Abstract
Kennedy and Pottier discovered that photodynamic therapy (PDT) could be carried out using a procedure consisting of topical application of the porphyrin-precursor, 5-aminolevulinic acid (ALA) to the skin, followed after some time by illumination with various light parameters in the 1980s. Since then, ALA-PDT has expanded enormously and now covers most aspects of dermatological disease. The purpose of this review is to discuss a range of ingenious strategies that investigators have devised for improving the overall outcome (higher efficiency and lower side effects) of ALA-PDT. The big advance of using ALA esters instead of the free acid to improve skin penetration was conceived in the 1990s. A variety of more recent innovative approaches can be divided into three broad groups: (a) those relying on improving delivery or penetration of ALA into the skin; (b) those relying on ways to increase the synthesis of protoporphyrin IX inside the skin; (c) those relying on modification of the illumination parameters. In the first group, we have improved delivery of ALA with penetration-enhancing chemicals, iontophoresis, intracutaneous injection, or fractionated laser. There is also a large group of nanotechnology-related approaches with ALA being delivered using liposomes/ethosomes, ALA dendrimers, niosomes, mesoporous silica nanoparticles, conjugated gold nanoparticles, polymer nanoparticles, fullerene nanoparticles, and carbon nanotubes. In the second group, we can find the use of cellular differentiating agents, the use of iron chelators, and the effect of increasing the temperature. In the third group, we find methods designed to reduce pain as well as improve efficiency including fractionated light, daylight PDT, and wearable light sources for ambulatory PDT. This active area of research is expected to continue to provide a range of intriguing possibilities.
Keywords: 5-Aminolevulinic acid, Protoporphyrin IX, Heme biosynthesis, Skin penetration, Photodynamic therapy, Nanotechnology
Introduction: PDT with ALA and ALA Esters
The birth of ALA-PDT can be traced back to studies that were concerned with the biochemistry of porphyria [1••]. The term “porphyria” describes a range of rare genetic diseases that are characterized by disturbances to the heme biosynthetic cycle (see Fig. 1) that provides all the tetrapyrrole molecules required, not just for the oxygen carrying capability of hemoglobin and myoglobin, but for a myriad of enzymes and pathways that require heme-based co-factors and cytochromes. Since all mammalian cells rely on oxidative phosphorylation for (at least part) of their energy supply, the heme biosynthesis pathway must be present to some degree in all the tissues of the body. There are many varieties of the inherited disease called “porphyria,” but the one that is relevant to this review is erythropoietic protoporphyria (EPP) caused by a deficiency (genetic or acquired) in the last enzyme in the heme synthesis chain, ferrochelatase. Since the immediate precursor of heme, protoporphyrin IX (PPIX) is a photosensitizer, sun-exposed areas of the skin can suffer severe photodamage in this disease. 5-Aminolevulinic acid (ALA) is a stable small molecule precursor of the pathway, and if this compound is added exogenously to cells in culture, or to experimental animals (either systemically or topically), some degree of additional free porphyrin synthesis is observed (frequently assessed by the characteristic red fluorescence of PPIX). In the 1950s, four human volunteers orally consumed an ALA solution and suffered from dose-related phototoxic skin reactions especially after sun exposure [2]. These observation provided Kennedy and Pottier with the inspiration to test whether topical application of ALA to the skin followed by illumination could carry out photodynamic therapy (PDT) [3•]. The idea was that there was a feedback inhibition loop involving excess heme inhibiting ALA-synthetase that serves to prevent the heme biosynthetic cycle from producing more porphyrins than were required to make the amounts of heme the cell needs. However, the addition of exogenous ALA bypasses the feedback control, and an excess of free PPIX accumulates since ferrochelatase (which insets the ferrous iron into heme) is the slowest acting enzyme in the cycle, and is therefore rate limiting.
Fig. 1.
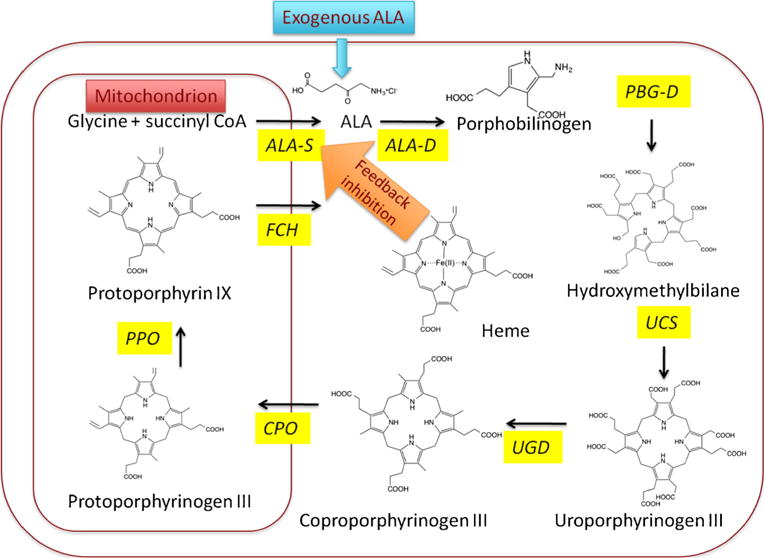
Heme biosynthetic cycle in eukaryotic cells. Exogenously administered ALA bypasses the feedback control mechanism whereby heme inhibits ALA synthase which is designed to prevent excess accumulation of PPIX in normal cells. Consequently, excess free PPIX accumulates in the cells since ferrochelatase is now rate limiting. ALA-S 5-aminolevulinic acid synthase, ALA-D 5-aminolevulinic acid dehydratase, PBG-D porphobilinogen deaminase, UCS uroporphyrinogen co-synthase, UGD uroporphyrinogen decarboxylase, CPO coproporphyrinogen oxidase, PPO protoporphyrinogen oxidase, FCH ferrochelatase
The principle of PDT had been discovered as early as 1900, but in 1978, Dougherty et al. published striking results in which they treated 113 cutaneous or subcutaneous malignant tumors by intravenous injection of a hematoporphyrin derivative and observed a total or partial response in 111 cases [4••]. Photofrin-PDT was first approved in the USA in 1993 for treatment of bladder cancer [5]. Kennedy and collaborators in the 1990s treated superficial skin cancers (mainly basal cell carcinomas) with topically applied ALA and subsequent illumination using broad-band white light from a slide projector [3•].
ALA-PDT was first used on a large scale for treating actinic keratosis (AK) when activated by blue light and received US approval as Levulan Kerastick with BLU-U activation in 1999 [6]. One big advance in ALA-PDT was made when the use of ALA esters was introduced. The idea here was that the more lipophilic structure of the esters (compared to the free carboxylic acid) would increase the speed and amount of uptake into cells, and therefore, more PPIX would be formed faster [7]. The methyl ester of ALA was the first to be approved for treating BCC and AK in 2001 as Metvix or MAL [8]. MAL-PDT when activated by red light was approved in the USA for treating AK. The hexyl ester of ALA was approved for use in the bladder (for fluorescence diagnosis) as Hexvix (now Cysview) in 2010. Numerous other esters of ALA have been investigated in the laboratory setting [9].
Improvements in ALA Penetration
Since the most important limiting factor in ALA-PDT is the penetration of the relatively polar molecule ALA through the permeability barrier of the skin, the lipophilic stratum corneum, many approaches have concentrated on increasing penetration. The approaches tested for increasing ALA penetration are schematically illustrated in Fig. 2.
Fig. 2.
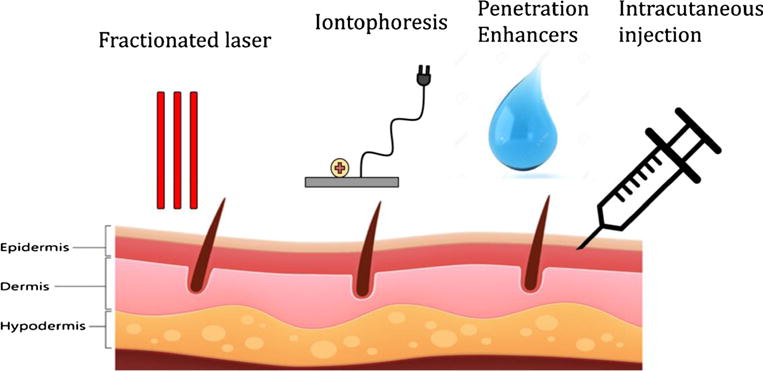
Methods of increasing ALA penetration into the skin. Fractional laser, iontophoresis, penetration enhancers, and intracutaneous injection are all methods/techniques to either improve the permeability of the skin or increase the penetration ability of ALA. Fractional laser irradiates the skin to remove skin layers in microscopic holes so ALA can reach deeper structures underneath. Iontophoresis is a technique to push compounds into the body through the skin by applying a local electric current and creating a repulsion force. Penetration enhancers work in combination with ALA (usually as an emulsion of other substances) to increase the ability of ALA to move transdermally across the lipophilic stratum corneum. Intracutaneous injection is the simple approach to inject ALA into the skin
Intracutaneous ALA
The simplest way to get more ALA into the skin is simply to inject it into the skin where it is meant to be. This seemingly obvious approach was not actually tested until 2001. De Blois et al. [10] administered ALA to the normal skin of 6-week-old female Dutch pigs by intracutaneous injection of an aqueous solution of ALA (pH 5.0) in volumes of 0.1–0.5 ml and concentrations ranging from 0.5 to 2 % and by topical administration of a 20 % ALA cream. All injected doses of 3 mg ALA or more led to a faster initial increase in the rate of PpIX synthesis and significantly greater fluorescence than that measured after topical administration of ALA. Irradiation (60 J/cm2 of 600–730 nm) of the spots was performed at 3.5 h after ALA administration. Injection of 2 mg ALA or more followed by light gave more PDT damage than after topical application of 20 % ALA.
Sakamoto et al. [11] in 2011 obtained similar data in a pig model looking at intracutaneous injection of ALA and illumination with a 635 nm LED array at a fluence of 200 J/cm2.
Combination with Fractional Laser
A different approach to ALA delivery into the skin is through manipulating the skin surface with lasers. Applying ALA in combination with ablative and non-ablative fractional laser treatment helped increase the penetration of ALA into deeper structures of the skin. Ablative fractional laser is used as a pretreatment before ALA-PDT by removing skin material through irradiating the skin surface with a small scanned laser spot. This process creates small holes (see Fig. 2) in the skin in which ALA can more easily pass through. Non-ablative fractional laser is also used as a precursor to ALA-PDT but is less invasive and instead of removing the skin layer to create holes; it simply heats the skin (thermal denaturation) to increase penetration of ALA. The beneficial feature of fractional laser treatment is the fact that healing of the microscopic lesions in the skin is much faster than comparable amounts of damage produced in macroscopic lesions.
Haedersdal et al. [12] showed the success of ablative fractional laser (AFXL) first in an animal model. They showed that in Yorkshire, swine skin pretreated with the CO2 AFXL (10.6 μm, 1850 μm ablation depth) produced increased porphyrin fluorescence versus skin that was left intact after the topical application of ALA and MAL cream (20 %). This increase in topical drug uptake was also seen for non-ablative fractional laser pretreatment. Lim et al. [13] pretreated 12 (1 cm2) areas on the backs of 10 healthy male patients and followed the pretreatment with application of ALA. Sites pretreated with non-ablative fractional laser showed a significant increase in porphyrin fluorescence when compared with non-pretreated areas. The results also showed that laser energy strength and ALA incubation time were positively correlated with ALA absorption in the skin.
Fractional laser treatment in combination with ALA and MAL was also shown to have clinical effectiveness. Previously, ALA-PDT could not be used as a treatment for nodular basal cell carcinoma because these tumors were thicker than 2 mm. However, Lippert et al. [14] showed that ALA-PDT with ablative laser therapy could overcome the limit caused by tumor thickness in patients with nBCC as the increased penetration allowed ALA to reach deep enough into the tumor layers. Yin et al. [15] evaluated the effects of erbium: yttrium-aluminum-garnet (Er:YAG) ablative fractional laser resurfacing for treatment of severe acne. Severe acne is known to be difficult to treat by conventional methods, but with fractional laser resurfacing combined with ALA-PDT, the depth of penetration and unique ability to stimulate wound healing is promising as a new treatment approach. Forty subjects with severe acne were treated with 15 % ALA-PDT, 4 times over 40 days (10 day intervals) and then received ablative fractional Er:YAG laser treatment five times at 4 week intervals. After 6 months post-treatment, all subjects showed an overall improvement in acne inflammatory lesions and an 80 % improvement in acne scars.
Forster et al. [16•] investigated how ALA and MAL treatments were affected when combined with continuous versus fractional ablation lasers. The stratum corneum (SC) was irradiated with continuous and fractional ablation with 2940-nm Er:YAG laser systems (Continuous: 0.5–1.5 J/cm2; Fractional: 4–24 J/cm2). The mean fluoresces intensity enhanced 13.8-fold with continuous ablation and 7.3-fold with fractional ablation when compared against skin without laser pretreatment showing that continuous ablation increased ALA and MAL penetration by the greatest amount.
Lee et al. [17] looked at another parameter of fractional laser pretreatment with ALA-PDT. Lee and colleagues examined how the magnitude of fluences of fractional laser affected ALA permeation in nude mouse skin and porcine skin and found that laser-treated nude mouse skin versus intact skin had a 27–124-fold increase in ALA flux while porcine skin versus intact skin had a 3–260-fold increase. Compared to a conventional laser, the low fluence lasers showed less severe skin disruption, a shorter recovery duration and were overall safer.
Penetration Enhancement
Methods of increasing the permeability of ALA through the skin encompass a much wider variety than just ablative and non-ablative fractional laser treatments. In fact, subtleties exist between the different combinations of therapies with laser treatments, how to most effectively use these lasers as well as a plenty of other techniques that all help enhance the transdermal penetration of ALA.
Combinations of multiple different penetration enhancement techniques were examined by Fang et al. [18]. They investigated how electrically assisted methods of iontophoresis and electroporation used on laser- or microdermabrasion-treated skin affected ALA permeation. First, Fang et al. measured how Er:YAG laser and microdermabrasion affected ALA permeation through skin separately from other techniques. Microdermabrasion increased ALA permeation 5–15 times more than intact skin, while Er:YAG laser-treated skin had the greatest enhancement of ALA permeability by enhancing penetration by 4–246 times versus controls. In combination with iontophoresis, ALA permeation increased by another 15-fold and electroporation increased permeation by 2-fold. This combination of electrically assisted techniques along with resurfacing methods caused a strong synergistic effect on ALA permeation.
There exist other penetration enhancers that work in ways other than physically damaging the skin structure. Delivery vehicles and ALA mixtures are an alternative to combination therapies listed above. De Rosa et al. [19] studied how ALA emulsions containing dimethyl sulfoxide (DMSO) and ethylenediaminetetraacetic acid disodium salt (EDTA) affected ALA-induced protoporphyrin IX. De Rosa et al. used a 20 % DMSO oil-in-water emulsion that increased the in vitro permeation of ALA, while an oil-in-water emulsion contain 10 % ALA, 3 % EDTA and 20 % DMSO significantly increased PpIX. However, an EDTA emulsion had no effect on short-term protoporphyrin IX accumulation in skin. Maisch et al. [20] found similar data when analyzing the effects of a nanoemulsion-based formulation of ALA (BF-200 ALA) for increased penetration of ALA. ALA penetration was 4.8-fold and 5.0-fold stronger after 8 and 12 h, respectively, for BF-200 ALA use than after use of the control ALA cream. These delivery formulations have clinical applications, just as the laser therapies did. Fang et al. [21] looked at how ALA-PDT in tandem with acid-encapsulated ethosomes could help treat the inflammatory skin disease of psoriasis. Because the ALA precursor is hydrophilic, it has limited skin penetration. However, by using an ethosomal carrier for ALA, this hydrophilic barrier can be overcome and thus increase the penetration of ALA into the skin. Results of this paper found that the application of this ethosomal carrier produced increases of cumulative PpIX by 5–26-fold and ultimately lead to better outcomes in patients with psoriasis.
Iontophoresis
Iontophoresis is the technique for pushing or driving charged compounds into the body through the skin by applying a local electric current and creating a repulsion force. As mentioned earlier, iontophoresis can be an effective method for increasing the penetration of ALA through the skin. Krishnan et al. [22] first looked at using iontophoresis to deliver small peptide compounds (ALA and L-alanine-L-tryptophan) across human skin. Compared to passive diffusion, iontophoresis increased the rate of transport of L-alanine-L-tryptophan and ALA by 15- and 22-fold, respectively. Similar to the nanoemulsions discussed previously, Merclin et al. [23] investigated how iontophoresis interacted with ALA dissolved in a lipid sponge phase of monoolein, propylene glycol, and an aqueous buffer. The sponge phase mixture resulted in a higher passive flux (140 nmol/cm2/h at 5 h) and a lower iontophoretic flux (800 nmol/cm2/h at 5 h). These numbers were comparable to passive fluxes from more concentrated vehicles and show the potential this method has to be a transdermal drug delivery vehicle.
Improvements in ALA Delivery Using Nanotechnology
One of the most important limitations of ALA-PDT is the low permeability of ALA through the stratum corneum (SC) of the skin. The hydrophilicity of ALA prevents meaningful diffusion through the highly hydrophobic stratum corneum, which is known to be a major barrier against transcutaneous drug delivery. To overcome this hydrophobic barrier and to enhance ALA penetration, the new field of nanotechnology and drug delivery has been harnessed. New synthetic and lipophilic vehicles such as liposomes, ALA dendrimers, and niosomes along with other nanotechnology-based vehicles are being investigated. However, despite the promising future of nanoparticles as delivery vehicles for ALA (and in nanomedicine in general), we must not forget the concerns about the possible toxicity to both the patients themselves and to the wider environment that we all inhabit. Table 1 lists some of the nanodrug-delivery vehicles that have been investigated with ALA.
Table 1.
Reports of improved ALA delivery using nanotechnology and nanomedicine
| Nanoparticle | Structure | Type of Experiment/Animal Model | Advantages | Clinical Application | Ref |
|---|---|---|---|---|---|
| Liposomes |
|
In vitro and in vivo in rats | Compatible with lipid bilayer structure May employ surface ligands to attach to tissue |
Drug delivery to treat: Skin cancer, Acne, Wrinkles |
[17, 24, 25] |
| Ethosomes |
|
Mice | Greater penetration ability than liposomes, smaller particle size | Skin cancer treatment | [21, 26] |
| Niosomes |
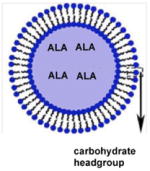
|
Excised human skin | 80 and 40% increased permeation of drug and 50 to 100% of the drug retained into the skin when compared to aqueous drug solution | Therapeutic treatment for skin malignancies, hidradenitis suppurativa | [27] |
| Polymeric nanoparticles |
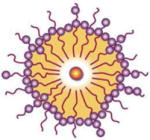
|
HeLa cancer cells Ex vivo skin transport |
10× higher transdermal transport Increased cell killing by PDT |
[28–30] | |
| Mesoporous Silica |

|
Mice | Bypasses the lipophilic barrier to directly enter into cancer cells Exhibited high photocytotoxicity to cancer cells in vitro |
[31] | |
| Conjugated Gold Particles |
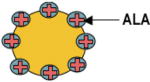
|
Human chronic myeloid leukemia K562 cells, biodistribution in rabbits Human fibroblast and A431 cells |
Used to circumvent multidrug resistance mechanisms Singlet oxygen generation by localized surface plasmon resonance of GNPs |
Early diagnosis and therapy of atherosclerosis | [32–36] |
| Fullerene Nanoparticles |
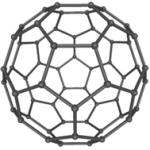
|
Mice | No detectable toxicity Compared to ALA, significant improvement in antitumor efficacy |
[37] | |
| ALA Dendrimers |
|
LM3 mammary carcinoma cells, PAM 212 murine keratinocyte and A431 human epidermoid carcinoma cell lines | More efficient at low concentrations compared to equimolar ALA for porphyrin production, most lipophilic, minimal dark toxicity, high drug payload | [38–40] | |
| Carbon Nanotubes |
|
MGC-803 tumor cells | Increased PPIX formation Strong optical absorbance in NIR (near-infrared) light |
[41] |
Improvements in Cellular PPIX Synthesis
Another approach to improve ALA-PDT involves manipulation of the biochemical pathways of the heme biosynthetic cycle within the cells themselves (see Fig. 3).
Fig. 3.
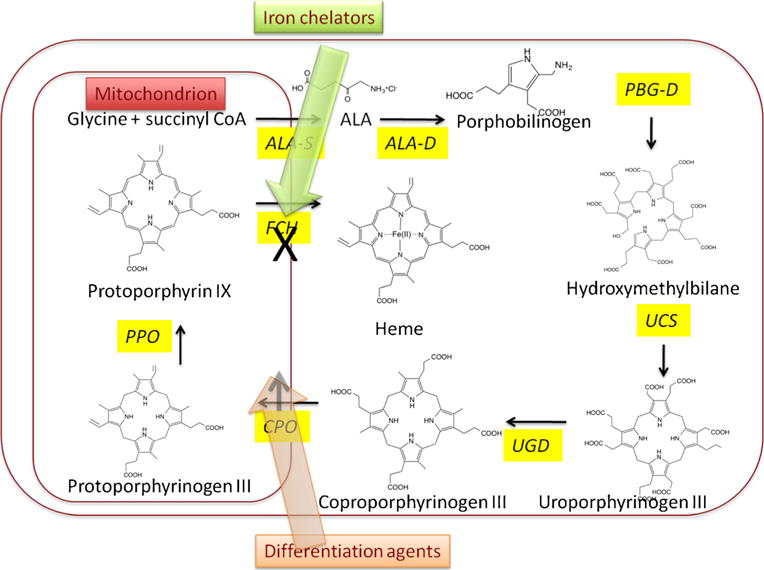
Methods of biochemically enhancing PPIX accumulation by modulating enzymes in heme biosynthesis cycle. Iron chelators can enhance PPIX accumulation by depriving the enzyme ferrochelatase of the ferrous iron it needs to form heme. Differentiation agents (vitamin D, retinoids, methotrexate, calcium, or androgens) can increase expression of coproporphyrinogen oxidase producing more PPIX
Combination with Iron Chelators
Due to its unique biochemical and biophysical properties, iron plays a role in most important processes within cells. Its availability is crucial for rapidly proliferating tumor cells to convert PPIX into heme. After the exogenous ALA has induced the PPIX production, ferrous iron (free Fe2+) is inserted into PPIX by the action of the ferrochelatase enzyme, which converts PPIX into heme. Iron chelators can combine with the ferrous iron within the cells and remove it from the heme synthesis system. Moreover, iron chelators also have the ability to inhibit ferrochelatase activity. Employing iron chelators prevents the formation of heme, and as a result, more PPIX accumulates inside the cells [42]. Up to now, the following iron chelators including ethylenediaminetetraacetic acid (EDTA), desferrioxamine (DFO), 1,2-diethyl-3-hydroxypyridin-4-one hydrochloride (CP94), and dexrazoxane (ICRF-187) [43•] have all been proved to increase PPIX accumulation and photosensitization in PDT. They can be divided into specific and nonspecific iron chelators. EDTA is a nonspecific chelator of metals, which has been found to have the potential to increase accumulation of endogenous porphyrins in the skin and ALA-induced PPIX fluorescence and phototoxicity in the Hep-2 cell line. By treating non-melanoma skin cancer patients with ALA-PDT combined with Ca2+Na+2 EDTA, Liu et al. found greater depth of PPIX production and PDT necrosis compared to ALA-PDTalone [44]. This observation suggested the clinical outcome could be improved. Desferrioxamine (DFO) is an iron-specific chelator which has greater specificity for iron than EDTA and has higher affinity to bind iron, with a large preference over other metal ions. DFO has the potential to accumulate in high concentrations in the lysosomes and to complex with intra-lysosomal iron. Yang et al. investigated the effectiveness of DFO in enhancing PPIX-based photodynamic reaction in different skin targets, including fibroblasts, HaCat cells, and Hep-2 cells, and found that DFO showed different effects on PPIX accumulation and PDT reaction in different cells lines [45]. CP94 is a novel hydroxypyridinone iron-chelating agent that was proved to be superior to DFO in the enhancement of PPIX accumulation using ALA and MAL in fetal lung fibroblasts, squamous carcinoma cells, and human glioma cells [46]. Dexrazoxane (ICRF-187) is a clinically approved cardioprotective agent against anthracycline-induced heart damage. Dexrazoxane can readily penetrate cell membranes and is converted into two one-ring open intermediates in cells followed by the formation of ADR-925, which can either remove iron from the iron-anthracycline complex that causes cardiotoxicity or else can bind to free iron. It has been reported that dexrazoxane and its hydrolysis products are all good iron-chelating agents that could increase PPIX formation [47].
Combination with Differentiating Agents
However, the cure rates of superficial skin carcinomas using ALA-PDT are still not sufficient, based on the fact that ALA-induced PPIX formation is related to the differentiation state in an erythroleukemia cell line [48•]. Ortel and his colleague studied how the effectiveness of ALA-dependent PDT depends on the differentiation state of the cell. They found that the more differentiated tumors with slowly cycling cells could actually accumulate more PPIX that less differentiated tumors with rapidly cycling cells and therefore could be better targets for ALA-dependent PDT [49•]. Several factors such as increased ALA uptake, enhanced PPIX production, and decreased PPIX export from the cell could all possibly contribute to the elevated level of PPIX, which results in an enhanced lethal PDT effect in differentiated cells. The conclusion was made that differentiation therapy, which is an approach to reverse the lack of differentiation in tumor cells using pharmacological agents, may play an important role in the enhancement of ALA-PDT. In a further study, Ortel and colleagues used the analogues of vitamin D, synthetic androgen R1881, and isomers of retinoic acid to produce a short-term differentiation therapy with 3 to 4 days in LNCaP cells. They found that not only the overall PPIX production was increased but also the fraction of cells that contained low PPIX levels was reduced after the treatment [50]. Until now, the following agents have been shown to improve PPIX accumulation and enhance the therapeutic response: (i) In patients, various carcinomas and psoriasis lesions pretreated with vitamin D and its derivatives [51, 52]; (ii) Skin or prostate carcinoma cells pretreated with methotrexate; (iii) Actinic keratosis lesions pretreated with retinoids [53]; (iv) Skin keratinocytes pretreated with calcium; (v) Hormone-responsive prostate cancer cells pretreated with androgens [54]. The molecular mechanism of combining differentiation therapy and ALA-PDT in cancer involves C/EBP (a transcription factor)-mediated cellular differentiation. Previous studies proved that differentiation-promoting agents upregulate CCAAT/enhancer-binding proteins (C/EBP) and increase expression of coproporphyrinogen oxidase (CPO). C/EBP transcription factors are powerful regulators of cellular differentiation. CPO is a heme-pathway enzyme responsible for the conversion of coproporphyrinogen into coproporphyrin and then into PPIX. The cooperative interactions between regularly spaced C/EBP sites is critical for CPO transcription, which regulated by differentiation [54].
Temperature Modulated ALA-PDT
Because the biosynthesis of PPIX is a metabolic process, it appeared reasonable to ask whether the amount of PPIX that was synthesized could be affected by the temperature of the skin. It had been shown as early as 1999 that both in cells [55] and in mouse skin [56], there was a strong dependence of the amount of PPIX synthesized on temperature during the incubation. Willey et al. carried out a clinical trial in 20 patients with actinic keratoses (AK) on the arms or legs. They found that by raising the temperature of the skin on one limb from 29.4 to 38.8 °C during 1 h of application of ALA under occlusion, the response of the skin (reduction in number of AK lesions) to blue light-mediated PDT was significantly improved in the heated limb without a concomitant increase in side effects [57]. It is at present unclear what the exact mechanism of temperature modulated ALA-PDT actually is. It is possible that temperature increases ALA uptake, increases PPIX production inside the cells, or increases the oxygen supply during PPIX synthesis or during PDT. A recent paper suggests that increased temperature can produce increases in total apoptosis and elevate superoxide ROS generation between 33 and 42 °C [58].
Improvements Relying on Illumination
The last group of methods of improving the effectiveness of ALA-PDT that will be discussed focuses on manipulating the parameters of light delivery used to excite the PPIX formed in the skin after ALA application. These approaches are graphically illustrated in Fig. 4.
Fig. 4.
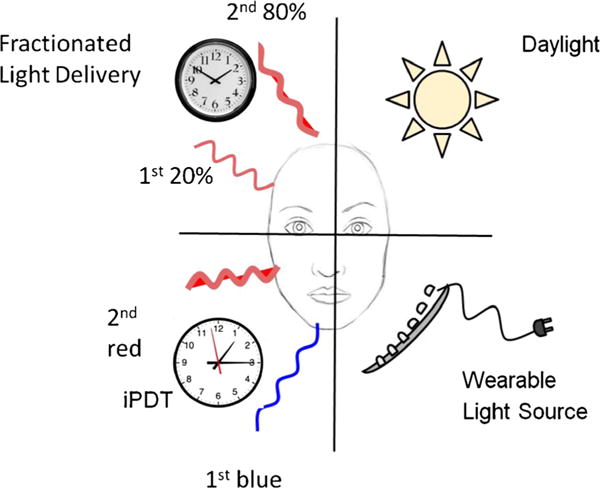
Methods of improving ALA-PDT by manipulating the light delivery parameters. Fractionated light delivery applies 20 % of the calculated red light dose after 4 h of ALA, incubation followed after 2 h by the remaining 80 % of the light dose. Daylight PDT involves exposing the patient to daylight for several hours as soon as the ALA has been applied. Wearable light sources allow a very low irradiance of red light to be applied for a longer time to reduce pain and make the treatment more patient friendly. iPDT uses an initial low dose of blue light exposure followed by red light to reduce the pain of treatment
Light Fractionation
The use of fractioned light delivery for ALA-PDT has been developed over the years by investigators in Rotterdam in the Netherlands [59]. The first report was as early as 1994 where it was shown that PDT damage in a mouse tumor was increased when the light was fractionated [60]. A clinical trial was then conducted in which 91 patients with 299 superficial BCC lesions were treated with a 2-fold illumination scheme with 2 light fractions of 20 and 80 J/cm2 delivered 4 and 6 h after a single application of 20 % ALA, and 106 patients with 274 lesions were treated with a single illumination of 75 J/cm2 4 h after ALA. The complete response rate was significantly greater following the 2-fold illumination than the single illumination [61•]. Similar results were obtained in a clinical trial of actinic keratoses (AKs) using the same parameters [62].
In order to understand the mechanism by which a fractionated light dose is superior, de Bruijn et al. [63] investigated PAM 212 cells in vitro. With a low (0.2 mM) ALA concentration (but not a high 2 mM ALA concentration), there was approximately an order of magnitude greater reduction in cell survival when two light fractions were delivered at 2 and 4 h compared to a single illumination with the same cumulative fluence at either time point. This data suggests that there is a mechanism by which cells are rendered more sensitive to light 2 h after the first sub-lethal light delivery. Very recently, de Bruijn et al. reported that light fractionation increased the PDT effect found with BF-200 (a nanoemulsion-based gel formulation of ALA) on mouse skin [64•].
Daylight ALA-PDT
The use of daylight PDT originated in Denmark [65]. The idea was that using daylight exposure to activate the PPIX during the incubation period would reduce the total amount of PPIX that accumulated within the skin at any one time, and this reduction would have a big effect on the level of pain suffered by patients during the procedure. Moreover for patients with large areas of the skin affected by AKs (field cancerization), daylight exposure would be much more practicable and convenient than using a medical light source to illuminate multiple sites on the skin. Rubel and colleagues [66] carried out a 24-week randomized, controlled, investigator-blinded, multi-center clinical trial of 100 subjects with AKs on the face/scalp. Patients received daylight (DL-PDT) on one side and conventional (c-PDT) on the contralateral side. At week 12, the complete lesion response rate with DL-PDT was non-inferior to c-PDT (89.2 vs. 92.8 %). For DL-PDT, subject-reported pain was significantly lower (0.8 vs. 5.7, respectively; p < 0.001), with better tolerability and significantly higher subject satisfaction regarding convenience and outcome.
Wearable Red Light Sources
With similar motivation to using daylight ALA-PDT, Moseley et al. came up with a new concept called “ambulatory PDT” [67]. Again, the idea was to allow long illumination times which would reduce total accumulation of PPIX in the skin and thereby reduce pain.
A prototype diode array, comprising 37 AlGaInP LEDs cast in epoxy with a diffuser, and driven by a battery pack, was designed to be attached to the patients’ skin with microporous tape. A pilot study was carried out in five patients with histologically proven Bowen’s disease who were referred for ALA-PDT. In accordance with the usual practice, patients received two treatments at a 4-week interval. Each treatment lasted for 1 h 40 min to deliver 75 J/cm2 at an irradiance of 12 mW/cm2. Pain levels were reduced to mild (3) or moderate (2) at the first treatment and mild (4) or none (1) at the second treatment. The same group went on to test a low-irradiance (5 mW/cm2), potentially disposable, lightweight, organic light-emitting diode (OLED), which is a light source (2 cm diameter), suitable for ambulatory PDT [68].
Cochrane and colleagues reported the design of flexible light sources that could be worn as fabrics [69]. The integration of plastic optical fiber (POF) and polyester yarn were woven into textile structures using a hand-loom, then both ends of the POF were coupled to a laser diode (5 W, 635 nm). Predetermined POF macrobending led to side emission of light when the critical angle is exceeded. The illumination was homogeneous over a fabric area of 100 cm2 with a fluence rate of 18 ± 2.5 mW/cm2. Optical losses were very low and the temperature elevation was only 0.6 °C after 10 min of illumination.
Inhibitory PDT with an Initial Blue Light Irradiation
The pain and swelling associated with ALA-PDT make it a poor choice for acne patients, particularly those with facial acne. In an effort to maintain efficacy but reduce pain, Sakamoto et al. [70] performed a randomized, double-blind controlled study to compare conventional ALA-PDT to a new method called “inhibitory PDT” (iPDT) that involves an initial irradiation with low levels of blue light during the incubation period. They found that low-level blue light exposure during ALA metabolism prevented the accumulation of PPIX in the superficial epidermis, while allowing for accumulation in deeper tissues, including the sebaceous glands. Both methods (conventional and iPDT) demonstrated significant improvement in the 18 subjects that finished the study (out of 29 original participants), but the intense pain and immediate inflammatory side effects were reduced in iPDT-treated sites.
Conclusion
ALA-PDT continues to fascinate both clinical dermatologists, alongside biomedical researchers and nanotechnologists because of the ingenuity of the approach. To take advantage of the body’s own intrinsic biochemical metabolic pathways to make a light-activated therapeutic molecule localized within the tissue where the ALA is actually applied is considered intellectually satisfying. As can be seen from our review, there is also no shortage of ingenious ways of improving its effectiveness. These range from methods familiar to all dermatologists such as fractionated laser, intracutaneous injection, and iontophoresis to approaches that are common in nanotechnology and drug delivery laboratories such as various liposomes, gold or silica nanoparticles, nanocarbon allotropes, etc. Because the cells are actually making the molecule that will eventually kill them, there is the opportunity to modulate the actual enzyme activity levels inside the cells to increase the amount of PPIX that accumulates inside the target cells. Finally, since nothing happens in PDT without the final coup de grace of light delivery, there is also an opportunity to play with the illumination parameters. This can help to reduce the pain suffered on light delivery, which is by far the most dominating side effect of ALA-PDT, to say nothing of improving the patient friendliness of the procedure. We expect that efforts will continue to be made to improve the effectiveness of ALA-PDT, which will become an even more fertile field for researchers.
Acknowledgments
MR Hamblin was supported by US NIH grant R01AI050875.
Footnotes
Conflict of Interest Dr. Connor Thunshelle, Dr. Rui Yin, Dr. Qiquan Chen, and Dr. Michael R Hamblin declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
This article is part of the Topical Collection on Laser Therapy
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1••.Krammer B, Plaetzer K. ALA and its clinical impact, from bench to bedside. Photochem Photobiol Sci. 2008;7(3):283–9. doi: 10.1039/b712847a. Good comprehensive review of the development of ALA-PDT, and its range of clinical applications. [DOI] [PubMed] [Google Scholar]
- 2.Berlin NI, Neuberger A, Scott JJ. The metabolism of delta-aminolaevulic acid. 1. Normal pathways, studied with the aid of 15N. Biochem J. 1956;64:80–90. doi: 10.1042/bj0640080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Kennedy JC, Pottier RH, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B. 1990;6(1–2):143–8. doi: 10.1016/1011-1344(90)85083-9. An early review by the original discoverers of ALA-PDT. [DOI] [PubMed] [Google Scholar]
- 4••.Dougherty TJ, Kaufman JE, Goldfarb A, Weishaupt KR, Boyle D, Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38(8):2628–35. An important landmark paper in the history of PDT used as a cancer therapy. [PubMed] [Google Scholar]
- 5.Marcus SL, Dugan MH. Global status of clinical photodynamic therapy: the registration process for a new therapy. Lasers Surg Med. 1992;12(3):318–24. doi: 10.1002/lsm.1900120313. [DOI] [PubMed] [Google Scholar]
- 6.Lang K, Schulte KW, Ruzicka T, Fritsch C. Aminolevulinic acid (Levulan) in photodynamic therapy of actinic keratoses. Skin Therapy Lett. 2001;6(10):1–2. 5. [PubMed] [Google Scholar]
- 7.Gaullier JM, Berg K, Peng Q, Anholt H, Selbo PK, Ma LW, et al. Use of 5-aminolevulinic acid esters to improve photodynamic therapy on cells in culture. Cancer Res. 1997;57(8):1481–6. [PubMed] [Google Scholar]
- 8.Christensen E, Warloe T, Kroon S, Funk J, Helsing P, Soler AM, et al. Guidelines for practical use of MAL-PDT in non-melanoma skin cancer. J Eur Acad Dermatol Venereol. 2010;24(5):505–12. doi: 10.1111/j.1468-3083.2009.03430.x. [DOI] [PubMed] [Google Scholar]
- 9.Fotinos N, Campo MA, Popowycz F, Gurny R, Lange N. 5-Aminolevulinic acid derivatives in photomedicine: characteristics, application and perspectives. Photochem Photobiol. 2006;82(4):994–1015. doi: 10.1562/2006-02-03-IR-794. [DOI] [PubMed] [Google Scholar]
- 10.de Blois AW, Grouls RJ, Ackerman EW, Wijdeven WJ. Development of a stable solution of 5-aminolaevulinic acid for intracutaneous injection in photodynamic therapy. Lasers Med Sci. 2002;17(3):208–15. doi: 10.1007/s101030200030. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto FH, Doukas AG, Farinelli WA, Tannous Z, Su Y, Smith NA, et al. Intracutaneous ALA photodynamic therapy: dose-dependent targeting of skin structures. Lasers Surg Med. 2011;43(7):621–31. doi: 10.1002/lsm.21073. [DOI] [PubMed] [Google Scholar]
- 12.Haedersdal M, Sakamoto FH, Farinelli WA, Doukas AG, Tam J, Anderson RR. Pretreatment with ablative fractional laser changes kinetics and biodistribution of topical 5-aminolevulinic acid (ALA) and methyl aminolevulinate (MAL) Lasers Surg Med. 2014;46(6):462–9. doi: 10.1002/lsm.22259. [DOI] [PubMed] [Google Scholar]
- 13.Lim HK, Jeong KH, Kim NI, Shin MK. Nonablative fractional laser as a tool to facilitate skin penetration of 5-aminolaevulinic acid with minimal skin disruption: a preliminary study. Br J Dermatol. 2014;170(6):1336–40. doi: 10.1111/bjd.12817. [DOI] [PubMed] [Google Scholar]
- 14.Lippert J, Smucler R, Vlk M. Fractional carbon dioxide laser improves nodular basal cell carcinoma treatment with photodynamic therapy with methyl 5-aminolevulinate. Dermatol Surg. 2013;39(8):1202–8. doi: 10.1111/dsu.12242. [DOI] [PubMed] [Google Scholar]
- 15.Yin R, Lin L, Xiao Y, Hao F, Hamblin MR. Combination ALA-PDT and ablative fractional Er:YAG laser (2,940 nm) on the treatment of severe acne. Lasers Surg Med. 2014;46(3):165–72. doi: 10.1002/lsm.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Forster B, Klein A, Szeimies RM, Maisch T. Penetration enhancement of two topical 5-aminolaevulinic acid formulations for photodynamic therapy by erbium:YAG laser ablation of the stratum corneum: continuous versus fractional ablation. Exp Dermatol. 2010;19(9):806–12. doi: 10.1111/j.1600-0625.2010.01093.x. Shows that FRAXEL laser increases ALA penetration into the skin and enhances PPIX production. [DOI] [PubMed] [Google Scholar]
- 17.Lee WJ, Jung HJ, Kim JY, Lee SJ, Kim DW. Effect of photodynamic therapy on inflammatory acne using 3% liposomal 5-aminolevulinic acid emulsion and intense-pulsed light: a pilot study. J Dermatol. 2012;39(8):728–9. doi: 10.1111/j.1346-8138.2012.01509.x. [DOI] [PubMed] [Google Scholar]
- 18.Fang JY, Lee WR, Shen SC, Fang YP, Hu CH. Enhancement of topical 5-aminolaevulinic acid delivery by erbium:YAG laser and microdermabrasion: a comparison with iontophoresis and electroporation. Br J Dermatol. 2004;151(1):132–40. doi: 10.1111/j.1365-2133.2004.06051.x. [DOI] [PubMed] [Google Scholar]
- 19.De Rosa FS, Marchetti JM, Thomazini JA, Tedesco AC, Bentley MV. A vehicle for photodynamic therapy of skin cancer: influence of dimethylsulphoxide on 5-aminolevulinic acid in vitro cutaneous permeation and in vivo protoporphyrin IX accumulation determined by confocal microscopy. J Control Release. 2000;65(3):359–66. doi: 10.1016/s0168-3659(99)00213-8. [DOI] [PubMed] [Google Scholar]
- 20.Maisch T, Santarelli F, Schreml S, Babilas P, Szeimies RM. Fluorescence induction of protoporphyrin IX by a new 5-aminolevulinic acid nanoemulsion used for photodynamic therapy in a full-thickness ex vivo skin model. Exp Dermatol. 2010;19(8):e302–5. doi: 10.1111/j.1600-0625.2009.01001.x. [DOI] [PubMed] [Google Scholar]
- 21.Fang YP, Huang YB, Wu PC, Tsai YH. Topical delivery of 5-aminolevulinic acid-encapsulated ethosomes in a hyperproliferative skin animal model using the CLSM technique to evaluate the penetration behavior. Eur J Pharm Biopharm. 2009;73(3):391–8. doi: 10.1016/j.ejpb.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan G, Roberts MS, Grice J, Anissimov YG, Benson HA. Enhanced transdermal delivery of 5-aminolevulinic acid and a dipeptide by iontophoresis. Biopolymers. 2011;96(2):166–71. doi: 10.1002/bip.21520. [DOI] [PubMed] [Google Scholar]
- 23.Merclin N, Bender J, Sparr E, Guy RH, Ehrsson H, Engstrom S. Transdermal delivery from a lipid sponge phase—iontophoretic and passive transport in vitro of 5-aminolevulinic acid and its methyl ester. J Control Release. 2004;100(2):191–8. doi: 10.1016/j.jconrel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Piccioni A, Fargnoli MC, Schoinas S, Suppa M, Frascione P, Ginebri A, et al. Efficacy and tolerability of 5-aminolevulinic acid 0.5% liposomal spray and intense pulsed light in wrinkle reduction of photodamaged skin. J Dermatolog Treat. 2011;22(5):247–53. doi: 10.3109/09546634.2011.590791. [DOI] [PubMed] [Google Scholar]
- 25.Plaunt AJ, Harmatys KM, Hendrie KA, Musso AJ, Smith BD. Chemically triggered release of 5-aminolevulinic acid from liposomes. RSC Adv. 2014;4(101):57983–90. doi: 10.1039/C4RA10340H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang YP, Tsai YH, Wu PC, Huang YB. Comparison of 5-aminolevulinic acid-encapsulated liposome versus ethosome for skin delivery for photodynamic therapy. Int J Pharm. 2008;356(1–2):144–52. doi: 10.1016/j.ijpharm.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Bragagni M, Scozzafava A, Mastrolorenzo A, Supuran CT, Mura P. Development and ex vivo evaluation of 5-aminolevulinic acid-loaded niosomal formulations for topical photodynamic therapy. Int J Pharm. 2015;494(1):258–63. doi: 10.1016/j.ijpharm.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 28.Shi L, Wang X, Zhao F, Luan H, Tu Q, Huang Z, et al. In vitro evaluation of 5-aminolevulinic acid (ALA) loaded PLGA nanoparticles. Int J Nanomedicine. 2013;8:2669–76. doi: 10.2147/IJN.S45821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung CW, Chung KD, Jeong YI, Kang DH. 5-aminolevulinic acid-incorporated nanoparticles of methoxy poly(ethylene glycol)-chitosan copolymer for photodynamic therapy. Int J Nanomedicine. 2013;8:809–19. doi: 10.2147/IJN.S39615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez C, Benito M, Katime I, Teijon JM, Blanco MD. In vitro transdermal and biological evaluation of ALA-loaded poly(N-isopropylacrylamide) and poly(N-isopropylacrylamide-co-acrylic acid) microgels for photodynamic therapy. J Microencapsul. 2012;29(7):626–35. doi: 10.3109/02652048.2012.676091. [DOI] [PubMed] [Google Scholar]
- 31.Ma X, Qu Q, Zhao Y. Targeted delivery of 5-aminolevulinic acid by multifunctional hollow mesoporous silica nanoparticles for photodynamic skin cancer therapy. ACS Appl Mater Interfaces. 2015;7(20):10671–6. doi: 10.1021/acsami.5b03087. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Wang S, Xu H, Wang B, Yao C. Role of 5-aminolevulinic acid-conjugated gold nanoparticles for photodynamic therapy of cancer. J Biomed Opt. 2015;20(5):51043. doi: 10.1117/1.JBO.20.5.051043. [DOI] [PubMed] [Google Scholar]
- 33.de Oliveira Goncalves K, da Silva MN, Sicchieri LB, de Oliveira Silva FR, de Matos RA, Courrol LC. Aminolevulinic acid with gold nanoparticles: a novel theranostic agent for atherosclerosis. Analyst. 2015;140(6):1974–80. doi: 10.1039/c4an02166e. [DOI] [PubMed] [Google Scholar]
- 34.Hadizadeh M, Fateh M. Synergistic cytotoxic effect of gold nanoparticles and 5-aminolevulinic acid-mediated photodynamic therapy against skin cancer cells. Iran J Med Sci. 2014;39(5):452–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammadi Z, Sazgarnia A, Rajabi O, Soudmand S, Esmaily H, Sadeghi HR. An in vitro study on the photosensitivity of 5-aminolevulinic acid conjugated gold nanoparticles. Photodiagn Photodyn Ther. 2013;10(4):382–8. doi: 10.1016/j.pdpdt.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Benito M, Martin V, Blanco MD, Teijon JM, Gomez C. Cooperative effect of 5-aminolevulinic acid and gold nanoparticles for photodynamic therapy of cancer. J Pharm Sci. 2013;102(8):2760–9. doi: 10.1002/jps.23621. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Pan LL, Zhang FL, Zhu XL, Liu Y, Zhang ZZ. 5-Aminolevulinic acid-loaded fullerene nanoparticles for in vitro and in vivo photodynamic therapy. Photochem Photobiol. 2014;90(5):1144–9. doi: 10.1111/php.12299. [DOI] [PubMed] [Google Scholar]
- 38••.Battah S, O’Neill S, Edwards C, Balaratnam S, Dobbin P, MacRobert AJ. Enhanced porphyrin accumulation using dendritic derivatives of 5-aminolaevulinic acid for photodynamic therapy: an in vitro study. Int J Biochem Cell Biol. 2006;38(8):1382–92. doi: 10.1016/j.biocel.2006.02.001. Important paper showing that ALA-dendrimers can enhance ALA delivery and improve PDT killing after illumination. [DOI] [PubMed] [Google Scholar]
- 39.Battah SH, Chee CE, Nakanishi H, Gerscher S, MacRobert AJ, Edwards C. Synthesis and biological studies of 5-aminolevulinic acid-containing dendrimers for photodynamic therapy. Bioconjug Chem. 2001;12(6):980–8. doi: 10.1021/bc010027n. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez L, Vallecorsa P, Battah S, Di Venosa G, Calvo G, Mamone L, et al. Aminolevulinic acid dendrimers in photodynamic treatment of cancer and atheromatous disease. Photochem Photobiol Sci. 2015;14(9):1617–27. doi: 10.1039/c5pp00126a. [DOI] [PubMed] [Google Scholar]
- 41.Huang P, Lin J, Yang D, Zhang C, Li Z, Cui D. Photosensitizer-loaded dendrimer-modified multi-walled carbon nanotubes for photodynamic therapy. J Control Release. 2011;152(Suppl 1):e33–4. doi: 10.1016/j.jconrel.2011.08.105. [DOI] [PubMed] [Google Scholar]
- 42.Blake E, Allen J, Curnow A. The effects of protoporphyrin IX-induced photodynamic therapy with and without iron chelation on human squamous carcinoma cells cultured under normoxic, hypoxic and hyperoxic conditions. Photodiagn Photodyn Ther. 2013;10(4):575–82. doi: 10.1016/j.pdpdt.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 43•.Blake E, Allen J, Curnow A. An in vitro comparison of the effects of the iron-chelating agents, CP94 and dexrazoxane, on protoporphyrin IX accumulation for photodynamic therapy and/or fluorescence guided resection. Photochem Photobiol. 2011;87(6):1419–26. doi: 10.1111/j.1751-1097.2011.00985.x. Compares different iron chelating agents for increasing PPIX synthesis after application of ALA. [DOI] [PubMed] [Google Scholar]
- 44.Liu HF, Xu SZ, Zhang CR. Influence of CaNa2 EDTA on topical 5-aminolaevulinic acid photodynamic therapy. Chin Med J. 2004;117(6):922–6. [PubMed] [Google Scholar]
- 45.Yang J, Xia Y, Liu X, Jiang S, Xiong L. Desferrioxamine shows different potentials for enhancing 5-aminolaevulinic acid-based photodynamic therapy in several cutaneous cell lines. Lasers Med Sci. 2010;25(2):251–7. doi: 10.1007/s10103-009-0721-0. [DOI] [PubMed] [Google Scholar]
- 46.Blake E, Curnow A. The hydroxypyridinone iron chelator CP94 can enhance PpIX-induced PDT of cultured human glioma cells. Photochem Photobiol. 2010;86(5):1154–60. doi: 10.1111/j.1751-1097.2010.00770.x. [DOI] [PubMed] [Google Scholar]
- 47.Junjing Z, Yan Z, Baolu Z. Scavenging effects of dexrazoxane on free radicals. J Clin Biochem Nutr. 2010;47(3):238–45. doi: 10.3164/jcbn.10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Malik Z, Ehrenberg B, Faraggi A. Inactivation of erythrocytic, lymphocytic and myelocytic leukemic cells by photoexcitation of endogenous porphyrins. J Photochem Photobiol B. 1989;4(2):195–205. doi: 10.1016/1011-1344(89)80005-3. Another early report describing the observations that would lead to ALA-PDT. [DOI] [PubMed] [Google Scholar]
- 49•.Ortel B, Chen N, Brissette J, Dotto GP, Maytin E, Hasan T. Differentiation-specific increase in ALA-induced protoporphyrin IX accumulation in primary mouse keratinocytes. Br J Cancer. 1998;77(11):1744–51. doi: 10.1038/bjc.1998.292. First report that differentiation can increase PPIX formation by up-regulating coproporphyrinogen oxidase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ortel B, Sharlin D, O’Donnell D, Sinha AK, Maytin EV, Hasan T. Differentiation enhances aminolevulinic acid-dependent photodynamic treatment of LNCaP prostate cancer cells. Br J Cancer. 2002;87(11):1321–7. doi: 10.1038/sj.bjc.6600575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maytin EV, Honari G, Khachemoune A, Taylor CR, Ortel B, Pogue BW, et al. Vitamin D combined with aminolevulinate (ALA)-mediated photodynamic therapy (PDT) for human psoriasis: a proof-of-principle study. Isr J Chem. 2012;52(8–9):767–75. doi: 10.1002/ijch.201200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rollakanti KR, Anand S, Davis SC, Pogue BW, Maytin EV. Noninvasive optical imaging of UV-induced squamous cell carcinoma in murine skin: studies of early tumor development and vitamin D enhancement of protoporphyrin IX production. Photochem Photobiol. 2015 doi: 10.1111/php.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galitzer BI. Effect of retinoid pretreatment on outcomes of patients treated by photodynamic therapy for actinic keratosis of the hand and forearm. J Drugs Dermatol. 2011;10(10):1124–32. [PubMed] [Google Scholar]
- 54.Anand S, Hasan T, Maytin EV. Mechanism of differentiation-enhanced photodynamic therapy for cancer: upregulation of coproporphyrinogen oxidase by C/EBP transcription factors. Mol Cancer Ther. 2013;12(8):1638–50. doi: 10.1158/1535-7163.MCT-13-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moan J, Berg K, Gadmar OB, Iani V, Ma L, Juzenas P. The temperature dependence of protoporphyrin IX production in cells and tissues. Photochem Photobiol. 1999;70(4):669–73. [PubMed] [Google Scholar]
- 56.Juzenas P, Sorensen R, Iani V, Moan J. Uptake of topically applied 5-aminolevulinic acid and production of protoporphyrin IX in normal mouse skin: dependence on skin temperature. Photochem Photobiol. 1999;69(4):478–81. [PubMed] [Google Scholar]
- 57.Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40(10):1094–102. doi: 10.1097/01.DSS.0000452662.69539.57. [DOI] [PubMed] [Google Scholar]
- 58.Mamalis A, Koo E, Sckisel GD, Siegel DM, Jagdeo J. The temperature-dependent impact of thermal-ALA-PDT on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br J Dermatol. 2016 doi: 10.1111/bjd.14509. [DOI] [PubMed] [Google Scholar]
- 59.De Vijlder HC, Middelburg T, De Bruijn HS, Martino Neumann HA, Sterenborg HC, Robinson DJ, et al. Optimizing ALA-PDT in the management of non-melanoma skin cancer by fractionated illumination. G Ital Dermatol Venereol. 2009;144(4):433–9. [PubMed] [Google Scholar]
- 60.van der Veen N, van Leengoed HL, Star WM. In vivo fluorescence kinetics and photodynamic therapy using 5-aminolaevulinic acid-induced porphyrin: increased damage after multiple irradiations. Br J Cancer. 1994;70(5):867–72. doi: 10.1038/bjc.1994.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.de Vijlder HC, Sterenborg HJ, Neumann HA, Robinson DJ, de Haas ER. Light fractionation significantly improves the response of superficial basal cell carcinoma to aminolaevulinic acid photodynamic therapy: five-year follow-up of a randomized, prospective trial. Acta Derm Venereol. 2012;92(6):641–7. doi: 10.2340/00015555-1448. Clinical study showing that fractionated light delivery improves ALA-PDT outcome. [DOI] [PubMed] [Google Scholar]
- 62.Sotiriou E, Apalla Z, Chovarda E, Goussi C, Trigoni A, Ioannides D. Single vs. fractionated photodynamic therapy for face and scalp actinic keratoses: a randomized, intraindividual comparison trial with 12-month follow-up. J Eur Acad Dermatol Venereol. 2012;26(1):36–40. doi: 10.1111/j.1468-3083.2011.04003.x. [DOI] [PubMed] [Google Scholar]
- 63.de Bruijn HS, Casas AG, Di Venosa G, Gandara L, Sterenborg HJ, Batlle A, et al. Light fractionated ALA-PDT enhances therapeutic efficacy in vitro; the influence of PpIX concentration and illumination parameters. Photochem Photobiol Sci. 2013;12(2):241–5. doi: 10.1039/c2pp25287b. [DOI] [PubMed] [Google Scholar]
- 64•.de Bruijn HS, Brooks S, van der Ploeg-van den Heuvel A, Ten Hagen TL, de Haas ER, Robinson DJ. Light Fractionation significantly increases the efficacy of photodynamic therapy using BF-200 ALA in normal mouse skin. PLoS ONE. 2016;11(2):e0148850. doi: 10.1371/journal.pone.0148850. Clinical study showing that daylight-mediated ALA-PDT performs equally well to red light, but is better tolerated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wiegell SR, Fabricius S, Gniadecka M, Stender IM, Berne B, Kroon S, et al. Daylight-mediated photodynamic therapy of moderate to thick actinic keratoses of the face and scalp: a randomized multicentre study. Br J Dermatol. 2012;166(6):1327–32. doi: 10.1111/j.1365-2133.2012.10833.x. [DOI] [PubMed] [Google Scholar]
- 66.Rubel DM, Spelman L, Murrell DF, See JA, Hewitt D, Foley P, et al. Daylight photodynamic therapy with methyl aminolevulinate cream as a convenient, similarly effective, nearly painless alternative to conventional photodynamic therapy in actinic keratosis treatment: a randomized controlled trial. Br J Dermatol. 2014;171(5):1164–71. doi: 10.1111/bjd.13138. [DOI] [PubMed] [Google Scholar]
- 67.Moseley H, Allen JW, Ibbotson S, Lesar A, McNeill A, Camacho-Lopez MA, et al. Ambulatory photodynamic therapy: a new concept in delivering photodynamic therapy. Br J Dermatol. 2006;154(4):747–50. doi: 10.1111/j.1365-2133.2006.07145.x. [DOI] [PubMed] [Google Scholar]
- 68.Attili SK, Lesar A, McNeill A, Camacho-Lopez M, Moseley H, Ibbotson S, et al. An open pilot study of ambulatory photodynamic therapy using a wearable low-irradiance organic light-emitting diode light source in the treatment of nonmelanoma skin cancer. Br J Dermatol. 2009;161(1):170–3. doi: 10.1111/j.1365-2133.2009.09096.x. [DOI] [PubMed] [Google Scholar]
- 69.Cochrane C, Mordon SR, Lesage JC, Koncar V. New design of textile light diffusers for photodynamic therapy. Mater Sci Eng C Mater Biol Appl. 2013;33(3):1170–5. doi: 10.1016/j.msec.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 70.Ferrick B, Izikson L, Ibrahimi O, Jalian HR, Kroshinsky D, Anderson RR, et al. Quantitative volumetric changes after conventional ALA-PDT compared to a new inhibitory PDT method (i-PDT) to reduce inflammation in a preliminary study. Lasers Surg Med. 2014;46(S25):43–4. [Google Scholar]


