design mainly yielded mutant strains in which the gene of interest was not tagged. The transposons had inserted into genes nonessential for diazotrophic growth, and secondary mutations were apparently responsible for the bypass phenotype.
Because the majority of these bypass mutants exhibited a normal heterocyst pattern, which would be expected if the mutations effected the expression or copy number of the plasmid-borne patS, we concentrated on rare mutants that showed an abnormal heterocyst pattern because these were more likely to be impaired in components of the patS signaling pathway. The investigation of one such mutant, S2-45, revealed that the Pat Mch phenotype was caused by a mutation in the hetR gene.
The HetR protein has long been known to play a key role as an activator of heterocyst differentiation, but its role in pattern formation was less clear. Our data provide genetic evidence that HetR plays a central role not only in controlling the differentiation process, but also in processing heterocyst pattern formation signals, and that these functions can be separated by mutation. Reconstruction of the hetRR223W mutation in the wild-type background allowed us to show unambiguously that a single mutation is responsible for resistance to both known negative regulators of differentiation, PatS and HetN. A striking consequence of the hetRR223W mutation is an apparently random pattern of heterocysts along filaments. Thus, this hetR allele appears to be insensitive to the major signals governing initial heterocyst pattern formation.
The simplest explanation for the loss of resistance to PatS inhibition in our mutant is that HetR is a direct target of the PatS gene product. Recent results from Zhao and coworkers (10) show that this is very likely to be the case, because the PatS-5 pentapeptide inhibited HetR DNA-binding activity in mobility-shift assays (10). Simultaneous acquisition of resistance to inhibition by overexpression of patS and hetN indicates that both negative regulators work through HetR, which then acts as the central regulator of heterocyst pattern by determining which cells differentiate. However, PatS and HetN signaling pathways are apparently independent upstream of HetR because they show their strongest influence on different stages of pattern formation and they do not need each other to exert their inhibitory effect on differentiation, because a patS mutant is sensitive to inhibition by overexpressed hetN, and vice versa (S. Callahan, personal communication; I.Y.K., unpublished data). HetN apparently does not play a role in de novo pattern formation, because a strain with the chromosomal hetN gene under the control of the copper-regulated petE promoter in copper-free medium differentiates single heterocysts with normal spacing in the first round of differentiation upon nitrogen step-down (15). Only subsequent rounds of differentiation produce semiregularly spaced stretches of contiguous heterocysts. The same sequence of events was observed in the hetN mutant strain DR994a (14) after nitrogen step-down (I.Y.K., unpublished data). The de novo pattern in a patS mutant, although strongly disturbed, is not completely random and normalizes partially during diazotrophic growth (12). The phenotype of hetRR223W mutant strains could result from the inability to respond to the two different patterning signals that are normally superimposed on each other. It is not known whether PatS and HetN signaling, apart from the supply of fixed nitrogen (12), are the only pathways that regulate the heterocyst pattern. It would be interesting to determine whether the phenotype of a patS hetN double mutant mirrors the phenotype of the R223W mutation in HetR.
Ectopic overexpression of the hetRR223W allele in a hetRR223W background results in almost complete differentiation and a conditionally lethal phenotype in combined nitrogen-free medium. It is well known that a minimal filament length is required to allow heterocyst differentiation to proceed. Two explanations have been suggested for this phenomenon (33). The first is a nutritional one and implies a dependence of differentiating cells on photosynthate supplied by neighboring vegetative cells. An alternative explanation postulates that a minimum number of vegetative cells are required to inactivate a diffusible morphogen that will otherwise accumulate in presumptive heterocyst and inhibit differentiation. The ability of strain AMC1288 to differentiate almost 100% morphologically distinct heterocysts upon nitrogen step-down indicates a great degree of metabolic autonomy of differentiating heterocysts and makes intercellular signaling the more plausible explanation.
The PatS peptide, or a product of its cleavage, has been hypothesized to function as a diffusible morphogen (11). Because the localized induction of patS and hetR expression occurs in the same cells, HetR must not be inhibited by PatS in cells that successfully differentiate. One can envisage several ways in which HetR could avoid this inhibition. First, spatially localized induction of HetR expression probably precedes induction of PatS expression because patS transcription is HetR-dependent. No induction of a patS-gfp reporter (unpublished data) and no accumulation of patS message (10) occur in a hetR mutant. This could result in a high HetR/PatS ratio in differentiating cells. Second, patS message could be degraded rapidly or its translation blocked, or PatS could be inactivated in proheterocysts by a protease, an inhibitor, or through posttranslational modification. Third, posttranslational modification of HetR itself could render it insensitive to inhibition. Fourth, assuming a model in which PatS is a diffusible morphogen, PatS could be efficiently exported only from differentiating cells. However, there is currently no proof that PatS acts as a morphogen, which leaves the question of apparent PatS-mediated intercellular signaling open to alternative explanations. However, the formation of a normal pattern must depend on the sensitivity of HetR to PatS inhibition at early stages of differentiation in those cells that remain vegetative cells. The essentially random initial pattern of heterocysts produced by a hetRR223W mutant shows that these cells have lost normal sensitivity to the PatS inhibitory pathway.
In most heterocystous cyanobacteria, the differentiation process is controlled by the availability of external combined nitrogen. At first glance, the hetRR223W mutant behaves normally in this respect because it does not produce heterocysts constitutively on media with combined nitrogen. This is different from a patS mutant, which forms a few percent heterocysts in a medium containing nitrate (11, 12). However, the hetRR223W mutant appears to be altered in its sensitivity to nitrogen. Differentiation of this strain is sluggish, lagging behind the wild type by ≈8–12 h in liquid; in very dilute suspensions, it is inhibited at nitrate concentrations of 0.05 mM, approximately five times lower than in the wild type (unpublished data). In hetRR223W mutants, hetR expression appears to be activated and differentiation initiated only after filaments have exhausted extracellular and intracellular supplies of available nitrogen and a severe shift in C/N balance is perceived by the cells. This could explain the delay in development and why the bleaching of filaments before formation of functional heterocysts is far more pronounced in the hetRR223W mutant than in the wild type. Our preliminary data suggest that, unlike the wild type, in the hetRR223W mutant, the signal that induces differentiation is not a sudden step-down or gradual decrease below a threshold level of exogenous combined nitrogen, but the perception of nitrogen starvation by an intracellular sensor. These data might fit into a recently proposed model in which external combined nitrogen entering cells is recognized differently from fixed nitrogen produced by heterocysts, and the signal controlling differentiation is related to external availability of fixed nitrogen but not to the nitrogen sufficiency of individual cells (34). This model implies that the wild type employs a strategy that anticipates nitrogen starvation, and the decision to produce heterocysts is made before the filaments begin to experience actual nitrogen limitation. The benefits of this strategy are evident.
The most recent experimental evidence that HetR is a DNA-binding protein and that PatS-5 pentapeptide inhibits its DNA-binding activity (10) provides the first mechanistic view of HetR function and regulation. Our genetic data complement these findings and provide insights into the complex regulation of HetR activity. The ability to construct conditionally lethal strains that undergo complete differentiation after nitrogen step-down presents a powerful tool for screening for mutants specifically impaired in early stages of differentiation.
Acknowledgments
This work was supported by Public Health Service Grant GM36890 from the National Institutes of Health and Department of Energy Grant DE-FG03-98ER020309.
Author contributions: I.Y.K. and J.W.G. designed research; I.Y.K. performed research; I.Y.K. contributed new reagents/analytic tools; I.Y.K. and J.W.G. analyzed data; and I.Y.K. and J.W.G. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Herrero, A., Muro-Pastor, A. M. & Flores, E. (2001) J. Bacteriol. 183, 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frías, J. E., Flores, E. & Herrero, A. (1994) Mol. Microbiol. 14, 823–832. [DOI] [PubMed] [Google Scholar]
- 3.Wei, T.-F., Ramasubramanian, T. S. & Golden, J. W. (1994) J. Bacteriol. 176, 4473–4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khudyakov, I. & Wolk, C. P. (1996) J. Bacteriol. 178, 3572–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buikema, W. J. & Haselkorn, R. (1991) Genes Dev. 5, 321–330. [DOI] [PubMed] [Google Scholar]
- 6.Black, T. A., Cai, Y. & Wolk, C. P. (1993) Mol. Microbiol. 9, 77–84. [DOI] [PubMed] [Google Scholar]
- 7.Dong, Y., Huang, X., Wu, X. Y. & Zhao, J. (2000) J. Bacteriol. 182, 1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buikema, W. J. & Haselkorn, R. (2001) Proc. Natl. Acad. Sci. USA 98, 2729–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou, R., Cao, Z. & Zhao, J. (1998) Arch. Microbiol. 169, 417–426. [DOI] [PubMed] [Google Scholar]
- 10.Huang, X., Dong, Y. & Zhao, J. (2004) Proc. Natl. Acad. Sci. USA 101, 4848–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon, H.-S. & Golden, J. W. (1998) Science 282, 935–938. [DOI] [PubMed] [Google Scholar]
- 12.Yoon, H.-S. & Golden, J. W. (2001) J. Bacteriol. 183, 2605–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer, C. C., Ramaswamy, K. S., Endley, S., Scappino, L. A., Golden, J. W. & Haselkorn, R. (1997) FEMS Microbiol. Lett. 151, 23–30. [DOI] [PubMed] [Google Scholar]
- 14.Black, T. A. & Wolk, C. P. (1994) J. Bacteriol. 176, 2282–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callahan, S. M. & Buikema, W. J. (2001) Mol. Microbiol. 40, 941–950. [DOI] [PubMed] [Google Scholar]
- 16.Li, B., Huang, X. & Zhao, J. (2002) FEBS Lett. 517, 87–91. [DOI] [PubMed] [Google Scholar]
- 17.Wong, F. C. & Meeks, J. C. (2001) J. Bacteriol. 183, 2654–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meeks, J. C. & Elhai, J. (2002) Microbiol. Mol. Biol. Rev. 66, 94–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aravind, L. & Koonin, E. V. (2002) Proteins 46, 355–367. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Pinas, F., Leganes, F. & Wolk, C. P. (1994) J. Bacteriol. 176, 5277–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang, J., Scappino, L. & Haselkorn, R. (1992) Proc. Natl. Acad. Sci. USA 89, 5655–5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang, J., Scappino, L. & Haselkorn, R. (1993) J. Bacteriol. 175, 1697–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, K. M., Buikema, W. J. & Haselkorn, R. (2003) J. Bacteriol. 185, 2306–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golden, J. W., Whorff, L. L. & Wiest, D. R. (1991) J. Bacteriol. 173, 7098–7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khudyakov, I. Y. & Golden, J. W. (2001) J. Bacteriol. 183, 6667–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai, Y. & Wolk, C. P. (1990) J. Bacteriol. 172, 3138–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh, J. L., Erfle, M. & Wykes, E. J. (1984) Gene 32, 481–485. [DOI] [PubMed] [Google Scholar]
- 28.Lang, J. D. & Haselkorn, R. (1991) J. Bacteriol. 173, 2729–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cormack, B. P., Valdivia, R. H. & Falkow, S. (1996) Gene 173, 33–38. [DOI] [PubMed] [Google Scholar]
- 30.Ramaswamy, K. S., Carrasco, C. D., Fatma, T. & Golden, J. W. (1997) Mol. Microbiol. 23, 1241–1250. [DOI] [PubMed] [Google Scholar]
- 31.Wolk, C. P., Cai, Y. & Panoff, J.-M. (1991) Proc. Natl. Acad. Sci. USA 88, 5355–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, D. & Golden, J. W. (2002) J. Bacteriol. 184, 6873–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolk, C. P., Ernst, A. & Elhai, J. (1994) in The Molecular Biology of Cyanobacteria, ed. Bryant, D. A. (Kluwer Academic, Dordrecht, The Netherlands), Vol. 1, pp. 769–823. [Google Scholar]
- 34.Thiel, T. & Pratte, B. (2001) J. Bacteriol. 183, 280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]