Recent discoveries in biology and microbiology have highlighted the importance of the gastrointestinal (GI) microbiome in regulating human health and disease.[1] Thus, the delivery of probiotics to influence and modulate microbiome compositions can potentially impact the treatment of a number of human diseases.[2] Unfortunately, biological challenges encountered during oral delivery have limited the translation of many probiotic-delivering technologies.[3] Here, we report a layer-by-layer (LbL) method for the encapsulation of probiotics to directly address these challenges by protecting probiotics from GI tract insults while facilitating both mucoadhesion and direct growth on intestinal surfaces.
It has been established that the bacterial composition in the GI tract plays an essential role in the development and progression of a number of disorders, including cancer,[1a] obesity,[4] diabetes,[5] Clostridium difficile,[6] and depression,[7] among others.[1b] Given the diversity of an individual’s GI microbiome[8] and how environmental differences in diet,[9] medication usage (e.g., antibiotics),[10] and other factors[11] dramatically influence microbiome composition and subsequently disease progression, approaches, and technologies to introduce probiotic species into the microbiome are of pronounced interest.[2a] However, probiotic-introducing technologies face oral delivery challenges that are: (i) chemical-based, such as acidic stomach conditions and bile salts which are capable of deactivating probiotics,[12] and (ii) physical-based, such as rapid GI transit times that limit retention of probiotics on intestines, thus preventing the adhesion and growth of probiotics. A number of technologies such as nanoparticles,[13] pills,[14] polymer gels,[15] enteric coatings,[16] and patches[17] have been developed to address these challenges by preventing chemical degradation by acid or enzymes and facilitating mucoadhesion to ensure drug absorption and controlled release. While these approaches have been successful in improving the oral delivery of many small molecules and some biologics, few can address the specific challenges of delivering live-probiotics to the microbiome, due to their large size and viability/growth requirements.[18]
Typically, microencapsulation approaches have been used to address chemical-based and probiotic-specific delivery challenges.[3] These methods have been successful in preventing direct contact between probiotics and their environment, thereby providing survival benefits against the chemical challenges in the GI tract. Unfortunately, this also prevents probiotics from directly contacting intestinal surfaces, thus diminishing their adhesion and growth to sites of interest.[3c] Overwhelmingly, encapsulation approaches have not translated to survival advantages in animal models or in humans.[3a] This is a trend that extends to the probiotic industry; despite the abundance of probiotic products on the market, few of these have proven to provide health benefits in humans or animals,[2b,19] thus highlighting the need for advanced probiotic delivery systems. Here, we describe a novel probiotic encapsulation technology that improves probiotic in vivo delivery by directly addressing: (i) chemical, (ii) physical, and (iii) probiotic-specific encapsulation challenges. Specifically, a layer-by-layer approach[20] leveraging encapsulation of live-probiotics using minimal quantities of polymers is used to protect encapsulated probiotics from GI tract insults while providing a means to directly adhere, grow, and proliferate on intestinal surfaces without requiring release from the encapsulating matrix. These advantages translated to enhanced survival and persistence of probiotics in the small intestine in vivo. To our knowledge, the LbL encapsulation strategy described here represents one of the first broad approaches to introduce a probiotic species while simultaneously addressing the chemical, physical, and probiotic-specific encapsulation challenges.
The probiotic strain Bacillus coagulans (BC)[21] was successfully encapsulated using two biodegradable polysaccharides, chitosan and alginate via a LbL approach (Figure 1a). BC is a lactic acid producing probiotic[22] that has exhibited potential therapeutic benefits for treating colitis[23] and abdominal pain and bloating associated with irritable bowel syndrome.[21] Chitosan and alginate are widely used in oral delivery applications as they are both biocompatible and each has unique mucoadhesive properties.[24] Briefly, alternating layers of the cationic polysaccharide chitosan (CHI) and the anionic polysaccharide alginate (ALG) were sequentially layered on BC via electrostatic interactions for up to three bilayers (CHI/ALG)3. LbL facilitates an encapsulation method where the minimum amount of polymer can be used for complete encapsulation/coating. Bright field imaging of plain-BC (Figure 1b(i) and (CHI/ALG)2-BC (Figure 1b(ii) revealed that LbL templating can lead to probiotic aggregation. This occurred after adding the terminal alginate layer, independent of solution pH, and was likely due to assembly of alginate in NaCl solution.[25] Scanning electron microscopy (SEM) imaging of plain-BC (Figure 1b(iii) and (CHI/ALG)2-BC (Figure 1b(iv) showed that LbL encapsulation did not dramatically alter BC surface morphology, likely due to the presence of polysaccharides and sugars already present in the cell wall of gram-positive bacteria.[26] Zeta potential measurements (Figure 1c) confirmed the successful layering of chitosan and alginate. A linear relationship for each polysaccharide was observed (Figure 1d), implying uniform templating for up to three bilayers of chitosan/alginate.[27] Release of the encapsulating polymers was investigated in both simulated intestinal fluid (SIF; pH 7) and simulated gastric fluid (SGF; pH 2). After 4 h in SGF, ≈30% of the total alginate and ≈20% of the total chitosan is no longer associated with the encapsulated probiotic (Figure S1, Supporting Information). To demonstrate the ability of (CHI/ALG) LbL-probiotics to grow and proliferate while still-encapsulated, we examined the effect of layer number on BC growth for 0, 1, 2, and 3 bilayers of (CHI/ALG). In each case, LbL-probiotics maintained their ability to grow and proliferate while still-encapsulated, exhibiting lag, exponential, and stationary growth phases (Figure 1e). At three bilayers the exponential phase was delayed by over 10 h which highlights a key threshold for the growth of still-encapsulated probiotics; as such, a maximum of two bilayers were used for further experiments. LbL-probiotics also enhanced bacterial viability, where non-encapsulated BC exhibited over 1 log reduction in colony forming units (CFU) as compared to (CHI/ALG)2 LbL-BC in both refrigerated and room temperature conditions after one week in water (Figure S2, Supporting Information).
Figure 1.
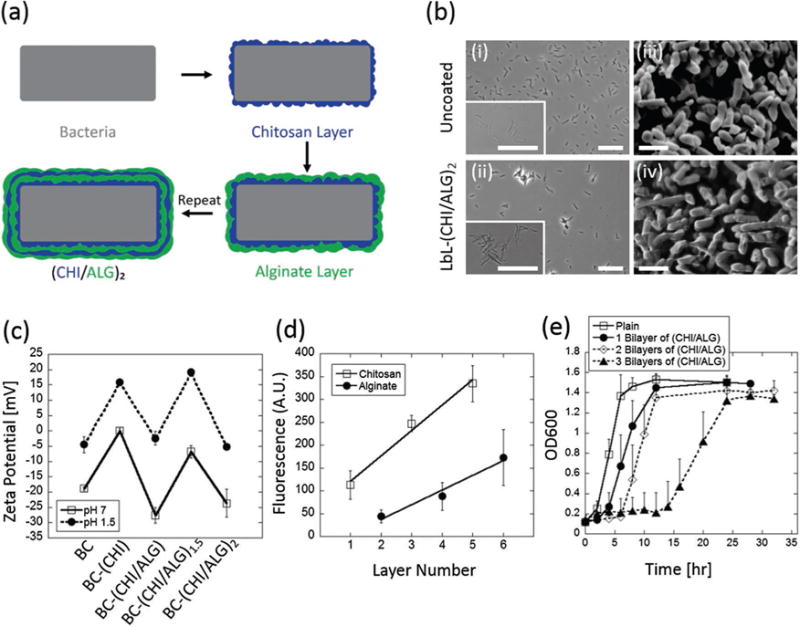
Layer-by-layer encapsulation of probiotics. a) Schematic LbL templating of chitosan and alginate on probiotic. b) Brightfield images of (i) uncoated-BC and (ii) LbL-(CHI/ALG)2-BC. SEM images of (iii) uncoated-BC and (iv) LbL-(CHI/ALG)2-BC. c) Zeta potential at each sequential layer, for up to two chitosan and alginate bilayers, (CHI/ALG)2, at pH 1.5 and 7. d) Uniform layer templating for up to three bilayers of chitosan and alginate was confirmed via measuring fluorescently labeled chitosan and alginate. e) Bilayer number modulates probiotic growth. As bilayer number increases, the time taken to reach the exponential growth phase is shifted to the right. Error bars represent standard deviation (n = 3). Bright field scale bars = 25 μm. SEM scale bars = 2 μm.
Passage through the GI tract involves encounters with several biological insults, including: (i) the acidic conditions of the stomach which can reach pH values as low as 1.5 and (ii) bile salts which are encountered throughout the intestines and are known to solubilize lipids[28] and thereby kill probiotic cells. Plain-BC were subjected to SGF (pH 2) (Figure 2a) to mimic acidic stomach conditions and 4% bile salt in PBS (pH 7.3) solutions (Figure 2b) to mimic small intestine conditions for up to 2 h at 37 °C. In both cases (Figure 2a,b), rapid cell death occurred after 30 min of exposure of BC to either SGF or bile salts. Complete reduction in CFU was observed in the case of an acidic insult after 2 h, indicating complete cell death (Figure 2a). This was expected, given that most probiotics, and BC specifically, cannot survive in acidic conditions.[29] While BC have shown to be resistant to low concentrations of bile salts,[29] they remain susceptible to higher bile salt concentrations, and in this case exhibited up to 6 log reduction in CFU in 4% bile salt solution at 2 h (Figure 2b). Five different LbL formulations, including: (i) chitosan alone (CHI)1, (ii) a single bilayer of chitosan/alginate (CHI/ALG)1, (iii) two bilayers of chitosan/alginate (CHI/ALG)2, (iv) a single bilayer of chitosan/L100 (an enteric polymer) (CHI/L100)1, and (v) two bilayers of chitosan/L100 (CHI/L100)2 were investigated for their potential to protect BC from acid and bile salt insults (Figure 2c). A single layer of chitosan, (CHI)1, was not sufficient in protecting BC from either bile salt or acid insults (Figure 2c). The anionic enteric polymer L100, widely used for its stability in acidic conditions,[30] was used as the terminal layer in (CHI/L100)1 and (CHI/L100)2 groups to investigate the role enteric polymers have in granting protection against both acidic and bile insults. In these cases, the enteric polymer protected against SGF insults, providing 3.5 and 1 log reductions in CFU in the cases of one and two bilayers, respectively (Figure 2c). However, these same enteric coatings were unsuccessful in protecting against bile insults (Figure 2c), likely because bile salts are present in the small intestine under neutral pH conditions, which are unstable conditions for L100. A single bilayer of chitosan and alginate, (CHI/ALG)1, protected against both bile salts and SGF, exhibiting 4 log reduction in CFU in both cases (Figure 2c). When BC were encapsulated in (CHI/ALG)2, less than 1 log reduction in CFU was observed in SGF conditions and less than 2 log reduction in CFU was observed in 4% bile salts after 2 h (Figure 2c). In this case, protection against acidic and bile salt conditions likely stem from the terminal alginate layer, given that alginate shrinks and forms an insoluble alginic skin-like structure[24] it is possible that this limits diffusion of H+ ions and bile salts into the bacteria cell.[31] (CHI/ALG)2 LbL-BC exhibited significant survival advantages against both acid and bile insults as compared to their plain, single-bilayer coated, and enteric-coated counterparts; as such, (CHI/ALG)2 was further investigated for adhesion and growth on intestinal tissues.
Figure 2.
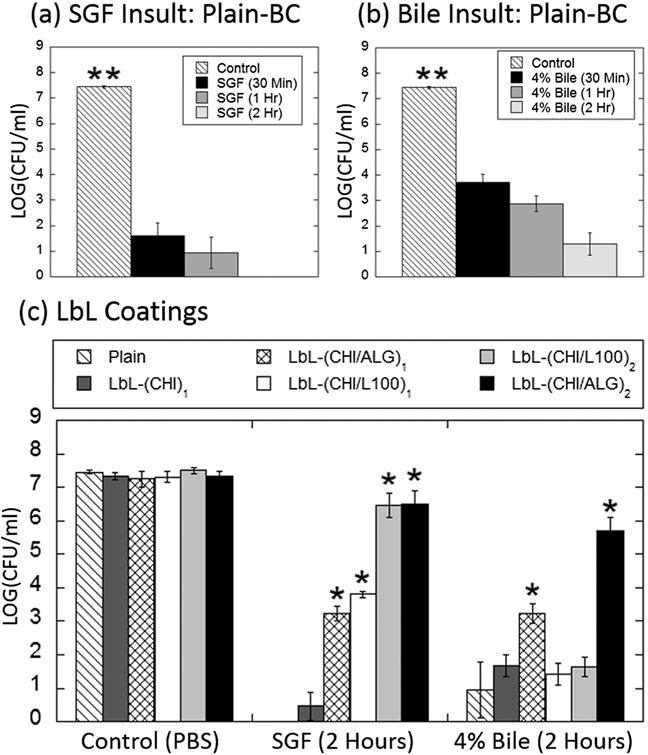
Effect of LbL coatings on probiotic survival against acid and bile insults. a) Plain, non-layered, BC rapidly die when exposed to simulated gastric fluid at 37 °C. b) Plain, non-formulated, BC rapidly die when exposed to 4% bile salt solution at 37 °C. c) LbL-formulated (CHI/ALG)2 (black bars) BC are protected against both acidic and bile salt insults at 37 °C for up to 2 h. LbL coatings of chitosan (dark gray bars), (CHI/L100)1 (white bars), (CHI/L100)2 (light gray bars), and CHI/ALG)1 (cross-hatched bars) are less effective at protecting BC against both acid and bile insults. Error bars represent standard deviation (n = 3). *denotes statistical difference (P < 0.05) using Student’s t-test between plain and LbL groups. **denotes statistical difference (P < 0.05) using individual Student’s t-test between the designated group and each other group.
The impact of (CHI/ALG)2 LbL-coatings on probiotic mucoadhesion and growth on intestinal tissues was investigated using freshly isolated sections of porcine small intestines. After 1 h incubation at 37 °C, and subsequent removal of non-adhered probiotics, (CHI/ALG)2 LbL-BC exhibited nearly 1.5-fold higher adherence to the mucosal surface of porcine intestine as compared to plain-BC (Figure 3a,b). Mucoadhesion advantages for (CHI/ALG)2 LbL-BC possibly arise from the mucoadhesive properties of the chitosan layers, which are likely exposed as soon as 30 min, as the terminal alginate layer begins to be released (Figure S1, Supporting Information).[32] Additionally, the terminal alginate layer also provides mucoadhesive advantages via its ionic strength and swelling properties.[24,33] Given the rapid passage of microbes (<2 h) through the small intestine,[34] mucoadhesion at short timepoints is essential to ensure the attachment and subsequent growth of probiotics in target areas. Intestine-mimicking tissues from humans (MatTek EpiIntestinal) were used to compare the growth of plain-BC and LbL-BC on live mammalian intestine tissues. The EpiIntestinal system is an isolated intestinal model proven to recreate physiological intestine structures.[35] Unlike the porcine intestine model, the EpiIntestinal system is a long-term cultured sterile human model that is better suited for studies tracking probiotic growth since no interference from other microbes will occur. The main similarity between these two systems includes the presence of a mucoadhesive layer and the presence of many similar intestinal cells. Bioluminescent plain-BC and (CHI/ALG)2 LbL-BC were placed in direct contact with EpiIntestinal tissue for 1 h, then washed, imaged (Figure 3c), and analyzed for total emitted radiance (Figure 3d) to track and compare their intestinal-adhesion and growth kinetics for up to 12 h. Since unbound BC pass through the GI tract in physiological situations, unbound BC were washed from the EpiIntestinal surface at each timepoint. After the first washing step at 1 h, a near threefold increase in BC was observed for the LbL formulation (Figure S3, Supporting Information), highlighting the mucoadhesive advantages this LbL system offers at short timepoints. Beyond this initial timepoint, signal for LbL-BC remains over twofold higher at 2 and 6 h (Figure S3, Supporting Information). However, at 12 h, these differences diminish as the intestinal surface begins to saturate as evidenced by the non-significant increase in p between 6 and 12 h for LbL-(CHI/ALG)2. Collectively, these results indicate that the enhanced mucoadhesion provided by LbL-BC leads to growth advantages during the first 6 h. This is likely because more probiotics adhere directly to the intestine tissue at short timepoints, and thus they replicate and reach the exponential growth phase faster.
Figure 3.
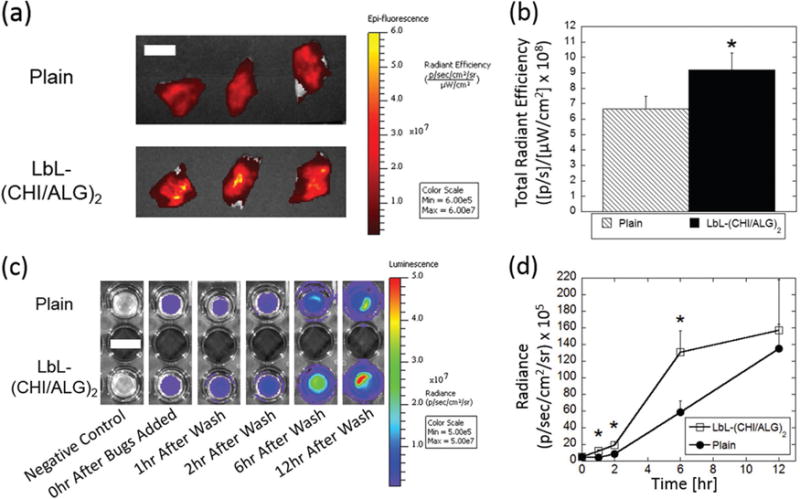
LbL coatings enhance physical retention with intestines. a) IVIS images of porcine intestine with plain- and (CHI/ALG)2-probiotics. b) Total radiant efficiency as measured by IVIS of plain-BC (hatched) and (CHI/ALG)2-BC (black). LbL-BC exhibit more rapid growth after 1 h incubation on intestine-mimicking tissues. Non-adherent BC were washed at 1 h and at each timepoint thereafter to ensure signal is exclusively from intestine-bound BC. c) Representative bioluminescent images of plain (top) and LbL (bottom) BC at each timepoint. d) Radiance of LbL- (open squares) and plain-(closed circles) BC as measured by IVIS up to 12 h. Error bars represent standard deviation (n = 3). *denotes statistical difference (P < 0.05) using Student’s t-test between plain and LbL groups. (a) Scale bar = 1.5 cm. (c) Scale bar = 1 cm.
The role of LbL coatings on survival and delivery of probiotics in vivo was investigated by delivering an identical number of bioluminescent plain-BC and (CHI/ALG)2 LbL-BC via oral gavage (Figure S4, Supporting Information). Single-layer chitosan/alginate and enteric-layer formulations were not evaluated in vivo, as they did not provide protective benefits against both SGF and bile insults (Figure 2c). 1 h after administration, bioluminescent (CHI/ALG)2 LbL-BC emitted over sixfold enhanced signal over background in the GI tract as compared to plain-BC (Figure 4a–c). Representative images (see Figure S5, Supporting Information, for individual animal images) highlight how (CHI/ALG)2 LbL-BC (Figure 4b) were capable of surviving harsh stomach conditions and reaching the bile-rich small intestine.[28] In vivo, LbL-BC exhibited significant survival advantages as compared to plain-BC, likely because (CHI/ALG)2 LbL-BC dramatically outperform plain-BC in terms of: (i) survival against acid and bile insults (Figure 2), (ii) mucoadhesion to, and growth on, intestinal surfaces at short timepoints (Figure 3), and (iii) direct growth on intestinal surfaces (Figure 3).
Figure 4.
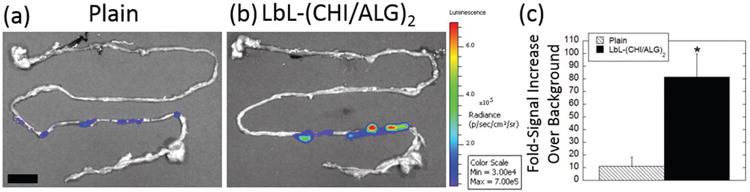
LbL coatings lead to enhanced survival of probiotics in vivo. Representative IVIS images of a) plain-BC and b) LbL-BC 1 h after oral gavage. c) Fold-signal increase over background for plain (hatched) and LbL (black) BC 1 h after oral gavage of an identical number (8.5 × 108 CFU) of BC. Error bars represent standard deviation (n = 4). *denotes statistical difference (P < 0.05) using Student’s t-test between plain and LbL groups. Scale bar = 1.5 cm.
In conclusion, this work demonstrates that LbL templating of probiotics offers a promising strategy to introduce specific probiotic species into the GI tract. The LbL technique described here addresses the chemical, physical, and probiotic-specific oral delivery challenges by simultaneously enhancing:
survival of probiotics against acidic and bile salt insults,
mucoadhesion and growth on intestinal tissues, and
survival in vivo. Moreover, the LbL-probiotic encapsulation could potentially be used with virtually any charged polyelectrolyte, protein, or polysaccharide and in combination with any probiotic strain. In particular, this study lays the foundation for technologies designed to introduce viable probiotics into the gastrointestinal microbiome for improved human health.
Supplementary Material
Acknowledgments
This work was funded by the Bill & Melinda Gates Foundation and the NIH under grant No. EB000244.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
References
- 1.a) Schwabe RF, Jobin C. Nat Rev Cancer. 2013;13:800. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Cho I, Blaser MJ. Nat Rev Genetics. 2012;13:260. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Biteen JS, Blainey PC, Cardon ZG, Chun M, Church GM, Dorrestein PC, Fraser SE, Gilbert JA, Jansson JK, Knight R, Miller JF, Ozcan A, Prather KA, Quake SR, Ruby EG, Silver PA, Taha S, van den Engh G, Weiss PS, Wong GC, Wright AT, Young TD. ACS Nano. 2016;10:6. doi: 10.1021/acsnano.5b07826. [DOI] [PubMed] [Google Scholar]; b) Ash C, Mueller K. Science. 2016;352:530. doi: 10.1126/science.352.6285.530. [DOI] [PubMed] [Google Scholar]
- 3.a) Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. J Controlled Release. 2012;162:56. doi: 10.1016/j.jconrel.2012.06.003. [DOI] [PubMed] [Google Scholar]; b) Rokka S, Rantamäki P. Eur Food Res Technol. 2010;231:1. [Google Scholar]; c) Vidhyalakshmi R, Bhakyaraj R, Subhasree R. Adv Biol Res. 2009;3:96. [Google Scholar]
- 4.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. Nature. 2006;444:1027. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 5.Kostic AD, Gevers D, Siljander H, Vatanen T, Hyotylainen T, Hamalainen AM, Peet A, Tillmann V, Poho P, Mattila I, Lahdesmaki H, Franzosa EA, Vaarala O, de Goffau M, Harmsen H, Ilonen J, Virtanen SM, Clish CB, Oresic M, Huttenhower C, Knip M, D. S. Group. Xavier RJ. Cell Host Microbe. 2015;17:260. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JFWM, Tijssen JGP, Speelman P, Dijkgraaf MGW, Keller JJ. New Engl J Med. 2013;368:407. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 7.Foster JA, Neufeld KAM. Trends Neurosci. 2013;36:305. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Science. 2005;308:1635. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. Nature. 2012;488:621. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, Erdman SE, Alm EJ. Genome Biol. 2014;15:R89. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding W, Shah N. J Food Sci. 2007;72:M446. doi: 10.1111/j.1750-3841.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- 13.des Rieux A, Fievez V, Garinot M, Schneider Y-J, Préat V. J Controlled Release. 2006;116:1. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Theeuwes F. J Pharm Sci. 1975;64:1987. doi: 10.1002/jps.2600641218. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Bellinger AM, Glettig DL, Barman R, Lee YAL, Zhu J, Cleveland C, Montgomery VA, Gu L, Nash LD, Maitland DJ, Langer R, Traverso G. Nat Mater. 2015;14:1065. doi: 10.1038/nmat4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howden C. Aliment Pharmacol Ther. 2005;22:25. doi: 10.1111/j.1365-2036.2005.02709.x. [DOI] [PubMed] [Google Scholar]
- 17.Gupta V, Hwang BH, Lee J, Anselmo AC, Doshi N, Mitragotri S. J Controlled Release. 2013;172:753. doi: 10.1016/j.jconrel.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Culligan EP, Hill C, Sleator RD. Gut Pathog. 2009;1:1. doi: 10.1186/1757-4749-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pamer EG. Science. 2016;352:535. doi: 10.1126/science.aad9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.a) Hammond PT. Mater Today. 2012;15:196. [Google Scholar]; b) Becker AL, Johnston AP, Caruso F. Small. 2010;6:1836. doi: 10.1002/smll.201000379. [DOI] [PubMed] [Google Scholar]
- 21.Hun L. Postgrad Med. 2009;121:119. doi: 10.3810/pgm.2009.03.1984. [DOI] [PubMed] [Google Scholar]
- 22.Payot T, Chemaly Z, Fick M. Enzyme Microb Technol. 1999;24:191. [Google Scholar]
- 23.Fitzpatrick LR, Small JS, Greene WH, Karpa KD, Keller D. Gut Pathog. 2011;3:1. doi: 10.1186/1757-4749-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George M, Abraham TE. J Controlled Release. 2006;114:1. doi: 10.1016/j.jconrel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Chen KL, Mylon SE, Elimelech M. Environ Sci Technol. 2006;40:1516. doi: 10.1021/es0518068. [DOI] [PubMed] [Google Scholar]
- 26.Shockman GD, Barren J. Annu Rev Microbiol. 1983;37:501. doi: 10.1146/annurev.mi.37.100183.002441. [DOI] [PubMed] [Google Scholar]
- 27.Richardson JJ, Liang K, Kempe K, Ejima H, Cui J, Caruso F. Adv Mater. 2013;25:6874. doi: 10.1002/adma.201302696. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann A, Small D. Annu Rev Med. 1967;18:333. doi: 10.1146/annurev.me.18.020167.002001. [DOI] [PubMed] [Google Scholar]
- 29.Hyronimus B, Le Marrec C, Sassi AH, Deschamps A. Int J Food Microbiol. 2000;61:193. doi: 10.1016/s0168-1605(00)00366-4. [DOI] [PubMed] [Google Scholar]
- 30.Sonaje K, Chen YJ, Chen HL, Wey SP, Juang JH, Nguyen HN, Hsu CW, Lin KJ, Sung HW. Biomaterials. 2010;31:3384. doi: 10.1016/j.biomaterials.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 31.Strugala V, Avis J, Jolliffe IG, Johnstone LM, Dettmar PW. J Pharm Pharmacol. 2009;61:1021. doi: 10.1211/jpp/61.08.0005. [DOI] [PubMed] [Google Scholar]
- 32.Smart JD. Adv Drug Delivery Rev. 2005;57:1556. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Bernkop-Schnürch A, Kast CE, Richter MF. J Controlled Release. 2001;71:277. doi: 10.1016/s0168-3659(01)00227-9. [DOI] [PubMed] [Google Scholar]
- 34.Berlec A, Završnik J, Butinar M, Turk B, Štrukelj B. Microb Cell Fact. 2015;14:1. doi: 10.1186/s12934-015-0376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maschmeyer I, Lorenz AK, Schimek K, Hasenberg T, Ramme AP, Hubner J, Lindner M, Drewell C, Bauer S, Thomas A, Sambo NS, Sonntag F, Lauster R, Marx U. Lab Chip. 2015;15:2688. doi: 10.1039/c5lc00392j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


