Abstract
Batrachotoxins are neurotoxic steroidal alkaloids first isolated from a Colombian poison-dart frog and later found in certain passerine birds of New Guinea. Neither vertebrate group is thought to produce the toxins de novo, but instead they likely sequester them from dietary sources. Here we describe the presence of high levels of batrachotoxins in a little-studied group of beetles, genus Choresine (family Melyridae). These small beetles and their high toxin concentrations suggest that they might provide a toxin source for the New Guinea birds. Stomach content analyses of Pitohui birds revealed Choresine beetles in the diet, as well as numerous other small beetles and arthropods. The family Melyridae is cosmopolitan, and relatives in Colombian rain forests of South America could be the source of the batrachotoxins found in the highly toxic Phyllobates frogs of that region.
Keywords: Pitohui, Ifrita, Phyllobates, APCI mass spectrometry, dietary arthropods
Batrachotoxins (BTXs) were discovered in the mid-1960s in skin extracts from a Colombian poison-dart frog (family Dendrobatidae) (1, 2). The name for these unique steroidal alkaloids was derived from the Greek “batrachos,” meaning frog. During the following 25 years, BTXs were detected only in frogs of the dendrobatid genus Phyllobates and not in scores of other poison frogs (3). Only three species are toxic enough to be used by native Americans for poisoning blow-dart tips: Phyllobates terribilis, Phyllobates bicolor, and Phyllobates aurotaenia (4) where BTXs are responsible for their toxicity. BTXs bind with high affinity to voltage-gated sodium channels in nerve and muscle membranes, locking them in an open state (5). Because of their potency and specificity, batrachotoxins have been widely used to study the function of sodium channels (6).
Poison-dart frogs that have been raised in captivity do not contain detectable amounts of BTXs (7). This and other lines of evidence indicate that dendrobatid poison frogs do not produce batrachotoxins or other alkaloids de novo but that they likely sequester such alkaloids from their diet (8, 9). Although other frog-skin alkaloids have been identified in ants, millipedes, and beetles (10–12), BTXs have not been found before in any insect or plant source. Restrictions on field-work in Colombia have prevented any search for the dietary origin of batrachotoxins in the poison-dart frogs of that country. Thus, a putative dietary source for the BTXs remained a mystery.
In 1992, the toxic principle from feathers and skin of bird species of genus Pitohui, endemic to New Guinea, was isolated and, remarkably, proved to be mainly a batrachotoxin, homo-BTX (h-BTX) (13). BTXs were later found in New Guinea toxic birds of the genus Ifrita (14). On a weight basis, the batrachotoxins are among the most toxic natural substances known, being 250-fold more toxic than strychnine (4). It is believed that such toxins provide some protection against the birds' natural enemies, such as parasites (15, 16) and predators, including humans (17, 18).
We now report analysis of extracts of the beetle genus (Choresine: family Melyridae) suggested by New Guinean traditional village naturalists as a potential source of BTXs in Pitohui and Ifrita. Villagers from Herowana (Crater Mountain Wildlife Management Area, Eastern Highlands Province) identified beetles locally known as “nanisani.” Nanisani is also the local name for the Blue-Capped Ifrita (Ifrita kowaldi), which does carry BTXs. According to these local naturalists, the name “nanisani” refers specifically to the unusual tingling and numbing sensation to the lips and face caused by contact with either the beetles or the bird feathers.
Materials and Methods
Collected beetles were stored in glass vials in 100% ethanol for transport at ambient temperatures to the National Institutes of Health for analysis. The supernatant was withdrawn, and the beetles were washed with ≈1 ml of ethanol. This process was repeated twice more, and the washes were combined with the original supernatant. These washes were shown to extract virtually all BTXs. Sonication (see footnote to Table 1) proved to be superfluous in removing additional BTXs. The ethanol extract was concentrated to ≈200 μl under a nitrogen stream. Single-beetle collections were concentrated to 10 μl. Beetles were identified by A.S. and found to consist of four Choresine species (see below). Voucher specimens were deposited in the Bernice P. Bishop Museum.
Table 1. Summary of alkaloids detected in collections of Choresine beetles.
| Collection type (no. analyzed) | BTX-A 418 | BTX-A O-acetate (rearranged) 460 | BTX-A O-crotonate 486 | BTX 539 | h-BTX 553 | Other BTXs |
|---|---|---|---|---|---|---|
| I (29) | ++-+++ | +++ | ++-+++ | nd-++ | nd-+ | 514 (+-++) (2), 500 (+), 525 (+-+++), 583 (+), and others |
| II (5) | +++ | +++ | +++ | nd-+++ | nd-+ | 500 (+-++), 514 (++), 565 (+), 583 (+), 597 (+) |
| III (3) | +++ | +++ | +++ | 565 | ||
| IV (1) | ++ | nd | +++ | +++ | + | 525 (++), 514 (++), 436/438 (++) |
| V (1) | + | + | +++ | nd | nd | |
| VI* (1) | +++ | +++ | ++ | ++ | + | 500 (+), 514 (+) |
The protonated molecular ion is shown in bold. nd, not detectable; +, trace (detected with single ion profiling); ++, minor; +++, major. Contents ranged widely in different collections listed in I, II, and IV. For example, in the 29 collections reported as type I, BTX-A and BTX-A O-crotonates ranged from minor to major, BTX-A ranged from nd to minor, and h-BTX ranged from nd to trace.
This collection in ethanol (17 beetles, not identified as to species) was sonicated in fresh ethanol for a 30-min period at room temperature after the initial extract had been decanted. The original and sonicated extracts were combined for analysis. The sonication step added little to the BTXs extracted.
A Hewlett–Packard 1100 liquid chromatograph with variable wavelength detector set at 260 nm (the long-wavelength absorption maximum of the pyrrole moiety of BTXs), fitted with an AQUA (Phenomenex, Torrance, CA) C-18 RP column (4.6 mm × 25 cm; 5-μm particle size) and using a water–acetonitrile gradient (90% water/0.05% HOAc to 50% water/0.05% HOAc in 40 min; 0.5 ml/min), was interfaced with a Finnigan LCQ mass spectrometer operating in the atmospheric pressure chemical ionization mode. Interpretation of mass spectra and identification of the BTX alkaloids are presented in greater detail elsewhere (cf. ref. 14).
Results
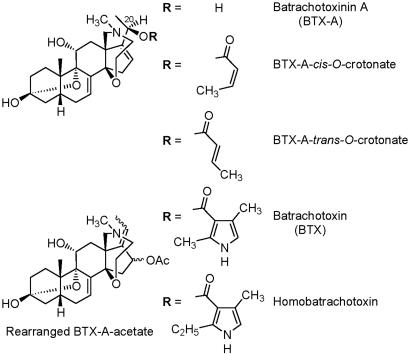
Alkaloid profiles of the beetles surveyed are presented in Table 1. Characteristic or major mass spectral peaks, [M+H]+, represented are the rearranged BTX-A acetate (m/z 460), batrachotoxinin A (BTX-A) (m/z 418), and the BTX-A crotonate (m/z 486), in an ≈4:2:1 ratio in most collections. The BTX-A crotonate usually emerged as two overlapping peaks of varying proportions, corresponding to the trans and cis BTX-A O-crotonate esters with the trans usually equivalent to or slightly predominating over cis. Minor peaks were present for BTX (m/z 539) and h-BTX (m/z 553). Structures of these alkaloids are shown in Fig. 1. In addition, several unknown BTX alkaloids were detected.
Fig. 1.
Structures of the most common batrachotoxins found in Choresine beetles.
Of the 391 beetles present in collections, 85% were Choresine pulchra (Pic) (CP) and 14% were Choresine semiopaca (Wittmer) (CS). There were also four Choresine sp. A (CA) and one Choresine rugiceps (Wittmer) (CR). Beetles were collected from May to September 2001 (mainly August) and from May to November 2002 (mainly November). All were collected in or within 2 km of the village of Herowana (elevation, ≈1,400 m). Some collections were made within the village, and others were made along the nearby Fio River. There is no discernable difference in collections made in 2001 or 2002 in the proportions of CS in the collections. A total of 40 separate collections were analyzed. A summary of the alkaloid data for the following collection types (I–VI) is presented in Table 1 (collections were segregated as types by species present in the beetle mixtures, although no evident pattern emerged in BTXs detected): type I, 29 separate collections of CP beetles; type II, 5 separate collections of CP+CS; type III, 3 separate collections of CP and CA or CR; type IV, one 3-beetle collection of CS (which originated within the village); type V, one single-beetle collection of CP; type VI, one 17-beetle collection (not positively identified as to species but likely all CP). One single-beetle collection of CP from November 2002 of type I had no detectable BTXs, whereas the single-beetle collection of CP (type V) from July 2001 had major amounts of the crotonate ester of BTX-A. Alkaloids were characterized by their protonated molecular ions [M+H]+, fragmentations, and liquid chromatography-retention times. Many had not been detected in prior studies of passerine birds of Papua New Guinea (13, 14).
Several previously uncharacterized BTX alkaloids are reported in Table 1 that had not been seen either in frog or bird: a molecular weight (MW) 524 alkaloid that lacks the N-methyl from the homomorpholine ring of the BTX nucleus; a MW 435/437 chloro analog of BTX-A, where the 20-hydroxyl group is replaced by chlorine; a MW 499 material, likely a homocrotonate of BTX-A, fragmenting to m/z 400; and two alkaloids of MW 513, one giving an m/z 400 fragment and the other giving a fragment at m/z 428 (perhaps an ethyl ketal of the O-crotonate of BTX-A) and perhaps a pyrrole-hydroxylated BTX (MW 564) and pyrrole-ethoxylated BTX (MW 582) or h-BTX (MW 596). Additional characterization will be required. Some of these alkaloids are likely to be artifacts.
It initially appeared that C. semiopaca (collection type IV) might be a richer source of batrachotoxin than other Choresine species. In a collection of type II where C. semiopaca was mixed with C. pulchra (25 CS and 28 CP, August 2001), BTX was a major alkaloid and h-BTX was also detected. Another mixed collection of type II (32 CS and 9 CP, September 2001), however, had no detectable BTX or h-BTX.
Discussion
Whereas the birds always had h-BTX >> BTX, the situation is reversed in the beetles, where h-BTX is scarcely detectable or absent. In frogs of the genus Phyllobates, BTX levels exceed those of h-BTX by ≈2-fold. The level of BTX in one collection of three C. semiopaca was found to be 1.8 μg per beetle or roughly one-tenth the amount present in a complete skin of either P. bicolor or P. aurotaenia but far less than that found in a P. terribilis skin, which has ca. 1 mg. The amounts of BTX in beetle collections were, however, extremely variable. Our atmospheric pressure chemical ionization mass spectrometer was sufficiently sensitive, even in the full scan mode (m/z 50–900 atomic mass units), to detect BTXs in a single beetle.
Beetles are not known to synthesize steroidal skeletons, such as that found in the BTXs, although they do use the mevalonate pathway to form terpenes. Instead, steroids and triterpenoids in beetles are presumed to originate from plant phytosterols (cholesterol, stigmasterol, sitosterol, ergosterol, etc.), which are modified by the beetle or possibly by a symbiont or collection of symbionts (19). The possible role of symbionts in the production of beetle BTXs is being investigated.
The four species of Choresine beetles in our collections range in body length from ≈5.4 to 6.2 mm and in body breadth (across elytra) from 2.25 to 2.65 mm and average, in dried specimens, ≈5.7 mm in length and 2.3 mm in breadth. Each of these species has a series of glandular vesicles that may protrude from the lateral margins of abdominal segments 1–6 (Fig. 2), plus additional large vesicles from the prothorax (anterior and lateral margins) and metathorax (lateral margins). The elytra are shaped such that vesicles can evert even when the elytra are closed. Such vesicles are characteristic of Melyrids of the subfamily Malachiinae to which Choresine belongs. The systematics of this group for the Pacific area, treated mainly by the late Walter Wittmer, now remain a challenge for the relatively few contemporary entomologists studying Melyrid beetle taxonomy. To our knowledge, there have been no reports of secondary, bioactive compounds from beetles of the family Melyridae.
Fig. 2.
A C. pulchra beetle with an insert showing vesicles.
Little is known about the natural history of these Choresine beetles. Males and females are distinguishable only with a dissecting microscope. Choresine beetles, although apparently not brightly colored, do display a metallic blue-violaceous colored elytra that contrasts strongly with the yellow and blackish coloration of the pronotum (Fig. 2). Contrasting colors have some aposematic value, and this contrast is probably more conspicuous in sunlight. In Herowana, where the local people captured and preserved hundreds, most beetles were caught flying early in the morning or were found under leaves of garden plants. It was uncommon for a collector to catch more than ≈10–15 beetles per day. No adult Choresine beetles were found aggregated in groups. Local naturalists were unable to provide any information about larvae, host plants, or other habits, other than that adults were more commonly found in or around fresh thatch bunches used for roofing material. Local men warned that when working in fields or with thatch, one should take precautions to prevent these beetles from alighting in an eye or in facial perspiration because the beetles cause a burning sensation that can be severe.
Collections in museums of the continental United States offered few additional clues. These beetles are often among insects collected when fogging canopies; however, it is rare to collect more than a few Choresine beetles per tree. Other collections found at the California Academy of Sciences contained specimens obtained by netting individuals in grass of Madang Province (D. Kavanaugh, personal communication).
BTXs have been identified from five Pitohui species as well as I. kowaldi (13, 14). We examined the stomach contents of 11 I. kowaldi, 17 Pitohui dichrous, 12 Pitohui ferrugineus, 8 Pitohui kirhocephalus, and 1 Pitohui nigrescens, for a total of 49 individual birds. Stomach contents were classified by arthropod group and insect order, where recognizable, and plant and seed material. We verified that Pitohui and Ifrita do feed on small Choresine-sized insects (present in up to 30% of all individuals) (Table 2). Furthermore, one Pitohui did contain a Choresine in its stomach that was identified as Choresine nigroviolacea (Champion). Although we have not surveyed the toxin content in C. nigroviolacea, these studies demonstrate that toxic birds do feed on a variety of insects of the same size and even from the same genus, and we anticipate that they probably do feed on C. pulchra and other Choresines in areas where those species are present. The present results suggest that Choresine beetles are potentially a direct source of batrachotoxins for toxic New Guinea birds. An alternative hypothesis, that both the birds and the beetles may get their toxins from some common third source, such as a plant, seems unlikely, because these stomach contents and other reports (18) indicate that Ifrita is primarily, probably exclusively, insectivorous, although plant matter was also found in their stomachs, most likely arising from accidental ingestion of mosses, etc. while foraging. Only Pitohui species had seeds in their stomachs.
Table 2. Summary of bird stomach contents.
| No. of individuals
|
Choresine-sized contents
|
||||
|---|---|---|---|---|---|
| Species | Arthropods | Insects | Choresine | Plant matter | |
| All species | 49 | 44 | 30 | 1 | 27 |
| P. dichrous | 17 | 15 | 10 | 1 | 14 |
| I. kowaldi | 11 | 10 | 8 | 0 | 4 |
| Other Pitohui | 21 | 19 | 12 | 0 | 12 |
Each column shows the number of individuals whose stomach contained the particular food type.
Over the last several years, we have used multiple methods to search for natural sources of BTXs, including radiotelemetry of Pitohui in New Guinea, examination of Pitohui and Ifrita stomach contents, survey of hundreds of insects and plants that might formulate Pitohui diets, and follow-up of leads provided by many local naturalists.
The present discovery emphasizes the value of traditional knowledge. Pitohui birds appear to be omnivorous and feed on a wide variety of insects, spiders, millipedes, other arthropods, fruit, and even smaller vertebrates. They do not appear to be diet specialists. In tropical rainforests, therefore, the diet of an omnivore, such as the Hooded Pitohui (P. dichrous), may contain literally thousands of species. Thus, the observations and folklore of traditional people can and did provide labor-saving leads and an incredible and essential store of knowledge and insights for scientists working in unfamiliar places.
Acknowledgments
We thank the people of Herowana Village for their hard work and interest; Greg Greene for assistance in the field; A. Mack, D. Wright, and R. Sinclair of the Wildlife Conservation Society for logistical assistance; S. Miller, T. Erwin, and W. Steiner, Jr., of the Smithsonian Institution for early entomological discussions and assistance with taxonomy; and D. Kavanaugh of the California Academy of Sciences for additional information on field observations and a critique of an earlier version of the manuscript. J.P.D. was supported by National Science Foundation Grant DEB 0108247.
Author contributions: J.P.D., S.R.D., and J.W.D. designed research; T.F.S. and J.W.D. analyzed chemical data; J.P.D. and J.W.D. wrote the paper; A.W. provided local expertise critical to the discovery; A.W. and J.P.D. conducted field collections; and A.S. identified beetles to species and provided entomological expertise.
Abbreviations: BTX, batrachotoxin; h-BTX, homo-batrachotoxin; BTX-A, batrachotoxinin A.
References
- 1.Daly, J. W., Witkop, B., Bommer, P. & Biemann, K. (1965) J. Am. Chem. Soc. 87, 124–126. [DOI] [PubMed] [Google Scholar]
- 2.Tokuyama, T., Daly, J. & Witkop, B. (1969) J. Am. Chem. Soc. 91, 3931–3938. [DOI] [PubMed] [Google Scholar]
- 3.Daly, J. W. (1998) J. Nat. Prod. 61, 162–172. [DOI] [PubMed] [Google Scholar]
- 4.Myers, C. W., Daly, J. W. & Malkin, B. (1978) Bull. Am. Mus. Nat. Hist. 161, 307–366. [Google Scholar]
- 5.Albuquerque, E. X., Daly, J. W. & Witkop, B. (1971) Science 172, 995–1002. [DOI] [PubMed] [Google Scholar]
- 6.Strichartz, G., Rando, T. & Wang, G. K. (1987) Annu. Rev. Neurosci. 10, 237–267. [DOI] [PubMed] [Google Scholar]
- 7.Daly, J. W., Myers, C. W., Warnick, J. E. & Albuquerque, E. X. (1980) Science 208, 1383–1385. [DOI] [PubMed] [Google Scholar]
- 8.Daly, J. W., Garraffo, H. M., Spande, T. F., Jaramillo, C. & Rand, A. S. (1994) J. Chem. Ecol. 20, 943–955. [DOI] [PubMed] [Google Scholar]
- 9.Daly, J. W., Secunda, S. I., Garraffo, H. M., Spande, T. F., Wisnieski, A. & Cover, J. F. J. (1994) Toxicon 32, 657–663. [DOI] [PubMed] [Google Scholar]
- 10.Jones, T. H., Gorman, J. S. T., Snelling, R. R., Delabie, J. H. C., Blum, M. S., Garraffo, H. M., Jain, P., Daly, J. W. & Spande, T. F. (1999) J. Chem. Ecol. 25, 1179–1193. [Google Scholar]
- 11.Saporito, R. A., Donnelly, M. A., Hoffman, R. L., Garraffo, H. M. & Daly, J. W. (2003) J. Chem. Ecol. 29, 2781–2786. [DOI] [PubMed] [Google Scholar]
- 12.Saporito, R. A., Garraffo, H. M., Donnelly, M. A., Edwards, A. L., Longino, J. T. & Daly, J. W. (2004) Proc. Natl. Acad. Sci. USA 101, 8045–8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumbacher, J. P., Beehler, B. M., Spande, T. F., Garraffo, H. M. & Daly, J. W. (1992) Science 258, 799–801. [DOI] [PubMed] [Google Scholar]
- 14.Dumbacher, J. P., Spande, T. & Daly, J. W. (2000) Proc. Natl. Acad. Sci. USA 97, 12970–12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumbacher, J. P. (1999) Auk 116, 957–963. [Google Scholar]
- 16.Mouritsen, K. N. & Madsen, J. (1994) Oikos 69, 357–358. [Google Scholar]
- 17.Kocher-Schmid, C. (1993) Muruk 6, 1–15. [Google Scholar]
- 18.Majnep, I. S. & Bulmer, R. (1977) Birds of My Kalam Country (Auckland Univ. Press, Auckland).
- 19.Jungnickel, H. & Dettner, K. (1997) Mitt. Dtsch. Ges. Angew. Entomol. 11, 895–898. [Google Scholar]