Abstract
Infectious disease transmission is a cost of sociality in humans and other animals. Nevertheless, the mechanisms linking social behaviour to infection risk are poorly known. We conducted a field experiment to examine how host intrinsic traits, behaviour and physiology affect infection of nonhuman primates with gastrointestinal parasites. We measured rate to reinfection in a social group of red-capped mangabeys, Cercocebus torquatus, following chemotherapeutic treatment for parasite infections. By measuring behaviour, infection and glucocorticoid levels, we compared the relative effects of space sharing, directional contact and physiological stress on risk of acquiring new infections. We found that, within proximity networks, individuals that were central and well connected and that had a tendency to switch groups were at increased risk of infection with helminths. Protozoan infections, however, were acquired more uniformly across the population. In general, position in the social network and, in particular, space sharing appears to be more important than the immunosuppressive effects of physiological stress or host traits in determining risk of infection. Our results suggest that future studies of disease ecology within wildlife populations should focus on measures of network association in addition to individual host traits.
Keywords: antiparasite treatment, gastrointestinal parasitehost trait, helminth, infectious disease transmission, physiological stress, primate, protozoa, red-capped mangabey, social network
In humans and other social animals, variation in behaviour and physiology can alter the risk of exposure to, and infection by, pathogens, ultimately affecting host fitness (Kappeler, Cremer, & Nunn, 2015; Nunn, Craft, Gillespie, Schaller, & Kappeler, 2015; Silk, 2014). Variation in parasitism often has direct links to host social behaviour, such that infection-related costs of sociality are considered important selective forces in human and animal evolution (Altizer et al., 2003; Kappeler et al., 2015; Møller, Dufval, & Allander, 1993). Clarifying the mechanisms whereby sociality translates to infection is important for our understanding of disease ecology and host–parasite coevolution. For example, it is currently unclear whether close proximity and high levels of contact or increased physiological stress resulting from within-group social dynamics is more important for infection in primates.
Macroparasites are generally aggregated within populations, with few hosts harbouring the majority of infections (Crofton, 1971; Poulin, 2007; Shaw & Dobson, 1995). Classic measures typically associated with infection include age, sex and dominance status (Nunn & Altizer, 2006). Behavioural and physiological mechanisms that influence encounter rates and immune status can vary with these measures, further explaining why certain individuals are at increased risk of infection. Focus on classic measures alone may therefore obscure important contributions of social connectivity and/or physiological stress (Cavigelli & Caruso, 2015; Kappeler et al., 2015).
In primates, trade-offs between sociality, encounter rates and immune function result in conflicting predictions for disease risk of individuals (Nunn & Altizer, 2006). For example, age can increase parasitism if larger-bodied individuals occupy more space, require more resources and contact contaminated foods and substrates disproportionately. Conversely, lack of acquired immunity in younger individuals may increase risk of parasitism in juveniles (Hudson & Dobson, 1997). Parasitism tends to be more common in males than in females across vertebrate taxa (Habig & Archie, 2015). However, male-biased parasitism is confounded by body size, such that the immunosuppressive effects of stress and testosterone are unclear (Zuk & McKean, 1996). In primate societies with dominance hierarchies, greater access to resources and rank-mediated social contact should increase risk for high-ranking individuals (MacIntosh et al., 2012; Rushmore et al., 2013). Meanwhile, immunosuppressive effects of stress hormones can increase susceptibility in either dominant or subordinate individuals depending on species-typical dynamics and hierarchical stability (Cavigelli & Caruso, 2015; Sapolsky, 2005).
Empirically, intraspecific differences in physical contact, proximity (González-Hernández et al., 2014; MacIntosh et al., 2012; Rimbach et al., 2015) and physiological stress (Chapman, Saj, & Snaith, 2007; Clough, Heistermann, & Kappeler, 2010; Muehlenbein, 2006) are associated with transmission of parasites within primate groups. In Japanese macaques, Macaca fuscata, for example, socially mediated exposure seems to be more important than the immunosuppressive effects of stress in explaining why dominant females have more infections from directly transmitted parasites (MacIntosh et al., 2012). Nevertheless, the relative importance of network connectivity versus physiological stress as mechanisms for facilitating pathogen spread is not well understood.
In this study, we investigated how social connectivity and physiological stress compare to host intrinsic factors with respect to explaining patterns of parasite aggregation in primates. To overcome confounding heterogeneities in exposure, susceptibility and resulting infection levels over time, we experimentally removed parasites and measured rate to reinfection. To date, experimental manipulations of parasite infections in wild animals have focused primarily on behavioural, immune and fitness responses to parasitism (Coster, Neve, Martín-Gálvez, Therry, & Lens, 2010; Hillegass, Waterman, & Roth, 2010; Raveh, Neuhaus, & Dobson, 2015). Here, we investigated patterns of parasite reacquisition following chemotherapeutic treatment of red-capped mangabeys, Cercocebus torquatus, for gastrointestinal helminth and protozoan parasites. Specifically, we examined how centrality within social networks and individual stress varied within the population according to sex, age and dominance. We then compared how these mechanistic explanations (e.g. contact, proximity and/or stress) performed against classic measures in predicting rate to reinfection. We compared reinfection from helminths and protozoans separately, given their inherent differences in time to infection and aggregation within hosts (Shaw & Dobson, 1995). By focusing on gastrointestinal parasites, which can be collected noninvasively and can be treated with oral medications, we were able to compare results from our field experiment to other observational studies that also investigated gastrointestinal parasites. We predicted that following experimental manipulation of infection, centrality would augment classic measures to more powerfully explain differences in infection rates.
METHODS
Study Site and Population
The study took place at Rhoko Research and Conservation Education Centre (41.21° N, 16.16° E), the forest site of the Centre for Education, Research and Conservation of Primates and Nature (CERCOPAN). Rhoko is located in the transition zone surrounding the Oban division of Cross River National Park in Cross River State, Nigeria. The vegetation is characteristic of lowland rainforest, forming a mosaic of disturbed and relatively undisturbed forest patches. Climate includes a long wet season from April to November and a short dry season from November to March.
We studied 49 red-capped mangabeys living in a multimale–multifemale social group that either had been rescued from the bushmeat and pet trades as young juveniles, or were first- to third-generation captive born. The group was housed in a 1 ha open-topped forest enclosure with full canopy cover and within the natural home range of the species. The population was provisioned daily but also ate wild foods opportunistically and drank from a stream running through the enclosure. Animals were vulnerable to natural predators (e.g. snakes and birds of prey) and parasites. All animals were well habituated and individually recognizable to the trained observer through individual differences in size, pelage and facial characteristics; all data were collected from animals where observers had achieved 100% agreement on identification. The age of each individual at the start of data collection (range 1.08–18.5 years) was known from birth records or estimated from tooth wear, pelage characteristics and sexual maturity at date of rescue. We categorized males as adult (≥6 years, N = 9), subadult (≥3 year, N = 9) and juvenile (<3 years, N = 7), and females as adult (≥4 years, N = 19) or juvenile (<4 years, N = 5). Cercocebus torquatus is currently listed as vulnerable by IUCN (Oates, Gippoliti, & Groves, 2008).
Chemotherapeutic Treatment
In June of 2012, the entire population was treated for gastrointestinal parasites via simultaneous administration of metronidazole (50 mg/kg for 7 days) for protozoans, mebendazole (50 mg/kg for 3 days) for nematodes, and praziquantel (20 mg/kg for 3 days) for cestodes and trematodes. For all drugs, a single dose was delivered in maize cereal to identified individuals to ensure that each animal received at least one dose. The remaining doses were dissolved in fruit and administered via group feeds following standard practice for the population-level treatment at CERCOPAN. The treatment period lasted 10 days in total.
Study Design
The study took advantage of a planned treatment event, providing a unique opportunity to measure patterns of infection following an intervention and minimizing additional risk. The treatment regimen was developed from standard treatment practices at CERCOPAN and in consultation with two wildlife veterinarians. Oral administration of drugs and noninvasive assessment of parasites were used to minimize adverse risks and enhance welfare. The Institutional Animal Care and Use Committee at University of Wisconsin-Madison approved all research activities (protocol V1490).
We collected faecal samples and behavioural and health data between May and September 2012. We conducted faecal sampling 1 month prior to treatment and parallelbehavioural sampling for 3 months immediately following treatment. We collected pre-treatment faecal samples in triplicate from each individual to increase detection of parasites shed intermittently. To assess variation in protozoan infection, which are infectious upon shedding and have short prepatent periods, we collected post-treatment faecal samples at the highest frequency for the month immediately following treatment (ca. every 3 days/individual). We measured time to infection from protozoans during the period of high sampling intensity only (30 days post treatment). We gradually decreased sampling intensity in the second month (ca. every 5 days) and third month (ca. every 10 days) to detect new infections from helminths, which develop in the external environment and have longer prepatent periods, such that we measured helminth reinfection over this longer time period (80 days post treatment). We extracted hormones from triplicate samples directly following treatment (ca. every 10 days), with sampling intensity reduced to twice a month (ca. every 15 days) for the second and third months.
Behavioural Data Collection
We collected associational data by recording all grooming partners, direction of grooming and nearest neighbours (within 2 m) of focal individuals during three observation periods daily: early morning (0700–1000 hours), mid-morning (1100–1500 hours) and evening (1600–1900 hours). The mangabeys were often dispersed in dense undergrowth throughout their enclosure, making it difficult to collect data on a predetermined schedule or in a specific order. We therefore selected individuals opportunistically. We allowed a minimum of 1.5 min to elapse between observations to reduce interdependence of data. We did not sample an individual if it was an associate or nearest neighbour in the preceding observation. We collected dominance data by recording all observed agonistic interactions as well as the directionality of submissions and supplants using structured ad libitum sampling, as conducted for similar species (Range & Noë, 2002). Three observers collected all data for 81 days over 3 months post treatment. We tested and accepted interobserver reliability by calculating Fleiss’s Kappa test for categorical agreements between multiple observers (Kappa = 0.89, P < 0.001 for observers 1 and 2; Kappa = 0.92, P < 0.001 for observers 1 and 3).
Faecal Collection and Preservation
We collected faecal samples immediately following defecation and fixed within 2 h of collection. We used separate aliquots of each sample for preservation of gastrointestinal parasites and hormones. First, we took a 2 g aliquot from within the faecal mass and stored it in a 3:1 ratio of 10% formalin to faeces for preservation of gastrointestinal parasites (Greiner & McIntosh, 2009). We then separated a second aliquot, mixed it thoroughly, and placed 0.50–0.55 g in a 15 ml tube. We mixed equal parts (2.5 ml) of distilled water and ethanol with the sample by shaking it vigorously for 5 min for preservation of hormones (Ziegler & Wittwer, 2005).
We extracted hormones in the field via solid-phase extraction (SPE) (Ziegler & Wittwer, 2005). Samples were stored for over 24 h until faecal material naturally sedimented at the bottom of the tube and a clear supernatant was evident. We decanted supernatant into a small weighing dish, removed and pushed it through the SPE columns (Prevail C18, Alltech, Deerfield, IL, U.S.A.) using a 5 ml disposable syringe at a flowrate of 1 ml/min. We then washed solid-phase extraction columns by pushing 1 ml of distilled water through the cartridges at the same rate to eliminate contaminants. We capped cartridges and stored them at room temperature out of direct sunlight until transport. We transported formalin-preserved samples and SPE cartridges to the University of Wisconsin, Madison following all applicable import, export and International Air Transport Association regulations.
Behavioural Data Analysis
We constructed dominance matrices from post-treatment dyadic supplants and aggressive and avoidance interactions between adults of the same sex using SOCPROG 2.6 (Whitehead, 2009). We used David’s scores (DS) to dichotomize individuals as ‘usually dominant’ (DS >0) or ‘usually subordinate’ (DS <0) (de Vries, Stevens, & Vervaecke, 2006). We assessed linearity of male and female hierarchies using de Vries’ test and h′ with 1000 permutations for dominance hierarchies containing unknown or tied relationships (de Vries et al., 2006).
We constructed adjacency matrices for social network analyses directly from observed post-treatment pairwise associations (‘proximity’ network) and directional grooming interactions (‘contact’ networks) using SOCPROG 2.6 (Whitehead, 2009). We imported matrices with attribute information into R v.3.2.2 (R Core Team, 2014), where we conducted all further analyses unless otherwise specified. We performed calculations of symmetric network metrics from proximity matrices and calculations of asymmetric metrics from directional contact matrices using the ‘sna’ package (Farine & Whitehead, 2015). For each individual in each network, we calculated node-based measures commonly used for modelling transmission of infectious diseases: (1) degree centrality: the number of associates or interactants (hereafter referred to as ‘centrality’); (2) strength: the number of associations or interactions; (3) closeness: the shortest number of paths needed to reach all other individuals; and (4) betweenness: the number of shortest paths going through an individual (Drewe & Perkins, 2015). We calculated in degree and out degree for directional networks, representing groom-receive and groom-give, respectively.
Given the nonindependence of data in network analyses, we compared network metrics to host characteristics using permutation tests. We built null models were built from data stream-based randomizations and measured significance by comparing the test statistic of the models fitted to the observed data with the test statistic calculated from 1000 permutations of the network using the ‘asnipe’ package (Farine, 2013; Farine & Whitehead, 2015). We calculated Cohen’s d effect size for all comparisons using the ‘compute.es’ package (Farine & Whitehead, 2015; Re, 2015). We constructed network diagrams using UCINET software’s NetDraw program (Borgatti, Everett, & Freeman, 2002), with node size representing individual centrality, weighted edges representing strength, and without filters.
Parasitology
We concentrated 1 g of formalin-preserved faeces via faecal sedimentation following the protocol of Greiner and McIntosh 2009 for assessment of gastrointestinal parasites of primates (Greiner & McIntosh, 2009). Briefly, we suspended 1 g of faeces in 40 ml of sedimentation solution (soapy water), mixed it gently to avoid formation of bubbles and filtered it twice through cheesecloth to remove large debris. We allowed the mixture to sediment for 10 min, after which we decanted the supernatant. We resuspended the remaining pellet in distilled water and allowed it to sediment for another 10 min. We then removed the supernatant with a transfer pipette and preserved the sediment in formalin until examination. We systematically examined the entirety of the sediment at 10× objective light magnification for helminth eggs and larvae. We then examined one drop of sediment from each sample at 40× for identification of protozoan cysts. We measured representative parasites with a calibrated ocular micrometer and photographed them at 40× magnification. All helminth eggs, larvae and protozoan cysts were assigned to taxa based on their size, shape, colour and contents. Parasite richness (number of different parasite taxa within a host) and prevalence (percentage of individuals infected with a particular parasite taxon) were calculated (Bush, Lafferty, Lotz, & Shostak, 1997). We evaluated the efficacy of treatment by examining changes in parasite richness over time (before and after treatment) using one-way ANOVA and Tukey HSD post hoc tests. Prior to this study, parasite communities had not been reported for this species.
Faecal Cortisol Analysis
We measured faecal cortisol levels via enzyme immunoassay at the Wisconsin National Primate Research Center Assay Services Unit. We eluted steroid hormones were using 2 ml of 100% methanol after washing cartridges with 1 ml of 5% methanol. We then evaporated eluted and rehydrated hormones in 1 ml of 100% ethanol and stored them at 4 °C. Prior to EIA, we removed and evaporated 25 μl from each sample. We performed all assays using R4866 (anti-cortisol-bovine serum albumin) developed by Stabenfeldt and Munro at the University of California, Davis, with 60% cross-reactivity to corticosterone (Ziegler, Scheffler, & Snowdon, 1995). We read plates using SpectraMax 340PC microplate reader. Recovery was 105.68 ± 3.15%. We demonstrated parallelism using serial dilution curves derived from high-value faecal extracts with no significant difference from the slope of the standard curve (t24 = 0.93, P > 0.05). Interassay variation was 18.3% for the high pool and 22.2% for the low pool, whereas intra-assay variation was 3.8% for the high pool and 7.9% for the low pool. We compared average post-treatment faecal cortisol levels (ng/g) across sex, age and dominance status using Spearman rank correlations and Mann–Whitney U tests. Prior to this study, cortisol had not been reported for this species.
Statistical Analysis
We used marginal Cox proportional hazards models for multiple events data to examine host traits, behaviour and physiology as predictors of time to reinfection (Kleinbaum & Klein, 2012; Wei, Lin, & Weissfeld, 1989). The marginal approach focuses on the total time from study entry to occurrence of each event, thereby combining time to infection and number of infections (i.e. richness) within a single model. In these models, we defined the baseline hazard function (i.e. dependent variable) as time to infection, stratified by parasite type. Stratification by parasite within models allowed the baseline hazard function to vary for each parasite taxon. We defined new infections as shedding of cysts/eggs in the post-treatment sampling period after testing negative for the entirety of the parasite-specific prepatent period. Because drug administration typically reduces burden but does not typically clear infections entirely (Pedersen & Fenton, 2015), we included only parasites that showed more than 50% reduction in prevalence and contributed to subsequent reinfections. Individuals that did not experience an event by the end of the study were right-censored (e.g. time to infection for these individuals was considered to be at least as long as the duration of the study).
We incorporated individual characteristics, including sex, age class, dominance status, faecal cortisol level and centrality, as covariates in maximal models. We assigned robust variance estimates to adjust for the likely correlation among multiple events on the same subject (Lin & Wei, 1989). To control for the increased opportunity for infection in individuals successfully treated for all parasites (compared to those that retained some infections following treatment), we forced a covariate into each model to represent the maximum number of events possible per subject.
We built separate maximal models for protozoan and helminth infections. Because juveniles and subadults were not assigned dominance values, we ran two models under each category: (1) all ages (dominance excluded) and (2) adults only (dominance included). We incorporated centrality measures into maximal models one at a time (i.e. each model only contained one centrality metric from one network) to avoid multicollinearity. Model selection was then carried out independently for each model using the likelihood ratio test selection criterion (Kleinbaum & Klein, 2012). We used backwards elimination of predictor variables to select models that retained only significant covariates (at the alpha < 0.05 level) and first-order interactions. We did not use permutation-based methods, which are often used in the statistical analysis of network data (Croft, Madden, Franks, & James, 2011), because the response variable was not based on relational data (VanderWaal, Atwill, Hooper, Buckle, & McCowan, 2013). We accepted if they had generalized variance inflation factors (GVIF) within reason (<4) (O’Brien, 2007), satisfied the proportional hazards assumption (i.e. residuals were not significantly correlated with time, ZPH: Pearson’s r: P > 0.05), and they explained significantly more variance than the null model (likelihood ratio test: P < 0.05) (Kleinbaum & Klein, 2012)
We report hazard ratios (HR), the ratio of the chance of parasite acquisition in one level of an explanatory variable relative to the other, for all significant predictors. We produced survival curves for significant predictors using the Kaplan–Meier method. We dichotomized entrality scores at the median because hazard ratios and Kaplan–Meier curves are more interpretable when comparing groups, and because in general, models with dichotomized variables outperformed those with continuous variables (i.e. produced higher log likelihoods and fewer ZPH violations). We performed all analyses with the ‘surv’, ‘coxph’ and ‘cox.zph’ functions in the ‘survival’ package using R v.3.2.2 (R Core Team, 2014; Therneau, 2015).
RESULTS
Dominance
We constructed dominance matrices were from 628 female (μ + SD = 66.11 + 30.94 per individual; μ + SD = 3.48 + 1.63 per dyad) and 368 male (μ + SD = 81.78 + 32.33 per individual, μ + SD = 9 + 3.59 per dyad) dyadic dominance interactions. For both male and female hierarchies, deVries test of linearity indicated that dominance was moderately linear and non-random for female (h′= 0.73, P<0.001) and male (h′= 0.84, P<0.01) hierarchies.
Centrality
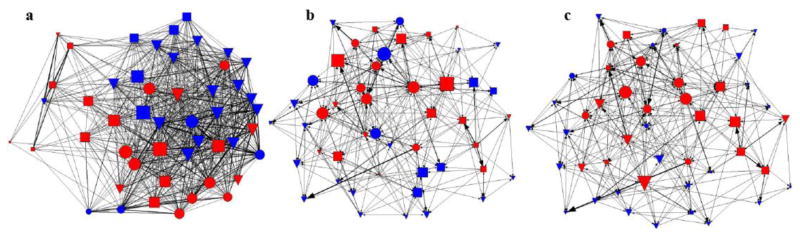
We constructed proximity networks from 2374 observed pairwise associations (μ + SD = 96.50 + 48.54 per individual; μ + SD = 1.97 + 0.98 per dyad) and contact networks from 555 dyadic grooming interactions (μ + SD = 11.32 + 9.37 per individual; μ + SD = 0.23 + 0.19 per dyad). Directed contact networks were more heterogeneous (varied across sex, age and dominance status) compared to proximity networks (Fig. 1). Females had higher closeness (t47 = 4.64, P < 0.001, d =1.33) and betweenness (t47 = 3.64, P < 0.001, d =1.04) in the contact networks compared to males, and higher centrality (t47 = 5.83, P < 0.001, d = 1.66) and strength (t47=6.17, P < 0.05, d = 1.76) measures in the groom-give network. Adults had significantly higher closeness (t47 = 3.63, P < 0.001, d = 1.05) in the contact networks and higher centrality (t47 = 6.15, P < 0.001, d = 1.78) and strength (t47 = 4.08, P < 0.05, d = 1.18) in groom-receive network. High-ranking males were more central in the groom-give network (t7 = 2.97, P < 0.001, d = 1.99) and had stronger connections in both proximity (t7 = 2.19, P < 0.05, d = 1.47) and groom-receive networks (t7 = 2.61, P < 0.01, d = 1.75). Dominant females had higher closeness (t17 = 2.58, P < 0.01, d = 1.2) and betweenness (t17 = 2.02, P < 0.001, d = 0.94) in contact networks and higher centrality (t17 = 2.14, P < 0.01, d = 0.99) and stronger connections in groom-give network (t17 = 3.05, P < 0.001, d = 1.42).
Figure 1.
Mangabey social networks showing the position of individuals according to host traits. Nodes represent individuals; edges (lines connecting nodes) represent (a) proximity, (b) directed grooming received (groom-receive) and (c) directed grooming given (groom-give). Node size and colour represent host characteristics, including sex (female = red; males = blue), age (juveniles and subadults = triangles) and dominance status (high ranking = circle; low raking = square). Individuals with more contacts (high degree centrality) are represented by larger nodes. Directed relationships are indicated by edges with arrows. Thickness of lines represents strength of the relationship (strength). No filters were applied.
Faecal Cortisol
We collected a total of 343 post-treatment faecal samples (7 per individual) for hormone analyses. Faecal cortisol levels ranged from 0.57 ng/g to 49.39 ng/g among individuals. Cortisol levels were positively related to age (rS = 0.55, P < 0.001), and low-ranking individuals had higher levels (mean = 12.77 ng/g) than high-ranking individuals (mean = 8.77 ng/g) (U = 50, N1 = 16, N2 = 12, P < 0.05). Females (mean = 9.95 ng/g) had marginally higher cortisol values than males (mean = 8.07 ng/g) (U = 215, N1 = 24, N2 = 25, P < 0.1). There was no relationship between centrality within social networks and average faecal cortisol levels.
Parasitism
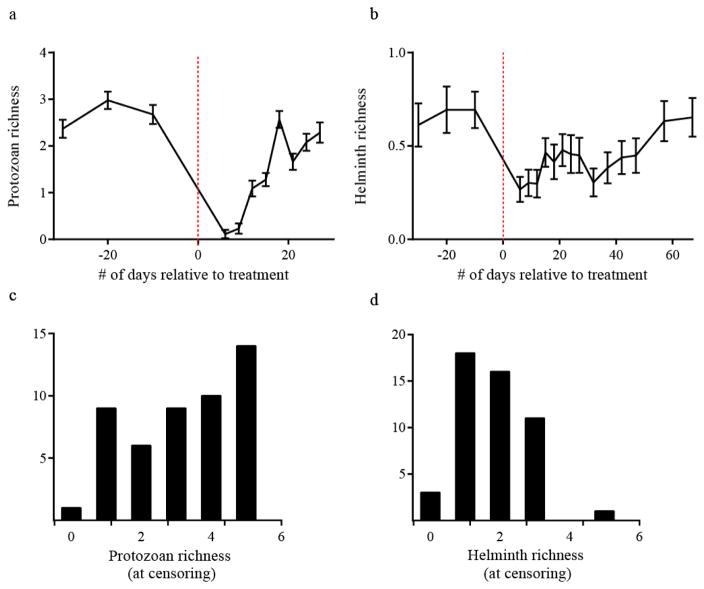
We collected a total of 982 faecal samples, for an average of 20 samples per individual. Mangabeys were infected with six protozoan and nine helminths prior to treatment (Table 1). Average protozoan (F5,287 = 39.22, P < 0.01) and helminth (F7,383 = 13.18, P < 0.01) richness differed significantly over time. Post hoc comparisons showed significant reductions in parasite richness between pre-treatment (helminth: μ + SD = 1.55 + 1.29, P < 0.001; protozoa: μ + SD = 2.77 + 1.36, P < 0.001) and post-treatment samples (helminth: μ + SD =.67 + .87; protozoa: μ + SD = 0.27 + 0.86). Protozoan infections were acquired more quickly than helminth infections. There was no significant difference between pre-treatment richness and richness at the final sample for helminths or protozoans, demonstrating return to baseline levels (Fig. 2a, b). Prevalence of five protozoans and four helminths was reduced by at least 50% and contributed to subsequent reinfections (Table 1). These nine parasites were therefore included in Cox proportional hazard models. Protozoans and helminths showed different distributions at the final time points, further supporting our decision to model infection separately for each group of parasites (Fig. 2c, d). A right-skewed distribution of helminth richness showed that a small number of individuals harboured many parasite taxa whereas most individuals were infected with only a few taxa (Fig. 2d).
Table 1.
Biological classifications of parasites recovered from faeces of red-capped mangabeys showing prevalence prior to and immediately following deworming, and at the final time point for protozoa and helminths
| Classification | Genus | Species | Life cycle | Mode of transmission | Prevalencea
|
||
|---|---|---|---|---|---|---|---|
| Pre | Post | Final | |||||
| Protozoab | Entamoeba* | hartmanni | Direct | Ingestion of trophozoites and cysts | 0.80 | 0.02 | 0.51 |
| Entamoeba* | histolytica/dispar | Direct | Ingestion of trophozoites and cysts | 0.98 | 0.02 | 0.78 | |
| Entamoeba* | coli+ | Direct | Ingestion of trophozoites and cysts | 0.69 | 0.02 | 0.67 | |
| Iodamoeba* | bütschlii | Direct | Ingestion of trophozoites and cysts | 0.69 | 0.02 | 0.61 | |
| Chilomastix* | mesnili | Direct | Ingestion of trophozoites and cysts | 0.47 | 0.00 | 0.55 | |
| Balantidium | coli | Direct | Ingestion of trophozoites and cysts | 0.88 | 0.02 | 0 | |
| Nematoda | Trichuris | trichiura | Direct | Ingestion of eggs | 0.02 | 0 | 0 |
| Capillaria | sp. | Unknown | Unknown | 0.06 | 0 | 0.04 | |
| Strongyloides * | sp. | Direct | Ingestion of eggs/penetration of skin by larvae | 0.22 | 0.06 | 0.35 | |
| Enterobius | sp. | Direct | Ingestion of eggs | 0.16 | 0.02 | 0 | |
| Hookworm* | Direct | Penetration of skin by larvae | 0.12 | 0.02 | 0.12 | ||
| Unknown* | sp. | Unknown | Unknown | 0.06 | 0 | 0.12 | |
| Abbreviata* | sp. | Indirect1 | Ingestion of arthropod | 0.87 | 0.28 | 0.59 | |
| Trematoda | Paragonimus | africanus | Indirect2 | Ingestion of crab | 0.43 | 0.35 | 0.43 |
| Cestoda | Bertiella | sp. | Indirect1 | Ingestion of mite | 0.04 | 0 | 0 |
Indicates that the parasite met the criteria for incorporation into Cox proportional hazards models.
Prevalence was calculated from triplicate samples collected prior to treatment, a single post-treatment sample (protozoan; N = 45) and triplicate samples collected at the end of the study.
Species identifications are putative based on size and morphological characteristics of cysts.
Figure 2.
Average parasite richness at each sample point relative to treatment (vertical dashed line) for (a) protozoans and (b) helminths. Error bars show standard error of the mean. Histograms show different distributions of (c) protozoan and (d) helminth parasites within the host population at censoring.
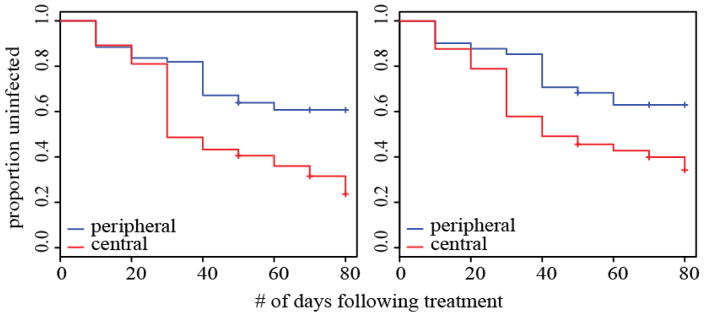
Following treatment (and prior to the end of the study), 81 new helminth infections (48 in adults) and 189 new protozoan infections (111 in adults) were detected. High centrality, closeness and betweenness in proximity networks were associated with rate to infection with helminth parasites in adult mangabeys (Fig. 3, Table 2). When all age classes were included, these patterns were in the same direction but marginal. No centrality measures explained time to reinfection with protozoans. Neither host traits (e.g. sex, age or dominance) nor average post-treatment cortisol levels were associated with rate to infection under either model.
Figure 3.
Kaplan–Meier curves showing rate to reinfection for peripheral and central individuals following treatment for parasites based on individuals’ (a) degree and closeness centrality and (b) betweenness in proximity networks.
Table 2.
Marginal Cox proportional hazard models associating host characteristics with rate to parasite infection
| Response variable | Network type | Predictorsa,b | P>|z| | HR (95% CI) | LRT | ZPH | GVIFc |
|---|---|---|---|---|---|---|---|
| Helminth reinfection | Proximity | Degree | <0.01 | 2.33 (1.39, 3.91) | <0.01 | 0.87 | <4 |
| Strength | NS | ||||||
| Betweenness | <0.05 | 1.91 (1.02, 3.57) | <0.05 | 0.96 | <4 | ||
| Closeness | <0.01 | 2.20 (1.33, 3.63) | <0.01 | 0.94 | <4 | ||
| Contactd | NS | ||||||
| Protozoan reinfection | Proximity | NS | |||||
| Contact | NS |
HR: hazard ratio; LRT: likelihood ratio test; ZPH: proportional hazard assumption (i.e. residuals not significantly correlated with time); GVIF: generalized variance inflation factor.
Sex, age, dominance status and faecal cortisol were not significant under any model.
Reference level is central.
Generalized variance inflation factors met the rule GVIF <4 for all maximal models.
In degree/out degree (groom-receive) and in strength/out strength (groom-give) were incorporated into models as measures of centrality within contact networks.
DISCUSSION
Measures of social centrality were more important than individual host traits (e.g. sex, age and dominance) or physiological stress for explaining rates of infection with helminth parasites in mangabeys. Specifically, we found that individuals with more associates (centrality) that were well connected (closeness) and that had a tendency to switch groups (betweenness) were at higher risk of acquiring helminth parasites. Number of associations (strength) and measures of connectivity within contact networks were not associated with infection risk. Acquisition of protozoan infections, which do not tend to aggregate in populations, did not vary according to centrality, stress or host traits. Together, our results show that parasite aggregation was determined primarily by host associations and that social connectivity, rather than the immunosuppressive effect of stress, may explain enhanced infection risk.
The probability of acquiring new helminth infections was higher in animals that were central in the proximity network. This finding demonstrates a potential cost associated with social connectivity. A growing body of evidence suggests that social centrality increases risk of infection from macroparasites. In primates, centrality within grooming networks has been indirectly associated with nematode infections in female Japanese macaques, Macaca fuscata yuki, and directly associated with parasite species richness in brown spider monkeys, Ateles hybridus (MacIntosh et al., 2012; Rimbach et al., 2015). Our findings differ from studies of brown spider monkeys, however, in which centrality in contact networks was more important than proximity for explaining elevated infection risk (Rimbach et al., 2015).
Helminths must develop in the external environment for days to months before they become infective. Risk associated with connectivity in proximity networks, rather than contact networks, may therefore reflect increased exposure to infectious life stages persisting on fomites and food items (Freeland, 1980). We cannot rule out the possibility that spatial associations serve as proxies for contact (Farine, 2015). However, this is unlikely given that grooming networks showed a more heterogeneous structure (Fig. 1) despite being based on fewer observations. Indeed, space sharing has been identified as an important predictor of risk for infection from macroparasites in other taxonomic groups (Fenner, Godfrey, & Bull, 2011; Godfrey, Moore, Nelson, & Bull, 2010; Perkins, Cagnacci, Stradiotto, Arnoldi, & Hudson, 2009).
We found that protozoans, which are immediately infective once shed into the environment, were acquired uniformly across the population. This result aligns with our current understanding of the transmission biology of protozoan parasites, which tend not to aggregate in hosts like macroparasites (Shaw & Dobson, 1995) (Fig. 2). Similarly, centrality was associated with parasite richness in brown spider monkeys, but these results were not significant for a protozoan (Entamoeba spp.) alone (Rimbach et al., 2015). In other taxa, however, contact appears to be important for predicting the spread of directly transmitted and immediately infectious pathogens such as Mycobacterium bovis in meerkats, Suricata suricatta (Drewe, 2009), and fungi in garden ant, Lasius neglectus, colonies (Theis, Ugelvig, Marr, & Cremer, 2015).
Unfortunately, low prevalence often reduces the power of transmission mode-specific models such as the ones we constructed. Nevertheless, systematic studies using aggregate models are important, since cumulative effects of multiple infections can have marked impacts on host health and fitness (Bordes & Morand, 2011). Identifying the hosts with high rates of infection from multiple parasites can therefore help identify fitness costs associated with social behaviour. Such models will be critical in improving our understanding of the role of social contact in pathogen spread (Craft, 2015; Grear, Luong, & Hudson, 2013).
We did not find an effect of glucocorticoid level on acquisition of parasites. Similarly, in female Japanese macaques, social contact was determined to be more important than the immunosuppressive effects of stress in mediating the relationship between dominance and parasite infection (MacIntosh et al., 2012). Parasitism in white-handed gibbons, Hylobates lar, and black howler monkeys, Alouatta pigra, is also not affected by faecal cortisol (Gillespie, Barelli, & Heistermann, 2013; Martinez-Mota, 2015), although positive associations between parasitism and faecal cortisol have been documented in other primate species (Arlet et al., 2015; Foerster, Kithome, Cords, & Monfort, 2015; Muehlenbein, 2006). The lack of predictable outcomes may be due, in part, to the dynamics of the stress response (Cavigelli & Caruso, 2015) and competing effects of acute and chronic cortisol elevations on immunity (Dhabhar & McEwen, 1999).
Our results show that the nature of connections within a network affect infection risk. In our study, dominant males had stronger relationships in proximity networks, and females, dominant animals and adults tended to be have higher connectivity in grooming networks. However, none of these centrality measures was associated with parasite acquisition. Attempts to link transmission of pathogens to certain host traits based on centrality metrics alone should therefore be interpreted with caution. Furthermore, risk associated with social connectivity is not likely to be static, but rather can vary over time (Rushmore et al., 2013). Heterogeneities in social connectivity, and resulting changes in infection over time, may therefore obscure the relationship between social network position and individual infection risk in cross-sectional studies.
Conclusions
In our study population of Nigerian red-capped mangabeys, animals central in the social network had a higher probability of acquiring macroparasites than did peripheral individuals. Individuals with high centrality may facilitate transmission throughout the population, perhaps acting as ‘super spreaders’ (Lloyd-Smith, Schreiber, Kopp, & Getz, 2005). We found no direct associations between intrinsic host traits and time to infection, which concords with the result of previous studies. In fact, individual predictors of parasite aggregation (e.g. sex, age and dominance) documented in previous studies may be confounders of the direct relationship between social connectivity and infection risk. Overall, our results suggest that being central in a social network confers costs in terms of infection risk. Variation in social networks structure and dynamics should be considered in studies of infection risk in social species.
Acknowledgments
We thank Festus Onajde, Rosemary Gbegbaje and CERCOPAN staff for their assistance with data collection and support in the field, and Mason Saari, Kelsey Brown, Nicholas Segel and Julia Slezak for their assistance in the lab. We also thank Dan Wittwer for his help with hormone assays, Dr Tim Yoshino for his assistance with parasite identifications, Dr Dorte Dopfer for her input on statistical analyses, Dr Sarah Paige for creating a map of our study area, and Bill Rohde for his careful edits to this manuscript. This research was funded by the Fulbright International Educational Exchange, the National Science Foundation’s Doctoral Dissertation Improvement Grant (DDIG: 1403861), National Institutes of Health (NIH) Parasitology and Vector Biology Training Program (T32AI007414; PI: T. Yoshino), Robert Wood Johnson Health Foundation Dissertation Grant, Graduate Women in Science Foundation, and John Ball Zoological Society Conservation Grant. Funding was provided to the Assay Services Unit of the Wisconsin National Primate Research Center by NIH (grant number P51OD011106) to provide cost-efficient sample analyses.
References
- Altizer S, Nunn CL, Thrall PH, Gittleman JL, Antonovics J, Cunningham AA, et al. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annual Review of Ecology, Evolution, and Systematics. 2003;34:517–547. [Google Scholar]
- Arlet ME, Chapman CA, Isbell LA, Molleman F, Mänd R, Hõrak P, et al. Social and ecological correlates of parasitic infections in adult male gray-cheeked mangabeys (Lophocebus albigena) International Journal of Primatology. 2015;36(5):967–986. http://doi.org/10.1007/s10764-015-9866-9. [Google Scholar]
- Bordes F, Morand S. The impact of multiple infections on wild animal hosts: a review. Infection Ecology & Epidemiology. 2011;1:7346. doi: 10.3402/iee.v1i0.7346. http://doi.org/10.3402/iee.v1i0.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgatti SP, Everett MG, Freeman LC. UCINET for Windows [Software] Harvard, MA: Analytic Technologies; 2002. Retrieved from https://sites.google.com/site/ucinetsoftware/home. [Google Scholar]
- Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology. 1997;83(4):575–583. http://doi.org/10.2307/3284227. [PubMed] [Google Scholar]
- Cavigelli SA, Caruso MJ. Sex, social status and physiological stress in primates: the importance of social and glucocorticoid dynamics. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1669):20140103. doi: 10.1098/rstb.2014.0103. http://doi.org/10.1098/rstb.2014.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CA, Saj TL, Snaith TV. Temporal dynamics of nutrition, parasitism, and stress in colobus monkeys: implications for population regulation and conservation. American Journal of Physical Anthropology. 2007;134(2):240–250. doi: 10.1002/ajpa.20664. http://doi.org/10.1002/ajpa.20664. [DOI] [PubMed] [Google Scholar]
- Clough D, Heistermann M, Kappeler PM. Host intrinsic determinants and potential consequences of parasite infection in free-ranging red-fronted lemurs (Eulemur fulvus rufus) American Journal of Physical Anthropology. 2010;142(3):441–452. doi: 10.1002/ajpa.21243. http://doi.org/10.1002/ajpa.21243. [DOI] [PubMed] [Google Scholar]
- Coster GD, Neve LD, Martín-Gálvez D, Therry L, Lens L. Variation in innate immunity in relation to ectoparasite load, age and season: a field experiment in great tits (Parus major) Journal of Experimental Biology. 2010;213(17):3012–3018. doi: 10.1242/jeb.042721. http://doi.org/10.1242/jeb.042721. [DOI] [PubMed] [Google Scholar]
- Craft ME. Infectious disease transmission and contact networks in wildlife and livestock. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1669):20140107. doi: 10.1098/rstb.2014.0107. http://doi.org/10.1098/rstb.2014.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft DP, Madden JR, Franks DW, James R. Hypothesis testing in animal social networks. Trends in Ecology & Evolution. 2011;26(10):502–507. doi: 10.1016/j.tree.2011.05.012. http://doi.org/10.1016/j.tree.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Crofton HD. A model of host–parasite relationships. Parasitology. 1971;63(03):343–364. doi: 10.1017/s0031182000079890. [DOI] [PubMed] [Google Scholar]
- de Vries H, Stevens JMG, Vervaecke H. Measuring and testing the steepness of dominance hierarchies. Animal Behaviour. 2006;71(3):585–592. http://doi.org/10.1016/j.anbehav.2005.05.015. [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(3):1059–1064. doi: 10.1073/pnas.96.3.1059. http://doi.org/10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewe JA. Who infects whom? Social networks and tuberculosis transmission in wild meerkats. Proceedings of the Royal Society B: Biological Sciences. 2009;277:633–642. doi: 10.1098/rspb.2009.1775. http://doi.org/10.1098/rspb.2009.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewe JA, Perkins SE. Disease transmission in animal social networks. In: Krause J, James R, Franks DW, Croft DP, editors. Animal social networks. Oxford, U.K: Oxford University Press; 2015. [Google Scholar]
- Farine DR. Animal social network inference and permutations for ecologists in R using asnipe. Methods in Ecology and Evolution. 2013;4(12):1187–1194. http://doi.org/10.1111/2041-210X.12121. [Google Scholar]
- Farine DR. Proximity as a proxy for interactions: issues of scale in social network analysis. Animal Behaviour. 2015;104(6):e1–e5. http://doi.org/10.1016/j.anbehav.2014.11.019. [Google Scholar]
- Farine DR, Whitehead H. Constructing, conducting and interpreting animal social network analysis. Journal of Animal Ecology. 2015;84(5):1144–1163. doi: 10.1111/1365-2656.12418. http://doi.org/10.1111/1365-2656.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner AL, Godfrey SS, Bull CM. Using social networks to deduce whether residents or dispersers spread parasites in a lizard population. Journal of Animal Ecology. 2011;80(4):835–843. doi: 10.1111/j.1365-2656.2011.01825.x. http://doi.org/10.1111/j.1365-2656.2011.01825.x. [DOI] [PubMed] [Google Scholar]
- Foerster S, Kithome K, Cords M, Monfort SL. Social status and helminth infections in female forest guenons (Cercopithecus mitis) American Journal of Physical Anthropology. 2015;158(1):55–66. doi: 10.1002/ajpa.22764. http://doi.org/10.1002/ajpa.22764. [DOI] [PubMed] [Google Scholar]
- Freeland WJ. Mangabey (Cerocebus albigena) movement patterns in relation to food availability and fecal contamination. Ecology. 1980;61(6):1297–1303. http://doi.org/10.2307/1939037. [Google Scholar]
- Gillespie TR, Barelli C, Heistermann M. Effects of social status and stress on patterns of gastrointestinal parasitism in wild white-handed gibbons (Hylobates lar) American Journal of Physical Anthropology. 2013;150(4):602–608. doi: 10.1002/ajpa.22232. http://doi.org/10.1002/ajpa.22232. [DOI] [PubMed] [Google Scholar]
- Godfrey SS, Moore JA, Nelson NJ, Bull CM. Social network structure and parasite infection patterns in a territorial reptile, the tuatara (Sphenodon punctatus) International Journal for Parasitology. 2010;40(13):1575–1585. doi: 10.1016/j.ijpara.2010.06.002. http://doi.org/10.1016/j.ijpara.2010.06.002. [DOI] [PubMed] [Google Scholar]
- González-Hernández M, Rangel-Negrín A, Schoof VAM, Chapman CA, Canales-Espinosa D, Dias PAD. Transmission patterns of pinworms in two sympatric congeneric primate species. International Journal of Primatology. 2014;35(2):445–462. http://doi.org/10.1007/s10764-014-9751-y. [Google Scholar]
- Grear DA, Luong LT, Hudson PJ. Network transmission inference: host behavior and parasite life cycle make social networks meaningful in disease ecology. Ecological Applications. 2013;23(8):1906–1914. doi: 10.1890/13-0907.1. http://doi.org/10.1890/13-0907.1. [DOI] [PubMed] [Google Scholar]
- Greiner EC, McIntosh A. Collection methods and diagnostic procedures for primate parasitology. In: Huffman MA, Chapman CA, editors. Primate parasite ecology : The dynamics and study of host–parasite relationships. Cambridge, U.K: Cambridge University Press; 2009. pp. 3–27. [Google Scholar]
- Habig B, Archie EA. Social status, immune response and parasitism in males: a meta-analysis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1669):20140109. doi: 10.1098/rstb.2014.0109. http://doi.org/10.1098/rstb.2014.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillegass MA, Waterman JM, Roth JD. Parasite removal increases reproductive success in a social African ground squirrel. Behavioral Ecology. 2010;21(4):696–700. http://doi.org/10.1093/beheco/arq041. [Google Scholar]
- Hudson PJ, Dobson AP. Host–parasite processes and demographic consequences. In: Clayton DH, Moore J, editors. Host–parasite evolution: General principles and avian models. Oxford, U.K: Oxford University Press; 1997. [Google Scholar]
- Kappeler PM, Cremer S, Nunn CL. Sociality and health: impacts of sociality on disease susceptibility and transmission in animal and human societies. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1669):20140116. doi: 10.1098/rstb.2014.0116. http://doi.org/10.1098/rstb.2014.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbaum DG, Klein M. Survival analysis: A self-learning text. 3. New York, NY: Springer; 2012. [Google Scholar]
- Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. Journal of the American Statistical Association. 1989;84(408):1074–1078. http://doi.org/10.1080/01621459.1989.10478874. [Google Scholar]
- Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438(7066):355–359. doi: 10.1038/nature04153. http://doi.org/10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh AJJ, Jacobs A, Garcia C, Shimizu K, Mouri K, Huffman MA, et al. Monkeys in the middle: parasite transmission through the social network of a wild primate. PLoS One. 2012;7(12):e51144. doi: 10.1371/journal.pone.0051144. http://doi.org/10.1371/journal.pone.0051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Mota R. Doctoral Dissertation. Champaign, IL: University of Illinois at Urbana-Champaign; 2015. The effects of habitat disturbance, host traits, and host physiology on patterns of gastrointestinal parasite infection in black howler monkeys (Alouatta pigra) Retrieved from https://www.ideals.illinois.edu/handle/2142/78434. [Google Scholar]
- Møller AP, Dufval R, Allander K. Parasites and the evolution of host social behavior. Advances in the Study of Behavior. 1993;22:65–102. [Google Scholar]
- Muehlenbein MP. Intestinal parasite infections and fecal steroid levels in wild chimpanzees. American Journal of Physical Anthropology. 2006;130(4):546–550. doi: 10.1002/ajpa.20391. http://doi.org/10.1002/ajpa.20391. [DOI] [PubMed] [Google Scholar]
- Nunn CL, Altizer S. Infectious diseases in primates: Behavior, ecology and evolution. Oxford, U.K: Oxford University Press; 2006. [Google Scholar]
- Nunn CL, Craft ME, Gillespie TR, Schaller M, Kappeler PM. The sociality–health–fitness nexus: synthesis, conclusions and future directions. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1669):20140115. doi: 10.1098/rstb.2014.0115. http://doi.org/10.1098/rstb.2014.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates JF, Gippoliti S, Groves CP. IUCN Red List of Threatened Species (Version 2015.2) Cambridge, U.K: IUCN Global Species Programme Red List Unit; 2008. Cercocebus torquatus. Retrieved from www.iucnredlist.org. [Google Scholar]
- O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Quality & Quantity. 2007;41(5):673–690. http://doi.org/10.1007/s11135-006-9018-6. [Google Scholar]
- Pedersen AB, Fenton A. The role of antiparasite treatment experiments in assessing the impact of parasites on wildlife. Trends in Parasitology. 2015;31(5):200–211. doi: 10.1016/j.pt.2015.02.004. http://doi.org/10.1016/j.pt.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Perkins SE, Cagnacci F, Stradiotto A, Arnoldi D, Hudson PJ. Comparison of social networks derived from ecological data: implications for inferring infectious disease dynamics. Journal of Animal Ecology. 2009;78(5):1015–1022. doi: 10.1111/j.1365-2656.2009.01557.x. http://doi.org/10.1111/j.1365-2656.2009.01557.x. [DOI] [PubMed] [Google Scholar]
- Poulin R. Are there general laws in parasite ecology? Parasitology. 2007;134(06):763. doi: 10.1017/S0031182006002150. http://doi.org/10.1017/S0031182006002150. [DOI] [PubMed] [Google Scholar]
- Range F, Noë R. Familiarity and dominance relations among female sooty mangabeys in the Tai National Park. American Journal of Primatology. 2002;56(3):137–153. doi: 10.1002/ajp.1070. http://doi.org/10.1002/ajp.1070. [DOI] [PubMed] [Google Scholar]
- Raveh S, Neuhaus P, Dobson FS. Ectoparasites and fitness of female Columbian ground squirrels. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1669):20140113. doi: 10.1098/rstb.2014.0113. http://doi.org/10.1098/rstb.2014.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. Retrieved from http://www.R-project.org. [Google Scholar]
- Re DAC. compute.es: Compute effect sizes (R package Version 0.2–4) 2015 Retrieved from http://CRAN.R-project.org/package=compute.es.
- Rimbach R, Bisanzio D, Galvis N, Link A, Fiore AD, Gillespie TR. Brown spider monkeys (Ateles hybridus): a model for differentiating the role of social networks and physical contact on parasite transmission dynamics. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1669):20140110. doi: 10.1098/rstb.2014.0110. http://doi.org/10.1098/rstb.2014.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore J, Caillaud D, Matamba L, Stumpf RM, Borgatti SP, Altizer S. Social network analysis of wild chimpanzees provides insights for predicting infectious disease risk. Journal of Animal Ecology. 2013;82(5):976–986. doi: 10.1111/1365-2656.12088. http://doi.org/10.1111/1365-2656.12088. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. http://doi.org/10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Shaw DJ, Dobson AP. Patterns of macroparasite abundance and aggregation in wildlife populations: a quantitative review. Parasitology. 1995;111(Suppl S1):S111–S133. doi: 10.1017/s0031182000075855. http://doi.org/10.1017/S0031182000075855. [DOI] [PubMed] [Google Scholar]
- Silk JB. Evolutionary perspectives on the links between close social bonds, health, and fitness. In: Weinstein M, Lane MA, editors. Sociality, hierarchy, health: Comparative biodemography: A colletction of papers. Washington, D.C: National Academies Press; 2014. pp. 120–143. [PubMed] [Google Scholar]
- Theis FJ, Ugelvig LV, Marr C, Cremer S. Opposing effects of allogrooming on disease transmission in ant societies. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1669):20140108. doi: 10.1098/rstb.2014.0108. http://doi.org/10.1098/rstb.2014.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau TM. survival: Survival Analysis (R package Version 2.38-3) 2015 https://cran.r-project.org/web/packages/survival.
- VanderWaal KL, Atwill ER, Hooper S, Buckle K, McCowan B. Network structure and prevalence of Cryptosporidium in Belding’s ground squirrels. Behavioral Ecology and Sociobiology. 2013;67(12):1951–1959. http://doi.org/10.1007/s00265-013-1602-x. [Google Scholar]
- Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. Journal of the American Statistical Association. 1989;84(408):1065–1073. http://doi.org/10.1080/01621459.1989.10478873. [Google Scholar]
- Whitehead H. SOCPROG programs: analysing animal social structures. Behavioral Ecology and Sociobiology. 2009;63(5):765–778. http://doi.org/10.1007/s00265-008-0697-y. [Google Scholar]
- Ziegler TE, Scheffler G, Snowdon CT. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Hormones and Behavior. 1995;29(3):407–424. doi: 10.1006/hbeh.1995.1028. http://doi.org/10.1006/hbeh.1995.1028. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Wittwer DJ. Fecal steroid research in the field and laboratory: improved methods for storage, transport, processing, and analysis. American Journal of Primatology. 2005;67(1):159–174. doi: 10.1002/ajp.20175. http://doi.org/10.1002/ajp.20175. [DOI] [PubMed] [Google Scholar]
- Zuk M, McKean KA. Sex differences in parasite infections: patterns and processes. International Journal for Parasitology. 1996;26(10):1009–1024. http://doi.org/10.1016/S0020-7519(96)80001-4. [PubMed] [Google Scholar]