Abstract
Previous studies demonstrated that neural progenitor cells (NPCs) transplanted into a subacute contusion injury improve motor, sensory, and bladder function. In this study we tested whether transplanted NPCs can also improve functional recovery after chronic spinal cord injury (SCI) alone or in combination with the reduction of glial scar and neurotrophic support. Adult rats received a T10 moderate contusion. Thirteen weeks after the injury they were divided into four groups and received either: 1. Medium (control), 2. NPC transplants, 3. NPC + lentivirus vector expressing chondroitinase, or 4. NPC + lentivirus vectors expressing chondroitinase and neurotrophic factors. During the 8 weeks post-transplantation the animals were tested for functional recovery and eventually analyzed by anatomical and immunohistochemical assays. The behavioral tests for motor and sensory function were performed before and after injury, and weekly after transplantation, with some animals also tested for bladder function at the end of the experiment. Transplant survival in the chronic injury model was variable and showed NPCs at the injury site in 60% of the animals in all transplantation groups. The NPC transplants comprised less than 40% of the injury site, without significant anatomical or histological differences among the groups. All groups also showed similar patterns of functional deficits and recovery in the 12 weeks after injury and in the 8 weeks after transplantation using the Basso, Beattie, and Bresnahan rating score, the grid test, and the Von Frey test for mechanical allodynia. A notable exception was group 4 (NPC together with chondroitinase and neurotrophins), which showed a significant improvement in bladder function. This study underscores the therapeutic challenges facing transplantation strategies in a chronic SCI in which even the inclusion of treatments designed to reduce scarring and increase neurotrophic support produce only modest functional improvements. Further studies will have to identify the combination of acute and chronic interventions that will augment the survival and efficacy of neural cell transplants.
Keywords: neural stem cell transplantation, chronic spinal cord injury, motor and sensory function, bladder function
INTRODUCTION
It is estimated that the annual incidence of spinal cord injury (SCI) is approximately 12,000 new cases each year (NSCISC, 2013). Injury to the spinal cord results in extensive axonal damage and degeneration, neuronal loss, and severe functional deficits. Three phases occur after SCI: acute (hours to days), sub-acute (days to weeks), and chronic (months to years). Many repair strategies have been proposed and tested in models of acute and sub-acute SCI such as tissue protection using corticosteroids and gangliosides (Hurlbert et al., 2013), anti-inflammatory reagents (Hawthorne and Popovich, 2011; Ren and Young, 2013), factors to promote axonal regeneration such as neurotrophic factors, blockage/removal of inhibitory factors, and tissue/cell transplantation (Cafferty et al., 2007; Hollis and Tuszynski, 2011; Tetzlaff et al., 2011; McCall et al., 2012; Cregg et al., 2014). Indeed, cell transplantation has been shown to be one of the most promising strategies to promote functional recovery in acute and subacute SCI. Transplants of Schwann cells, olfactory ensheathing cells, genetically modified fibroblasts, and various neural stem cells have demonstrated some axonal regeneration and partial functional recovery (Murray et al., 2002; Tuszynski et al., 2003; Oudega and Xu, 2006; Barnett and Riddell, 2007; Lopez-Vales et al., 2007; Louro and Pearse, 2008; Deng et al., 2013). Only a few studies, however, have addressed chronic SCI due to the experimental complexities and the daunting challenges associated with axonal growth through a chronic injury (Jin et al., 2002; Barakat et al., 2005; Lu et al., 2007; Tom et al., 2009; Karimi-Abdolrezaee et al., 2010). The glial and extracellular scar, which is formed within days after SCI, becomes well established after several weeks. This “chronic scar,” together with the formation of cystic cavities, creates a greater impediment to axonal regeneration at the chronic stage than immediately after injury. Some studies have shown that it is possible to achieve some axonal growth and even bridge the injury site using combination therapies (Barakat et al., 2005; Lu et al., 2007; Tom et al., 2009). Transplants of modified fibroblasts expressing BDNF in a chronic cervical hemisection lesion induced some supraspinal neurons to regenerate their axons into the transplants, but few bridged the injury site and re-entered the host (Jin et al., 2002). Similarly, transplants of modified bone marrow stromal cells (MSCs) expressing NT-3 promoted sensory axon growth through the chronic scar and into the cell graft but did not result in axon growth beyond the lesion (Lu et al., 2007). Combination treatments have shown that modifying the intrinsic growth state of neurons by using conditioning lesions, combined with introduction of genetically modified cells that provide neurotrophins and a gradient of neurotrophins rostral to the lesion site, can induce bridging of sensory axons one year after SCI (Kadoya et al., 2009). All data indicate that axonal regeneration through the lesion area and into host tissue at both the acute and chronic stages of SCI remains a significant challenge.
Our previous studies have demonstrated that transplants of neural progenitor cells (NPCs) into the acute and subacute injured spinal cord survive, generate neurons and glial cells and form a functional relay to connect the lesioned sensory pathway of dorsal column axons (Bonner et al., 2011) and improve function following a contusion injury (Mitsui et al., 2005). In the present study, we applied these approaches to the chronic SCI model and examined whether transplants of NPCs, together with lentivirus vectors expressing chondroitinase (Chase/LV) and lentivirus vectors expressing chondroitinase and growth factor treatments (Chase/LV + BDNF/NT-3/LV) could survive, generate neural cells, and improve functional recovery.
EXPERIMENTAL PROCEDURES
Animals
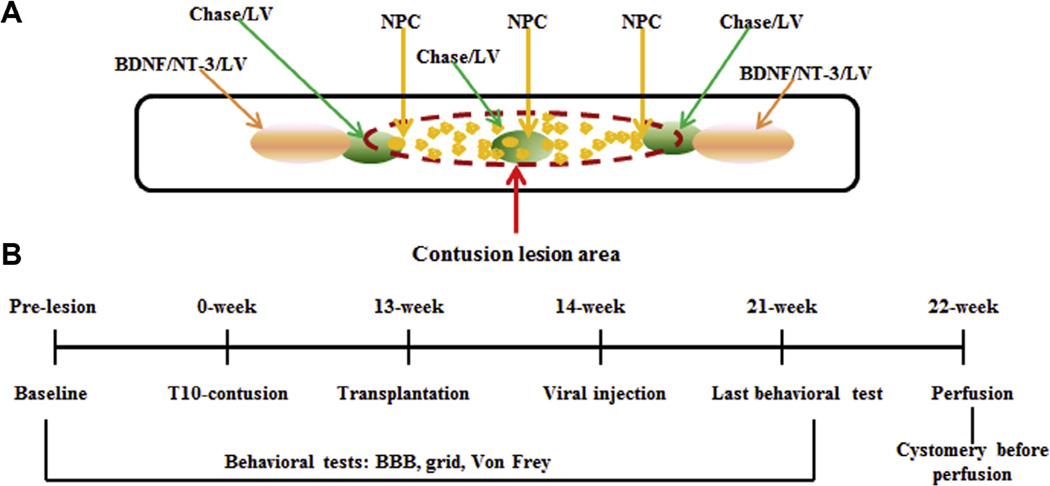
Adult female rats (225–250 g, Taconic, Germantown, NY, USA) were used in this study. They were housed in an environmentally controlled facility with a 12-h light/dark cycle. Food and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of Drexel University College of Medicine and were carried out according to the NIH Guide for the Care and Use of Laboratory Animals. Fig. 1 shows the outline of experimental design (A) and experimental time line (B).
Fig. 1.
Outline of experimental design, time line, and groups. Panel A shows the location of transplanted NPCs and viral injection. Panel B shows the time line. Four experimental groups were used in this study: Medium (Control, n = 9); NPC (n = 10); N/C (NPC + Chase/LV, n = 10); N/C/G (NPC + Chase/LV + BDNF/NT-3/LV, n = 8).
Surgical procedure
Forty-five rats were used in this study. Thirty-nine rats received a contusion lesion and were divided into four groups (Table 1): Medium (n = 9), NPC transplant (NPC, n = 10), NPC transplant with Chase/LV (N/C, n = 10), NPC transplant with Chase/LV + BDNF/NT-3/ LV (N/C/G, n = 10). Two rats from N/C/G were sacrificed early due to autophagia.
Table 1.
Experimental groups and treatments
| Groups | Contusion | NPC transplant |
Chase/ LV |
Growth factors |
Cyclosporine A |
Cystometry |
|---|---|---|---|---|---|---|
| Medium (control) n = 9 | + | − | − | − | + | + (n = 3) |
| NPC n = 10 | + | + | − | − | + | + (n = 4) |
| N/C (NPC + Chase/LV) n = 10 | + | + | + | − | + | + (n = 4) |
| N/C/G (NPC + Chase/LV + Growth factors) n = 10 |
+ | + | + | + | + | + (n = 4) |
| Intact n = 6 | − | − | − | − | − | + (n = 6) |
A laminectomy was performed at T10 after anesthesia with XAK containing Xylazine (6 mg/ml), Acepromazine maleate (0.05 mg/ml), and Ketamine (63 mg/ml) injected intraperitoneally. A contusion lesion was made with the NYU impactor (10 g, 25 mm). Muscle and skin were closed in layers. Thirteen weeks after contusion, animals were re-anesthetized with XAK and placed in a spinal stereotaxic frame and the lesioned region re-exposed without opening the dura. Using a 10-µl Nanofil syringe (World Precision Instruments, Sarasota, FL, USA) with a 33-gauge needle, HBSS (10 µl) or cells (10 µl, 1 × 105 cell/µl) were injected into the lesion center along the midline (4 µl), and rostral and caudal to the lesion along the midline (3 µl/each). HBSS or cells were injected at 20 nl/s using a nanoliter pump controller (World Precision Instruments, Sarasota, FL, USA). One minute after injection, the tip was slowly withdrawn. Rats were placed back in their cages on heating pads and closely observed until awakening. One week after transplantation, rats in the N/C and N/C/G groups received an additional injection of virus with the same procedure as the cell transplants. In the N/C group, rats received Chase/LV injected at lesion/transplant center and 1 mm rostral to caudal to the lesion (1 µl/injection, 2.0 × 106 TU/µ1) at midline. In the N/C/G group, rats received Chase/LV injections similar to the N/C group (within the lesion as well as 1 mm rostral and caudal to the lesion), and also received BDNF/LV and NT-3/LV 1 mm rostral and caudal to the lesion (2 µl/ injection; mixture of BDNF/LV and NT-3/LV, each 2.9 × 105 TU/µl). Rats received subcutaneous injection of saline immediately after injury, transplantation, and viral administration and were injected daily for 7 days. Cefazolin was injected daily for 7 days postoperatively. Bladders were manually expressed twice a day for 2 weeks and then once a day until recovery of bladder function.
Preparation of NPC for grafting
The preparation of NPC has been described previously (Han et al., 2002, 2004; Lepore and Fischer, 2005; Mitsui et al., 2005). Briefly, spinal cord from embryonic day 13.5 transgenic Fischer 344 rats that express the maker gene human placental alkaline phosphatase (AP) was dissected and cultured for 3–5 days in complete Medium [DMEM/F12, BSA (1 mg/ml; Sigma, St. Louis, MO, USA), B27 (Invitrogen, Carlsbad, CA, USA); basic FGF (bFGF, 30 ng/ml; Peprotech, Rocky Hill, NJ), Penicillin-streptomycin (100 IU/ml; Invitrogen), N2 (10 µl/ml; Invitrogen); and Neurotrophin-3 (NT-3, 10 µg/ml; Peprotech)] on poly-l-Lysine-coated (13.3 µg/ml; Sigma) and laminin-coated (15 µg/ml; Invitrogen) flasks. On the day of transplantation, NPCs were dissociated from culture flasks using 0.05% trypsin/EDTA (Invitrogen), washed, and re-suspended at a concentration of 1 × 105 cells/µl (in HBSS) for transplantation. Cells were kept on ice during transplantation.
Immunosuppression with cyclosporine A
All animals received subcutaneous injection of cyclosporine A (Sandimmune; Novartis Pharmaceuticals East Hanover, NJ, USA) at a dose of 1 mg/100 g per day beginning 2–3 days before transplantation and continuing to the end of the experiments.
Cystometry in awake rats
Eight weeks after transplantation, four rats from each group were anesthetized under 2% isoflurane inhalation, and a polyethylene catheter was implanted into the bladder. A midline lower abdominal incision exposed the bladder and a polyethylene catheter (PE-60; Clay Adams, Parsippany, NJ, USA) was implanted into the bladder through the dome, as described previously (Yoshiyama et al., 1999; Seki et al., 2002; Mitsui et al., 2003, 2005; Jin et al., 2011b). The bladder catheter was tunneled subcutaneously and exited through the skin on the back. After catheter implantation, rats were placed in a restraining cage (KN-326; Natsume, Tokyo, Japan) and allowed to recover for 1–2 h. The bladder catheter was connected to a pressure transducer (BLPR; World Precision Instruments) and a microinjection pump (STC-523; Terumo, Tokyo, Japan). Room-temperature saline was infused at a rate of 0.1 ml/min, and intravesicular pressure was recorded. Micturition cycles stabilized and became fairly regular after 1 h of saline infusion. Three micturition cycles were collected after stabilization. The averages of the maximal voiding pressure, duration of micturition, interval between micturition, frequency of non-voiding contraction (NVC, the number of NVC per micturition episode), post-void residual urine, bladder capacity, and voiding efficacy in these micturition cycles were compared among groups. Six intact rats of the same age as the chronically injured rats were used for cystometry at the end of the experiment as the control group.
Evaluation of motor and sensory function
All behavioral testing was conducted between 09:00 am and 12:00 pm, and sensory tests were performed on separate days from the motor tests. All observers and scorers of the behavioral tests were blinded to the group assignments.
Open-field locomotion
The rats were placed in an open field enclosure and scored by two observers according to the Basso, Beattie, and Bresnahan (BBB) rating scale (Basso et al., 1995; Cao et al., 2008). Testing was performed before contusion, 2–3 days after contusion, and once weekly after contusion for 12 weeks and continuing for an additional 8 weeks after transplantation.
Grid test
The rats walked on an elevated grid (36 cm L, 38 cm W, 30 cm H with 1.2 × 1.2 cm openings) for 2 min and were videotaped for later scoring. The total number of steps with correct paw placement over the total number of steps was counted for each hind paw. Grid tests were performed weekly on animals with a BBB score above 10.
Mechanical allodynia test
To determine the degree of tactile sensory changes present after SCI, Von Frey hair monofilaments (VFH; Stoelting Co., Wood Dale, IL, USA) were applied to the plantar surface of the hind paw using the up-down method (Chaplan et al., 1994; Detloff et al., 2010, 2012). Plantar VFH was not initiated unless hind limb weight support recovered, as determined by BBB testing (BBB score 10 or above). A total of 10 VFH stimulus applications were collected for each hind paw (Detloff et al., 2013). The response threshold was the lowest force (in grams) that elicited a paw withdrawal in at least 50% of the applications. Paw testing order was randomized to minimize an order effect.
Tissue preparation
At the end of the experiments, animals were anesthetized with an intraperitoneal injection of sodium pentobarbital (100 mg/kg; Abbot Laboratories, North Chicago, IL, USA) and transcardially perfused with 200 ml saline, followed by 500 ml of ice-cold 4% paraformaldehyde fixative in phosphate buffer (PB, pH 7.4). Spinal cords at the lesion/transplant area as well as at lumbar-sacral region (L6-S1) were dissected. The bladders from rats receiving cystometry testing were also dissected and the weight was measured. Tissues were post-fixed in the same fixative overnight and then transferred to a 30% sucrose solution (in 0.1 M PB) for 3–5 days. Tissues were embedded in M1 (Fisher Scientific, Pittsburgh, PA, USA). Sagittal sections at the lesion/transplant site were cut at 20 µm and mounted onto gelatin-coated slides. Cross sections at L6/S1 were cut at 30 µm and collected into 0.1 M PBS (pH 7.6) for free-floating staining. Both sections were collected in 10 serial sets.
AP histochemistry
One set of sections with NPC transplantation was analyzed using AP histochemistry to determine survival of grafted NPC. Sections were washed three times with PBS and heated at 60 °C in PBS for 1 h to inactive endogenous alkaline phosphates. Sections were briefly washed in AP buffer (100 mM Tris; 100 mM NaCl; and 50 mM MgCl, pH 9.5), and incubated in the dark at room temperature with AP buffer containing 1.0 mg/ml nitrobluetetrazolium-chloride, 0.1 mg/ml 5-bromo-4-chlor-indolyl-phophate and 5 mM levamisole (Sigma) for 2 h. Slides were coverslipped with Vectashield mounting media and visualized using a light microscope.
Immunohistochemistry
Immunohistochemical staining of sagittal sections at lesion/transplant was performed for the following antibodies: GFAP (1:1000, Chemicon) for reactive astrocytes, AP (1:200, AbD Serotec, Raleigh, NC) for transplanted cells, NeuN (1:100, Chemicon) for mature neurons, GFP (1:1000, Chemicon) for identifying viral expression, 2B6 (1:200, Seikagaku Biobusiness Corporation, Japan) specific for CSPG digestion, ED1 (1:150, Serotec/accurate) for macrophages, calcitonin gene-related peptide (CGRP) (1:2000, Peninsula Laboratories International, Inc., San Carlos, CA, USA) for ascending sensory fibers, and 5-HT (1:20,000, ImmunoStar, Inc., Hudson, WI, USA) for descending fibers. Sections were washed three times with PBS and incubated with blocking buffer for 1 h at room temperature, then incubated overnight with the appropriate antibody. On the following day, sections were washed 3 times for 10 min, incubated with goat anti-mouse or goat anti-rabbit conjugated to FITC or rhodamine red (Jackson ImmunoResearch, West Grove, PA, USA) for 2 h, and then cover-slipped with anti-fade Permount.
Free-floating cross sections (Mitsui et al., 2005; Jin et al., 2011b) from lumbosacral (L6-S1) tissue were immunostained with antibodies against serotonin (5-HT, 1:80,000, Immunostar), calcitonin gene-related peptide (CGRP, 1:6000, Peninsula), and vanilloid receptor type 1 (VR-1, 1:5000, Chemicon). Every 10th section in all groups was stained for each antibody. The sections were blocked with 10% goat serum in PBS for 2 h after incubation with 3% H2O2 in PBS for 30 min. Sections were then incubated with the appropriate primary antibodies and 2% goat serum in PBS containing 0.3% Triton X-100 at room temperature overnight, and reacted with a species-specific biotinylated secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA) and ABC reagent (Vector Laboratories, Inc., Burlingame, CA, USA), each for 2 h at room temperature. Staining was visualized with Sigma fast DAB (Sigma). The sections were mounted on gelatin-coated slides, dehydrated in graded ethanol, cleaned, and coverslipped.
Measurement of spared tissue and cyst area
The area of spared tissue and cyst were assessed using Stereo Investigator in sagittal sections with Nissl myelin staining in a blinded fashion. For each animal, the total area was determined for every section within a 16-mm-long segment centered at the contusion/transplant epicenter. Tissue was considered spared if areas contained healthy-looking spinal neurons and myelin. Cyst area was determined within the lesion area. Using Stereo Investigator, spared tissue, lesion area, and cysts can be marked with different colors. The software calculates the total area and provides the fraction of each part of each animal. Data were presented as percentages of total cord area for spared tissue, lesion/transplant, and cysts in lesion/transplant.
Measurement of relative density of transplanted AP + cells inside the lesion area
AP-stained tissue sections containing AP+ cells were visualized with a 1.25-× objective and images were captured. NIH Image J was subsequently used to outline the lesion area. The threshold was automatically set for each location for all groups. The labeled area within the outlined injury site was measured and is presented as area fraction (%).
Evaluation of changes in the lumbar-sacral spinal cord
Five sections of similar size, chosen at equal distances (every 10th section from L6-S1 were used for 5-HT, CGRP and VR-1 staining) were viewed with a 20-× objective and images were captured, as previously described (Jin et al., 2011b), with a few modifications. Briefly, images were converted to gray scale using NIH Image J. A fixed box for each location was set for all animals. Each image was sharpened twice to expose the fibers from the background. The threshold was set for each location for all groups. The labeled area within the fixed box was measured and is presented as area fraction (%).
Statistical analysis
A two-way analysis of variance (ANOVA) comparing control versus experimental groups over time, with time taken as a repeated measure, was used to analyze the behavioral data, with significance set at p < 0.05 for all comparisons. Analyses were performed for each outcome measure on the data collected within the 12 weeks post-injury and again within the 8 weeks post transplantation. Post-hoc analyses was performed where appropriate using the Bonferroni test. One-way ANOVA followed by the Bonferroni test was used to analyze lesion area and area fraction.
RESULTS
NPC at the lesion site
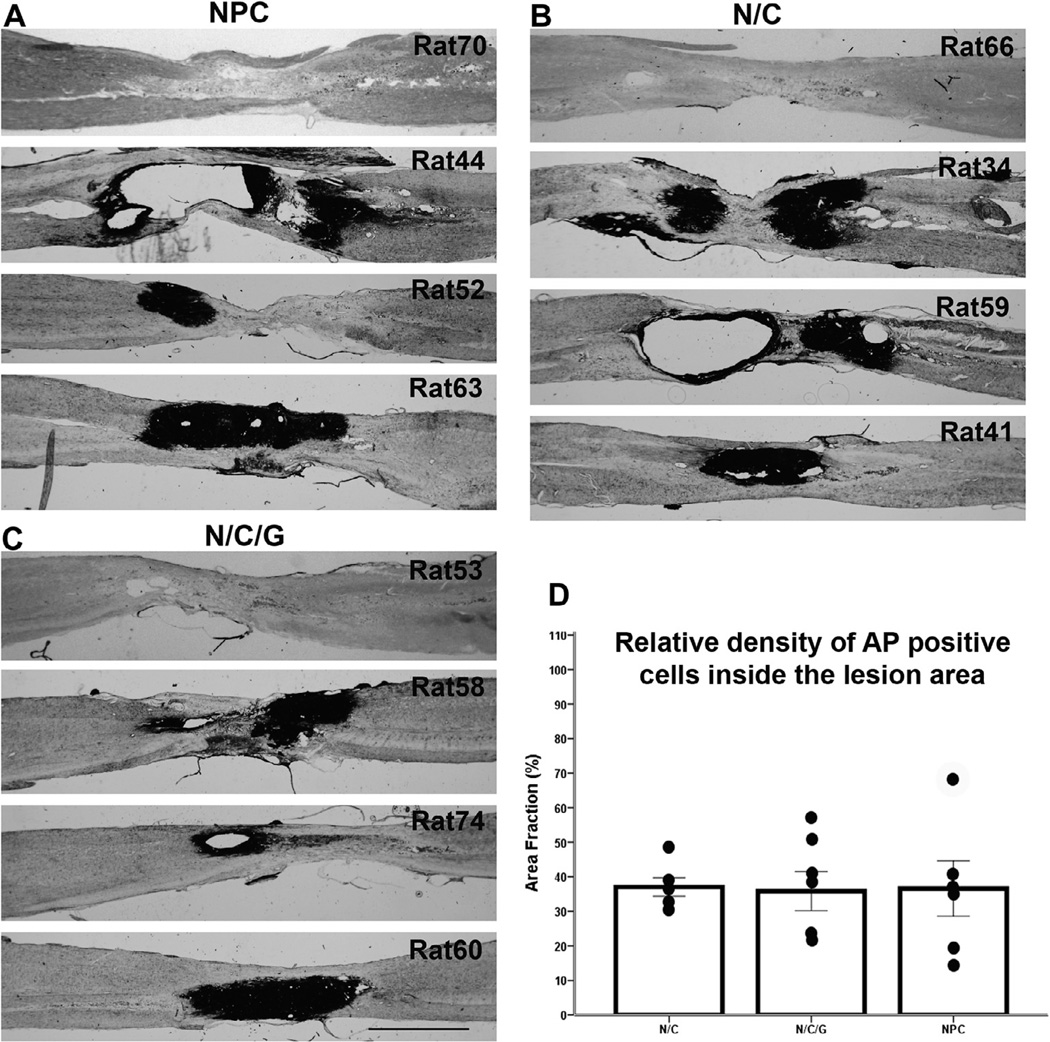
NPCs derived from transgenic AP+ rats were injected into lesion center, as well as locations rostral and caudal to the lesion 13 weeks after contusion (NPC group) and depending on the experimental groups, also received injections of Chase/LV (N/C group), together with BDNF/LV, and NT-3/LV (N/C/G group) one week after cell transplantation. Animals were sacrificed 9 weeks after transplantation and analyzed by AP histology to detect grafted cells. The analysis showed variable survival of the grafts within and around the lesion area. In the NPC group, 6 out of 10 rats showed the presence of AP+ cells at lesion site, and similar results were observed in the other experimental groups – 6 out of 10 rats in the N/C group and 5 out of 8 rats in the N/C/G group. These AP+ cells were located within either the lesion epicenter or rostral/caudal to the lesion. In a few cases, transplanted cells covered the entire lesion area (only one animal from each transplant group demonstrated such substantial and integrated survival). Fig. 2A–C showed variability of the injury site among individual animals with respect to formation of cysts and survival of the grafted cell in each of the experimental groups using AP histological staining to identify the transplants. The figure shows examples of animals with major cysts formed at the injury site (rats 44, 59), animals with no AP+ cells (rats 70, 66, 53), and animals with transplants filling the entire lesion area (rats 63, 41, 60). Quantitative analysis of the relative survival of the transplants calculated by area of AP+ cells demonstrates that on average the grafted cells covered less than 40% of the injury site with no significant differences among the groups (Fig 2D).
Fig. 2.
Transplanted cell survival. (A–C) Show AP histological staining identifying transplanted NPCs within the lesion area in three groups (NPC, N/C, and N/C/G). Images in panels A–C demonstrate the variability in AP+ cells survival at the lesion area, ranging from no survival to cells covering the entire lesion area. Scale bar = 2 mm. Panel D demonstrates the relative density of AP+ cells within the lesion area to visualize the extent of transplant survival. Data are presented as mean ± SEM.
Area of lesion/transplant and spared host spinal cord
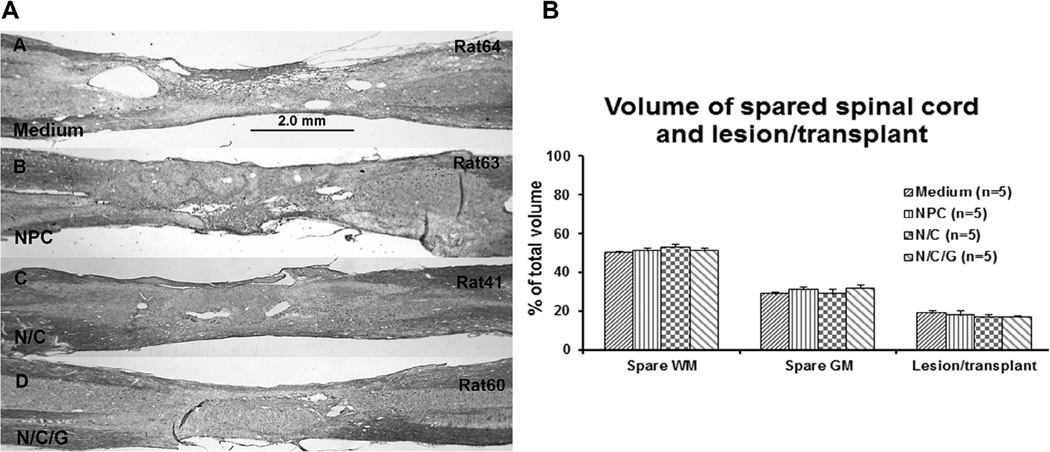
Nissl-myelin staining of the host and transplant region was carried out for morpholoaical evaluation of the injury site including the formation of cysts in all groups (Fig. 3A). Assessment of the spared tissue in the control group was 29% ± 0.77% (Mean ± SEM) for gray matter and 50% ± 1.12% for white matter with no significant differences among the NPC, N/C, and N/C/G groups (Fig. 3B). Similarly, assessment of the lesion area in the control group was 19% ± 1.3%, with the treatment groups NPC, N/C, N/C/G showing similar results without significant differences. Again, despite the variability between individual animals illustrated in Fig. 2, there were no significant changes in either cyst formation or transplant survival among the experimental groups.
Fig. 3.
Nissl/myelin staining (A) shows the lesion/transplant area in different experimental groups (Medium, NPC, N/C, and N/C/G). Sections from B-D were adjacent sections of same animals showed in Fig. 2A–C. Scale bar = 2.0 mm. Panel B presents the measurement of the volume of spared white matter (WM), spared gray matter (GM), and lesion/transplant. Data show mean ± SEM.
Viral expression in the spinal cord
At the end of the experiment, eight weeks after the injection of the virus (all viral vectors with GFP reporter), few GFP-expressing cells were found near or at the lesion/transplant. To examine the effectiveness of the Chase/LV vector in digesting proteoglycans, staining with the 2B6 antibody, which is specific for the digestion products of the enzyme, was performed. Staining showed 2B6 positivity at the center of transplant, as well as rostral and caudal to the lesion/transplant (Fig 4).
Fig. 4.
Chondroitinase activity at the lesion/transplant 8 weeks after virus injection. Chase/LV was injected into the center of lesion/transplant as well as rostral and caudal to the lesion/transplant one week after NPC transplantation. 2B6 antibody staining, which specifically identifies CSPG digestion products generated by Chase, was found rostral and caudal relative to the lesion/transplant as well as within the middle of transplant area. Panel A shows 2B6 positive staining rostral to the lesion/transplant region. Dashed lines and # indicate lesion/transplant region. Panel B shows 2B6 positive staining at the transplant region within a large and small cluster of transplanted cells, (**) and (*), respectively. Scale bar = 100um.
Scar formation and inflammation
To examine scar formation and the inflammatory process we performed immunohistochemical staining with GFAP and ED1. GFAP expression was intense around the injury site in the control group (Fig. 5, panel I, A). A similar pattern was observed in those transplant groups which demonstrated poor cell survival (Fig. 5, panel I, B) even in animals that were treated with chondroitinase. In contrast, in animals where the transplants survived and integrated with host tissue, GFAP staining showed a typical, non-activated astrocyte pattern at the interface (Fig. 5, panel I, C), indicating that the presence of NPCs can reduce the glial scar. Analysis of macrophages, identified by ED1 staining, showed accumulation of ED1 within and around the lesion/transplant area in all groups (Fig. 5, panel II).
Fig. 5.
Scar formation and inflammation around the lesion/transplant area. Panel I shows the scar formation stained by GFAP around the lesion/transplant area. Intense GFAP staining was present at the lesion/transplant area in Medium group (A) as well as in N/C group in animals where NPC transplanted cells did not survive at lesion area (B). In contrast, less intense GFAP staining was observed at the lesion/transplant area in NPC group (C) when NPC survived well and filled the lesion area, indicating that the presence of NPC reduced scar formation. Scale bar = 1 mm. Panel II demonstrates the inflammation stained by ED-1 around the lesion/transplant area. ED1 staining indicates that macrophages were around the lesion/transplant area with some inside the lesion/transplant region. A: Medium group, B: NPC group, C: N/C group, D: N/C/G group. Scale bar = 1 mm.
Hind limb locomotion
Open field locomotion was assessed using the BBB scale and was confirmed to be 21 in all animals before injury. All animal groups showed a major deficit in the BBB score (*p < 0.001) 2–3 days after injury (Fig. 6A). All groups then followed an identical pattern of recovery with no statistical differences among the groups. Locomotion gradually recovered and the BBB score significantly increased by week 4 post-injury in all groups compared to the initial post-injury values (**p < 0.001). All animals were able to achieve weight-supported occasional plantar stepping (BBB score = 10.3 ± 0.2). A limited number of animals showed increased recovery, demonstrating frequent to consistent plantar stepping and occasional coordination (BBB score = 11–12). By week 8 post-injury, all groups reached a plateau level where no further improvement was observed (BBB score = 11.0 ± 0.1) that continued to week 12 (just prior to transplantation). One week after transplantation, a slight decrease in BBB score was found in all groups (BBB score = 10.1 ± 0.1), which suggested that the surgery associated with cell transplantation did not cause substantial damage. Animals in all groups maintained their level of recovery without further improvement after treatments, with no significant differences at all points post-transplantation (BBB score = 10.5 ± 0.2 at week 8 after treatment).
Fig. 6.
Behavioral changes. Panel A: BBB test. Two to three days after contusion, all animals showed dramatic deficits in the BBB scores in all groups compared to the baseline (*p < 0.001). Recovery of locomotion was reached by 4 weeks post-injury to BBB scores of 10 in all groups, which significantly increased compared to 2–3 days after injury (**p < 0.001) and then remained at 10–11 by 12 weeks post-injury. The BBB scores were slightly, but not significantly, reduced after transplantation (week 13) but remained at the 10–11 until the end of the experiment. There were no significant differences among groups at all tested time points. Panel B: Grid test. Before injury, animals showed a baseline of 96% of correct hind limb paw placement in all groups. There was a significant reduction in the percentage of correct placement in all groups at 12 weeks post-injury with no differences among the groups compared to the baseline (*p < 0.001). Sensorimotor function did not change after transplantation and virus injection (analyzed at 2 weeks after transplantation). There were also no changes in functional recovery by the end of the experiments in all groups. Data were analyzed weekly, but are presented at alternate weeks for ease of presentation. Panel C: Von Frey test. Mechanical sensitivity did not change after injury in all groups. After transplantation, the response to mechanical stimuli remained similar for the Medium group and the N/C group. By week 8 post-transplant, the N/C/G and NPC group were significantly different from their baseline levels (*p < 0.05 for both N/C/G and NPC), but they were not significantly different from Medium and N/C groups at any time point.
Sensorimotor integration
All animals showed 96% ± 0.5% of correct hind limb paw placement before contusion lesion as a baseline in the grid test (Fig. 6B). Following injury, animals that were able to achieve plantar stepping (BBB score of 10 or higher) were tested weekly on the grid (Control 7 out of 9, NPC 8 out of 10, N/C 8 out of 10, N/C/G 6 out of 8). Twelve weeks after injury (immediately following transplantation), all animals showed a deficit in grid test performance (*p < 0.001) and there were no significant differences in correct hind limb placement among groups. Two weeks after transplantation, the percentage of correct hind limb placement did not significantly change in all groups, which confirmed that the second surgery did not further damage the tissue. No further recovery was observed in any group through 8 weeks after transplantation.
Mechanical allodynia
Baseline thresholds were averaged across both hind limbs. Following injury, animals that achieved plantar stepping (as shown in previous sections) were tested weekly. Following injury, all groups maintained their original scores with little variation for the 12 weeks prior to transplantation (Fig. 6C). By week 6 posttransplantation, the N/C/G and NPC groups showed increased sensitivity (decreased withdrawal latency), which continued until the end of the study. By week 8 post-transplantation, the N/C/G and NPC groups were found to be significantly different from their baseline levels (*p < 0.05 for each), while they were not significantly different from the control and N/C groups at any time point. It is interesting to note that the N/C group started to show a similar increase in sensitivity 8 weeks after transplantation, which may suggest a correlation between delayed allodynia and NPC transplantation.
Cystometry
Cystometry was performed in a subset of animals in each group at the end of the experiment, 22 weeks after initial injury. We recorded bladder activity from 6 intact animals of similar age and weight to establish a reference for normal bladder activity with the specific parameters shown in Fig. 7F. The voiding efficiency reached 95% in all experimental groups, with no differences among the groups, compared to the intact group (100%). This indicates that some functional bladder recovery occurred at the chronic stage of the injury. However, the cystometry graph showed residual bladder function deficits in all experimental groups compared to the intact group (Fig. 7A–E). The values for non-voiding contracts, voided volume, bladder capacity, bladder weight, and the ratio of bladder/body weight (Fig. 7F) increased in all experimental groups compared to the intact group (p < 0.05). The values for bladder contraction duration and amplitude of micturition also increased in 3 of 4 experimental groups compared to intact animals (p < 0.05), with the notable exception of the N/C/G group that showed recovery to normal values. These parameters were not significantly different from those of the intact group, underscoring the improved bladder recovery after the combined treatment in the N/C/G group.
Fig. 7.
Representative cystometry charts of intact and experimental animals. When vehicle solution was infused into the bladder, the intact group (A) showed normal voiding activity (*). However, each experimental group (B-E) developed non-voiding contractions that occurred prior to the actual voiding event, indicating bladder hyperactivity. Although the voiding efficiency in all experimental animals at the chronic stage was close to normal, most urodynamic parameters in experimental groups (F) were different from the intact group with the exception of bladder contraction duration and amplitude of micturition in N/C/G animals, which were similar to intact animals. *p < 0.05, compared to the intact group.
Plasticity in the lumbosacral spinal cord
We measured primary afferent (CGRP and VR-1) and descending (5-HT) axons in the lumbosacral spinal cord (L6-S1) to examine the reorganization of these fibers after chronic injury and treatment. Expression of CGRP in the dorsal horn was dense in the control and NPC groups, but there was no significant difference compared to the N/C and N/C/G groups (Fig. 8A). There were also no significant differences when comparing the intact group with the experimental groups. Expression of VR-1, which represents unmyelinated C-fibers with high sensitivity to the neurotoxin capsaicin and contributes to bladder hyperreflexia after SCI (Zhou et al., 2002; Mitsui et al., 2005), was increased in all experimental groups 22 weeks after injury/treatments. However, there were no significant differences when comparing the intact group to the experimental groups (Fig. 8B). For descending 5-HT fibers in the dorsal horn of L6-S1, a decreased expression was observed in all experimental groups compared to the intact group, with no significant differences between the experimental groups (Fig. 8C). 5-HT expression increased in the N/C/G group, with comparable levels to intact animals, in contrast to the Medium control, NPC, and N/C groups, a finding that may be associated with the observed bladder function recovery in the N/C/G group.
Fig. 8.
Expression of CGRP, VR-1 and 5-HT at L6-S1 dorsal horn. Panel A: Expression of CGRP was reduced in all experimental groups compared to the intact group. The upper line in the graph shows baseline CGRP expression in the intact group. The lower line indicates the expression of CGRP in the control injury group. There were no significant differences in CGRP expression among all groups. Panel B: VR-1 expression was increased in all experimental groups compared to the intact group, but no significant differences between the intact group and all experimental groups were observed. The lower line in the graph shows the baseline VR-1 level in the intact group. The upper line shows VR-1 expression in the control injury group. Panel C: 5-HT expression in the dorsal horn is reduced compared to the intact animals after injury. No significant differences were observed between the intact group and all experimental groups. The upper line shows the baseline value in intact animals. The lower line shows the value in the control injury group.
DISCUSSION
Cell transplantation is a promising therapeutic approach to SCI by bridging the lesion or forming relays, providing trophic factors to attract damaged axons, and protecting injured neurons. In a previous study we found that transplants of NPC into a contused spinal cord survived, filled the lesion site, differentiated into neurons and glia and improved motor, sensory, and autonomic functional recovery (Mitsui et al., 2005). We have extended that study by transplanting NPC into chronic contused spinal cord and combining NPC transplants with other treatments including scar formation reduction, by injection Chase/LV, and providing additional growth factors, by injection BDNF/LV and NT-3/LV. We found a wide variability in the survival of transplanted NPCs in the chronic stage when transplanted 13 weeks post-injury. Locomotion and sensory deficits caused by contusion injuries gradually recovered by 12 weeks after injury, but no further improvement was observed with the different treatments. Similarly, some degree of functional bladder recovery occurred in all experimental groups, but a variety of bladder function parameters remained impaired. Importantly the N/C/G combination group receiving NPC grafts, Chase/LV, BDNF/LV, and NT3/LV showed functional bladder contraction and micturition equivalent to that of intact animals.
NPC survival in chronic SCI
The major challenge of cell transplantation into CNS injury is cell survival. Cells exhibit poor survival after being acutely transplanted into a spinal cord lesion, which may be due to the microenvironment at the lesion site that induces necrosis, apoptosis (Hill et al., 2006, 2007), and the elevation of various inflammatory cytokines such as TNFa, IL-1a, IL1b, IL-6 (Okano et al., 2003; Stammers et al., 2012). The level of some inflammatory cytokines also showed correlations with injury severity (Stammers et al., 2012), offering an explanation as to why acutely transplanted cells survived well in a small lesion, such as the dorsal column lesion (Bonner et al., 2010, 2011; Haas et al., 2012; Haas and Fischer, 2013), while they did not survive well in a complete transection (Medalha et al., 2014). Improvement of cell survival occurred when cells were transplanted 7–14 days (at the sub-acute phase of SCI) after injury. Consequently, many cell transplants are performed in the sub-acute stage of SCI. Delayed transplantation of adult NPC demonstrated substantial survival 2 weeks after a spinal cord compression lesion with growth factor infusion and minocycline treatment (Karimi-Abdolrezaee et al., 2006). However, cell survival was poor after transplantation into the same lesion model (T10 contusion) at the chronic stage (8 weeks after injury) (Karimi-Abdolrezaee et al., 2006), indicating that the chronic injury environment may be more hostile than the sub-acute stage. Similarly, our previous study demonstrated that NPC transplants performed 9 days after a T10 contusion resulted in substantial cell survival (Mitsui et al., 2005). In the current study we wanted to investigate the chronic condition and therefore NPC that were transplanted 13 weeks after injury showed significant variability in survival. In all three transplant groups, about 60% of the animals from each group demonstrated cell survival, with some rats from each group failing to show any survival following 9 weeks post-transplantation. In animals with transplants, cells were located either within the lesion epicenter or at the rostral/caudal injury margins and in some cases, transplanted cells integrated throughout the entire lesion, filling the lesion epicenter and integrating with the rostral/caudal injury margins. Multiple factors may account for the variability of transplant survival: (1) the late chronic injury (cells were transplanted 13 weeks after SCI) - few papers have shown cell survival at late chronic injury, which in general remains poor in chronic injury, (2) the chronic transplantation requires a second surgery into a site that is difficult to localize and is surrounded by scar; (3) the use of multiple injections at the center and rostral and caudal regions of the transplants may lead to additional tissue damage with particular concern pertaining to damage to blood vessels, and (4) the accumulation of macrophage/microglia with a persistent inflammatory response contributing to cell loss.
Combination treatment effects on the chronic SCI environment
The chronic SCI microenvironment is complex as a consequence of the many acute and secondary processes that occur following the injury. A dense network of glial processes, a lesion cavity, and chronic inflammation infiltrated with microglia and macrophages impede transplanted cell survival and axonal growth through the lesion. The aim of our combination treatment was to reduce the glial scar, by supplying virally-delivered chondroitinase, and to promote axonal growth through the delivery of growth factors rostral and caudal to the lesion. Such a strategy was designed to allow the formation of relays with the transplanted NPC to reconnect the injured spinal cord and promote functional recovery. Because of the poor and variable survival of the NPC transplants even in the groups with the combined treatments we did not observe significant functional recovery compared to the control group. The glial scar was significantly reduced around lesion/transplant region only in animals where the NPC transplant survived and filled the injury site. This suggests that NPC transplants can help modify the scar in the chronic phase, but only if they survive and integrate with host tissue to a substantial degree. In animals with poor transplant survival, strong GFAP staining was found around the lesion area even in cases that included the injection of Chase/LV.
We previously developed a lentiviral vector encoding chondroitinase (Chase/LV), which can degrade chondroitin sulfate proteoglycan (CSPG) for at least 8 weeks when injected into normal spinal cord (Jin et al., 2011a), and utilized this virus in the current experiments, administering virus one week after cell transplantation (14 weeks after the initial injury). Although CSPG digestion was observed rostral and caudal to the lesion and between cell transplants, CSPG-degradation did not correlate with cell survival and scar reduction. It appears that in a chronic injury, where the injection of Chase/LV was performed 14 weeks after injury, when a major scar has already been formed, there was either a limited digestion of CSPG, which did not significantly reduce glial scar around the lesion area or that the scar was reformed in the absence of robust survival of the NPC transplant. Recent studies have demonstrated that the infusion of chondroitinase ABC (ChABC) one week before NPC together with a cocktail of PDGF-AA, bFGF and EGF for 7 d using a subarachnoid catheter increased NPC survival from 4.91% in vehicle-treated/NPC-transplanted animals to 28.27% in the ChABC-treated/NPC transplanted group after chronic injury (Karimi-Abdolrezaee et al., 2010). In that study, cells were injected 2 mm rostral and caudal to the injury and not into the injury site, with growth factor support provided simultaneously with cellular grafting, differing from our current study. In our study, Chase/LV and BDNF/NT-3/LV were injected one week after NPC transplantation, a time delay that may be too late to modulate the chronic environment for cell survival. Although cell survivability in that study (Karimi-Abdolrezaee et al., 2010) was still low, pre-administration of ChABC and growth factors may represent an alternative approach to enhance cell survival in chronic SCI. It is also important to note that we used NPC derived from embryonic spinal cord (together with neurotrophic factors BDNF and NT3), which produced mostly neurons and astrocytes in contrast to the Karimi-Abdolrezaee study, which used adult NPC derived from the brain SVZ and directed into the fate of oligodendroglia lineage.
The inflammatory microenvironment of the injured spinal cord represents another challenge for cell survival. We found that macrophages infiltrated in and around the lesion center in all groups without differences, which might be another reason for poor NPC survival. Our studies and others show substantial cell survival in sub-acute SCI (Mitsui et al., 2005; Parr et al., 2007; Jin et al., 2011b) although macrophages and microglia were also found at/around the lesion/ transplant. Microarray and histological analysis revealed that the infiltration of anti-inflammatory M2 macrophages was significantly higher at the sub-acute stage than the chronic stage, possibly contributing to enhanced transplant survival at this stage (Nishimura et al., 2013). Adaptation of molecular cues that enhance the relative concentration of these anti-inflammatory macrophages may be an alternative strategy to promote transplant survival. Furthermore, treatment with minocycline to reduce inflammation in Karimi-Abdolrezaee’s study may also be a factor that contributed to transplanted NPC cell survival in the chronic stage (Karimi-Abdolrezaee et al., 2010).
Combination treatment on functional recovery
Transplants of human or rodent neural stem/progenitor cells, with or without combination treatments, show functional recovery after sub-acute SCI. Locomotor function has been improved (BBB or BMS) with transplants of neural stem/progenitor cells, NPCs, multineurotrophin-expressing glial-restricted progenitor cells 7–9 days after SCI (Cao et al., 2005; Mitsui et al., 2005; Cusimano et al., 2012; Kumamaru et al., 2013; Nishimura et al., 2013). However, when the same cells were transplanted into the chronically injured spinal cord, there was no significant motor functional recovery (Cusimano et al., 2012; Kumamaru et al., 2013; Nishimura et al., 2013). Similar results were found in the present study. Our previous study demonstrated that NPC transplanted 9 days after contusion improved locomotor function (Mitsui et al., 2005). We did not find significant improvement in BBB and grid test in treated groups compared to the Medium control when NPCs were transplanted into the contusion lesion 13 weeks after SCI. The combination group (N/C/G) and NPC group exhibited increased sensitivity compared to their baseline, but no significant differences when compared to Medium control and N/C groups 8 weeks after NPC transplant and with Chase and growth factor treatment. These results may be partially explained by the LV delivery of BDNF in N/C/G group, but the underlying etiology is not clear in the NPC group. Some studies have demonstrated that overexpression of neurotrophic factors may result in worsening functional recovery and the development of neuropathic pain (Lu et al., 2012a; Lang et al., 2013). Indeed, transplants of MSCs genetically-engineered to secrete BDNF, when combined with AAV/BDNF injected caudal to the transplant, caused worsening motor functional recovery after partial cervical injury and worsening spasticity following complete injury (Lu et al., 2012a). Transplantation of OEC resulted in neuropathic pain, which has been shown to be associated with the phosphorylation of ERK and activated BDNF overexpression (Lang et al., 2013). Results from our laboratory and others indicate that the microenvironment of the spinal cord at the chronic stage of injury impedes functional recovery. Given these data, combinatorial strategies directed at axonal regeneration and functional recovery that incorporate the use of neurotrophic factors must be carefully designed and pay particular attention to the potential side effect of the development of neuropathic pain.
Combination treatment on autonomic function recovery and plasticity in lumbar spinal cord
Bladder function deficits have been shown to occur after SCI to produce a dyssynergia between the bladder and urethral sphincter resulting in bladder functional deficit, which is identified by urinary retention and increased micturition pressure (Mitsui et al., 2005). Studies on bladder function after SCI showed that deficits in bladder function vary depending on the level of the injury (David and Steward, 2010) and the extent of recovery of bladder detrusor and the external urethral sphincter (EUS) coordination after SCI depends on injury severity and the degree of residual connections with brainstem control center (Pikov and Wrathall, 2001). Our previous studies and others using urodynamic assessment of bladder function (cystometry) in unanesthetized, restrained rats demonstrate deficits of bladder function after contusion of the lower thoracic spinal cord in several parameters of cystometry such as contraction duration, micturition pressure, and voiding efficiency (Keirstead et al., 2005; Mitsui et al., 2005; Leung et al., 2007; David and Steward, 2010; Jin et al., 2011b). Our previous studies showed that transplants of NPC 9 days after contusion improve bladder function by lowering the micturition pressures and reducing the episodes of detrusor hyperreflexia compared to the control group, indicating amelioration of dyssynergia between bladder and urethral sphincter (Mitsui et al., 2005). These findings suggest that dyssynergia in SCI is associated with the loss of supraspinal projections into the lumbosacral spinal cord that normally modulate this function and that transplanting NPCs into an incomplete injury effectively reduces the damage by reducing secondary injury to these modulatory systems (Mitsui et al., 2005). Few studies, however, have examined the potential improvement of bladder function at the chronic stage of SCI (Penha et al., 2014; Sharma et al., 2014). In the present study, we found that the voiding efficiency in all experimental rats was similar to the intact animals 23 weeks after moderate thoracic contusion, which indicates significant recovery of bladder function. Nevertheless, persistent deficits in most cystometry parameters were noted in all experimental groups with the exception of the combination-treated group (N/C/G), which displayed improvement in bladder contraction duration and amplitude of micturition. The amplitude of micturition in the N/C/G group was similar to the intact group, while the amplitude of micturition in the other three experimental groups was significantly increased compared to the intact group, indicating an amelioration of dyssynergia between the bladder and urethral sphincter after the combination treatment in the N/C/G group (Seki et al., 2002; Mitsui et al., 2003, 2005).
To examine plasticity in the spinal cord, sections from lumbosacral cord were stained with CGRP and VR1, as markers of primary afferent pathways, and 5-HT for descending modulatory pathways. In comparing the findings from previous studies to the data presented here, staining patterns for CGRP, 5-HT, and VR-1 differ between the sub-acute and chronic stages. The expression of CGRP and VR-1 within the dorsal horn was significantly increased 3 days to 9 weeks after SCI (Krenz and Weaver, 1998; Zhou et al., 2002; Mitsui et al., 2005; Zinck et al., 2007; Jin et al., 2011b). In the chronic stage (22 weeks after injury), we found that expression of CGRP and VR-1 within the dorsal horn was not significantly different from the intact group. There were also no significant differences between the treated groups compared to the control group. These results may indicate a process of reorganization of the primary afferent fibers within the dorsal horn at the chronic stage of SCI. No differences in expression of CGRP and VR-1 within the dorsal horn in experimental groups compared to the intact group were observed, potentially contributing to the observation of preserved voiding efficiency in the experimental groups. We also examined the descending 5-HT modulatory pathway within the L6/S1 dorsal horn and found decreased expression in all experimental groups that failed to reach statistical significance compared to the intact group. However, the combination group (N/C/G) showed a trend of increased expression of 5-HT. Previous studies (Pikov and Wrathall, 2001) indicate that normal micturition requires coordinated activation of smooth muscle of the bladder (detrusor) and striated muscle of the EUS, which is controlled by spinal and supraspinal circuitry. The extent of recovery of detrusor–EUS coordination after SCI depends on injury severity and the degree of residual connections with brainstem control centers (Pikov and Wrathall, 2001). The raphe magnus and raphe pallidus are among brainstem nuclei with identified connections to the bladder and EUS pathway (Marson, 1997; Vizzard et al., 1997). Previous studies have established that serotonin immunoreactivity distal to the injury site is a good indicator of both SCI severity and the degree of recovery of somatic sensorimotor function (Faden et al., 1988; Wrathall et al., 1994; Teng and Wrathall, 1997; Pikov and Wrathall, 2001). Measurement of serotonin immunoreactivity (5-HT) suggests that recovery of detrusor–EUS coordination is associated with sparing of supraspinal projections to areas in the lumbosacral spinal cord that control bladder and EUS function (Pikov and Wrathall, 2001). A possible explanation of bladder function recovery in N/C/G group may be that the increase of 5-HT in the lumbosacral area indicates more preservation of long-descending projections that may increase supraspinal coordination of the detrusor-EUS from the brainstem.
CONCLUSIONS
We have demonstrated limited functional recovery with combination treatments after chronic contused SCI. Transplanted cell survival is still a significant challenge, limiting further recovery in chronic SCI. Without successful and substantial cell survival, especially within the lesion area, no bridging of the lesion cavity is possible, precluding axonal regeneration through the lesion cavity or the formation of a relay to reconnect host pathways. Furthermore, the complicated, chronic SCI microenvironment, characterized by glial scarring and chronic inflammation remains a significant obstacle to integration and regeneration. The temporal relationship between scar formation/reduction and cell survival may represent a critical factor in therapeutic strategies relying on cell transplantation for chronic SCI (Karimi-Abdolrezaee et al., 2010). The delivery of a growth factor cocktail to cells before transplantation may represent an additional strategy to enhance cell survival at the chronic stage (Lu et al., 2012b). A combination of tissue protection at the acute stage and multimodal interventions at the chronic stage may represent a therapeutic alternative to improve axonal regeneration and functional recovery. Our future studies will be focused on combinatorial strategies including early tissue protection, addition of a growth factor cocktail to the cells, scar and chronic inflammation reduction prior cell transplantation, and rehabilitation by physical training for chronic SCI to increase cell survival and improve functional recovery.
Acknowledgments
This work was founded by grants from The Craig H. Neilsen Foundation (#160746) and NIH (SP01NS055976). We thank Dr. B. Timothy Himes for assistance of cystometry. We also thank Theresa Conner and Maria Obrocka for their technical support.
Abbreviations
- AP
alkaline phosphatase
- BBB
Basso, Beattie, and Bresnahan
- CGRP
calcitonin gene-related peptide
- EUS
external urethral sphincter
- MSCs
marrow stromal cells
- NPCs
neural progenitor cells
- NVC
non-voiding contraction
- SCI
spinal cord injury
- VFH
Von Frey hair
- VR-1
vanilloid receptor type 1
REFERENCES
- Barakat DJ, Gaglani SM, Neravetla SR, Sanchez AR, Andrade CM, Pressman Y, Puzis R, Garg MS, Bunge MB, Pearse DD. Survival, integration, and axon growth support of glia transplanted into the chronically contused spinal cord. Cell Transplant. 2005;14:225–240. doi: 10.3727/000000005783983106. [DOI] [PubMed] [Google Scholar]
- Barnett SC, Riddell JS. Olfactory ensheathing cell transplantation as a strategy for spinal cord repair - what can it achieve? Nat Clin Pract Neurol. 2007;3:152–161. doi: 10.1038/ncpneuro0447. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bonner JF, Blesch A, Neuhuber B, Fischer I. Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J Neurosci Res. 2010;88:1182–1192. doi: 10.1002/jnr.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31:4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Yang SH, Duffy PJ, Li S, Strittmatter SM. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007;27:2176–2185. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Xu XM, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Shumsky JS, Sabol MA, Kushner RA, Strittmatter S, Hamers FP, Lee DH, Rabacchi SA, Murray M. Nogo-66 receptor antagonist peptide (NEP1-40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat. Neurorehabil Neural Repair. 2008;22:262–278. doi: 10.1177/1545968307308550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusimano M, Biziato D, Brambilla E, Donega M, Alfaro-Cervello C, Snider S, Salani G, Pucci F, Comi G, Garcia-Verdugo JM, De Palma M, Martino G, Pluchino S. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 2012;135:447–460. doi: 10.1093/brain/awr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David BT, Steward O. Deficits in bladder function following spinal cord injury vary depending on the level of the injury. Exp Neurol. 2010;226:128–135. doi: 10.1016/j.expneurol.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng LX, Deng P, Ruan Y, Xu ZC, Liu NK, Wen X, Smith GM, Xu XM. A novel growth-promoting pathway formed by GDNF-overexpressing Schwann cells promotes propriospinal axonal regeneration, synapse formation, and partial recovery of function after spinal cord injury. J Neurosci. 2013;33:5655–5667. doi: 10.1523/JNEUROSCI.2973-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff MR, Clark LM, Hutchinson KJ, Kloos AD, Fisher LC, Basso DM. Validity of acute and chronic tactile sensory testing after spinal cord injury in rats. Exp Neurol. 2010;225:366–376. doi: 10.1016/j.expneurol.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff MR, Fisher LC, Deibert RJ, Basso DM. Acute and chronic tactile sensory testing after spinal cord injury in rats. J Vis Exp. 2012:e3247. doi: 10.3791/3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff MR, Wade RE, Jr, Houle JD. Chronic at- and below-level pain after moderate unilateral cervical spinal cord contusion in rats. J Neurotrauma. 2013;30:884–890. doi: 10.1089/neu.2012.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden Al, Gannon A, Basbaum Al. Use of serotonin immunocytochemistry as a marker of injury severity after experimental spinal trauma in rats. Brain Res. 1988;450:94–100. doi: 10.1016/0006-8993(88)91548-x. [DOI] [PubMed] [Google Scholar]
- Haas C, Fischer I. Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. J Neurotrauma. 2013;30:1035–1052. doi: 10.1089/neu.2013.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C, Neuhuber B, Yamagami T, Rao M, Fischer I. Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration. Exp Neurol. 2012;233:717–732. doi: 10.1016/j.expneurol.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SS, Kang DY, Mujtaba T, Rao MS, Fischer I. Grafted lineage-restricted precursors differentiate exclusively into neurons in the adult spinal cord. Exp Neurol. 2002;177:360–375. doi: 10.1006/exnr.2002.7995. [DOI] [PubMed] [Google Scholar]
- Han SS, Liu Y, Tyler-Polsz C, Rao MS, Fischer I. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation, and preferential migration in white matter. Glia. 2004;45:1–16. doi: 10.1002/glia.10282. [DOI] [PubMed] [Google Scholar]
- Hawthorne AL, Popovich PG. Emerging concepts in myeloid cell biology after spinal cord injury. Neurotherapeutics. 2011;8:252–261. doi: 10.1007/s13311-011-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CE, Hurtado A, Blits B, Bahr BA, Wood PM, Bartlett Bunge M, Oudega M. Early necrosis and apoptosis of Schwann cells transplanted into the injured rat spinal cord. Eur J Neurosci. 2007;26:1433–1445. doi: 10.1111/j.1460-9568.2007.05771.x. [DOI] [PubMed] [Google Scholar]
- Hill CE, Moon LDF, Wood PM, Bunge MB. Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia. 2006;53:338–343. doi: 10.1002/glia.20287. [DOI] [PubMed] [Google Scholar]
- Hollis 2nd ER, Tuszynski MH. Neurotrophins: potential therapeutic tools for the treatment of spinal cord injury. Neurotherapeutics. 2011;8:694–703. doi: 10.1007/s13311-011-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbert RJ, Hadley MN, Walters BC, Aarabi B, Dhall SS, Gelb DE, Rozzelle CJ, Ryken TC, Theodore N. Pharmacological therapy for acute spinal cord injury. Neurosurgery. 2013;72(Suppl 2):93–105. doi: 10.1227/NEU.0b013e31827765c6. [DOI] [PubMed] [Google Scholar]
- Jin Y, Fischer I, Tessler A, Houle JD. Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol. 2002;177:265–275. doi: 10.1006/exnr.2002.7980. [DOI] [PubMed] [Google Scholar]
- Jin Y, Ketschek A, Jiang Z, Smith G, Fischer I. Chondroitinase activity can be transduced by a lentiviral vector in vitro and in vivo. J Neurosci Methods. 2011a;199:208–213. doi: 10.1016/j.jneumeth.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Neuhuber B, Singh A, Bouyer J, Lepore A, Bonner J, Himes T, Campanelli JT, Fischer I. Transplantation of human glial restricted progenitors and derived astrocytes into a contusion model of spinal cord injury. J Neurotrauma. 2011b;28:579–594. doi: 10.1089/neu.2010.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin MT, Blesch A, Tuszynski MH. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/ progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Fedulov V, Cloutier F, Steward O, Duel BP. A noninvasive ultrasonographic method to evaluate bladder function recovery in spinal cord injured rats. Exp Neurol. 2005;194:120–127. doi: 10.1016/j.expneurol.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience. 1998;85:443–458. doi: 10.1016/s0306-4522(97)00622-2. [DOI] [PubMed] [Google Scholar]
- Kumamaru H, Kobayakawa K, Saiwai H, Kubota K, Yokota K, Ohkawa Y, Shiba K, Iwamoto Y, Okada S. The therapeutic activities of engrafted neural stem/precursor cells are not dormant in the chronically injured spinal cord. Stem Cells. 2013 doi: 10.1002/stem.1404. [DOI] [PubMed] [Google Scholar]
- Lang BC, Zhang Z, Lv LY, Liu J, Wang TY, Yang LH, Liao DQ, Zhang WS, Wang TH. OECs transplantation results in neuropathic pain associated with BDNF regulating ERK activity in rats following cord hemisection. BMC Neurosci. 2013;14:80. doi: 10.1186/1471-2202-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol. 2005;194:230–242. doi: 10.1016/j.expneurol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Leung PY, Johnson CS, Wrathall JR. Comparison of the effects of complete and incomplete spinal cord injury on lower urinary tract function as evaluated in unanesthetized rats. Exp Neurol. 2007;208:80–91. doi: 10.1016/j.expneurol.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vales R, Fores J, Navarro X, Verdu E. Chronic transplantation of olfactory ensheathing cells promotes partial recovery after complete spinal cord transection in the rat. Glia. 2007;55:303–311. doi: 10.1002/glia.20457. [DOI] [PubMed] [Google Scholar]
- Louro J, Pearse DD. Stem and progenitor cell therapies: recent progress for spinal cord injury repair. Neurol Res. 2008;30:5–16. doi: 10.1179/174313208X284070. [DOI] [PubMed] [Google Scholar]
- Lu P, Blesch A, Graham L, Wang Y, Samara R, Banos K, Haringer V, Havton L, Weishaupt N, Bennett D, Fouad K, Tuszynski MH. Motor axonal regeneration after partial and complete spinal cord transection. J Neurosci. 2012a;32:8208–8218. doi: 10.1523/JNEUROSCI.0308-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Jones LL, Tuszynski MH. Axon regeneration through scars and into sites of chronic spinal cord injury. Exp Neurol. 2007;203:8–21. doi: 10.1016/j.expneurol.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Lu P, Wang YZ, Graham L, McHale K, Gao MY, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng BH, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012b;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson L. Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: a transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1997;389:584–602. [PubMed] [Google Scholar]
- McCall J, Weidner N, Blesch A. Neurotrophic factors in combinatorial approaches for spinal cord regeneration. Cell Tissue Res. 2012;349:27–37. doi: 10.1007/s00441-012-1388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalha CC, Jin Y, Yamagami T, Haas C, Fischer I. Transplanting neural progenitors into a complete transection model of spinal cord injury. J Neurosci Res. 2014;92:607–618. doi: 10.1002/jnr.23340. [DOI] [PubMed] [Google Scholar]
- Mitsui T, Kakizaki H, Tanaka H, Shibata T, Matsuoka I, Koyanagi T. Immortalized neural stem cells transplanted into the injured spinal cord promote recovery of voiding function in the rat. J Urol. 2003;170:1421–1425. doi: 10.1097/01.ju.0000075501.05758.33. [DOI] [PubMed] [Google Scholar]
- Mitsui T, Shumsky JS, Lepore AC, Murray M, Fischer I. Transplantation of neuronal and glial restricted precursors into contused spinal cord improves bladder and motor functions, decreases thermal hypersensitivity, and modifies intraspinal circuitry. J Neurosci. 2005;25:9624–9636. doi: 10.1523/JNEUROSCI.2175-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M, Kim D, Liu Y, Tobias C, Tessler A, Fischer I. Transplantation of genetically modified cells contributes to repair and recovery from spinal injury. Brain Res Brain Res Rev. 2002;40:292–300. doi: 10.1016/s0165-0173(02)00211-4. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Yasuda A, Iwai H, Takano M, Kobayashi Y, Nori S, Tsuji O, Fujiyoshi K, Ebise H, Toyama Y, Okano H, Nakamura M. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6:3. doi: 10.1186/1756-6606-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H, Ogawa Y, Nakamura M, Kaneko S, Iwanami A, Toyama Y. Transplantation of neural stem cells into the spinal cord after injury. Semin Cell Dev Biol. 2003;14:191–198. doi: 10.1016/s1084-9521(03)00011-9. [DOI] [PubMed] [Google Scholar]
- Oudega M, Xu XM. Schwann cell transplantation for repair of the adult spinal cord. J Neurotrauma. 2006;23:453–467. doi: 10.1089/neu.2006.23.453. [DOI] [PubMed] [Google Scholar]
- Parr AM, Kulbatski I, Tator CH. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J Neurotrauma. 2007;24:835–845. doi: 10.1089/neu.2006.3771. [DOI] [PubMed] [Google Scholar]
- Penha EM, Meira CS, Guimaraes ET, Mendonca MV, Gravely FA, Pinheiro CM, Pinheiro TM, Barrouin-Melo SM, Ribeiro-Dos-Santos R, Soares MB. Use of autologous mesenchymal stem cells derived from bone marrow for the treatment of naturally injured spinal cord in dogs. Stem Cells Int. 2014;2014:437521. doi: 10.1155/2014/437521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikov V, Wrathall JR. Coordination of the bladder detrusor and the external urethral sphincter in a rat model of spinal cord injury: effect of injury severity. J Neurosci. 2001;21:559–569. doi: 10.1523/JNEUROSCI.21-02-00559.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Young W. Managing inflammation after spinal cord injury through manipulation of macrophage function. Neural Plast. 2013;2013:945034. doi: 10.1155/2013/945034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol. 2002;168:2269–2274. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- Sharma A, Sane H, Khopkar D, Gokulchandran N, Jacob VC, Joseph J, Badhe P. Functional recovery in chronic stage of spinal cord injury by neurorestorative approach: a case report. Case Rep Surg. 2014;2014:404207. doi: 10.1155/2014/404207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stammers AT, Liu J, Kwon BK. Expression of inflammatory cytokines following acute spinal cord injury in a rodent model. J Neurosci Res. 2012;90:782–790. doi: 10.1002/jnr.22820. [DOI] [PubMed] [Google Scholar]
- Teng YD, Wrathall JR. Local blockade of sodium channels by tetrodotoxin ameliorates tissue loss and long-term functional deficits resulting from experimental spinal cord injury. J Neurosci. 1997;17:4359–4366. doi: 10.1523/JNEUROSCI.17-11-04359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, Houle JD. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci. 2009;29:14881–14890. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, Grill R, Jones LL, Brant A, Blesch A, Low K, Lacroix S, Lu P. NT-3 gene delivery elicits growth of chronically injured corticospinal axons and modestly improves functional deficits after chronic scar resection. Exp Neurol. 2003;181:47–56. doi: 10.1016/s0014-4886(02)00055-9. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Erickson K, de Groat WC. Localization of NADPH diaphorase in the thoracolumbar and sacrococcygeal spinal cord of the dog. J Auton Nerv Syst. 1997;64:128–142. doi: 10.1016/s0165-1838(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Wrathall JR, Choiniere D, Teng YD. Dose-dependent reduction of tissue loss and functional impairment after spinal cord trauma with the AMPA/kainate antagonist NBQX. J Neurosci. 1994;14:6598–6607. doi: 10.1523/JNEUROSCI.14-11-06598.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama M, Nezu FM, Yokoyama O, de Groat WC, Chancellor MB. Changes in micturition after spinal cord injury in conscious rats. Urology. 1999;54:929–933. doi: 10.1016/s0090-4295(99)00234-4. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Abdelhady M, Mourad MS, Hassouna MM. Change of vanilloid receptor 1 following neuromodulation in rats with spinal cord injury. J Surg Res. 2002;107:140–144. doi: 10.1006/jsre.2002.6481. [DOI] [PubMed] [Google Scholar]
- Zinck ND, Rafuse VF, Downie JW. Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp Neurol. 2007;204:777–790. doi: 10.1016/j.expneurol.2007.01.011. [DOI] [PubMed] [Google Scholar]