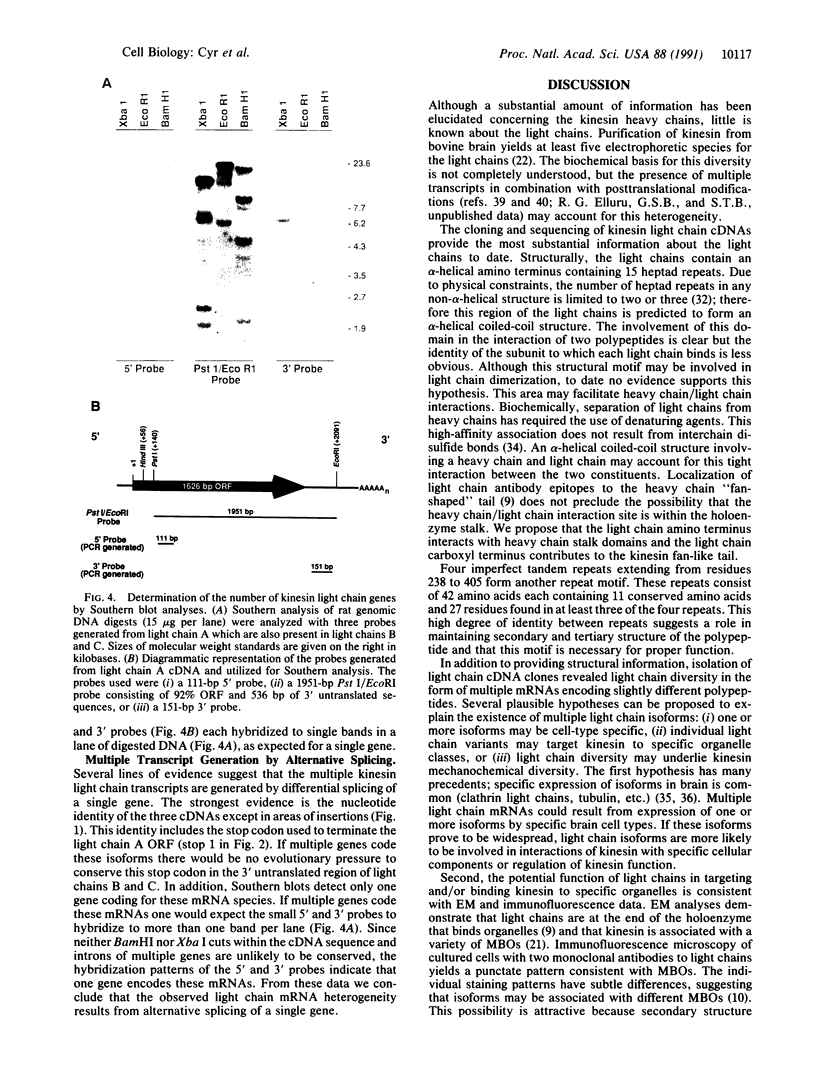
Abstract
Movement of membrane-bounded organelles to intracellular destinations requires properly oriented microtubules and force-generating enzymes, such as the microtubule-stimulated ATPase kinesin. Kinesin is a heterotetramer with two heavy chain (approximately 124-kDa) and two light chain (approximately 64-kDa) subunits. Kinesin heavy chains contain both ATP- and microtubule-binding domains and are capable of force generation in vitro. Functions of the light chains are undetermined, although evidence suggests they interact with membrane surfaces. We have used molecular genetic approaches to dissect the kinesin light chain structure. Three distinct kinesin light chain cDNAs were cloned and sequenced from rat brain, and they were found to result from alternative splicing of a single gene. Polypeptides encoded by these cDNAs are identical except for their carboxyl ends. Synthesis of multiple light chains, differing from one another in primary structure, could provide a means of generating multiple, functionally specialized forms of the kinesin holoenzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom G. S., Wagner M. C., Pfister K. K., Brady S. T. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry. 1988 May 3;27(9):3409–3416. doi: 10.1021/bi00409a043. [DOI] [PubMed] [Google Scholar]
- Brady S. T. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985 Sep 5;317(6032):73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Pfister K. K., Bloom G. S. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Cambray-Deakin M. A., Lewis S. A., Sarkar S., Cowan N. J. Differential distribution of beta-tubulin isotypes in cerebellum. EMBO J. 1988 Aug;7(8):2311–2319. doi: 10.1002/j.1460-2075.1988.tb03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M. G., McCammon M. T., Vassarotti A. Targeting proteins into mitochondria. Microbiol Rev. 1986 Jun;50(2):166–178. doi: 10.1128/mr.50.2.166-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S. P., Allen R. D., Sloboda R. D. Translocation of vesicles from squid axoplasm on flagellar microtubules. Nature. 1985 May 16;315(6016):245–248. doi: 10.1038/315245a0. [DOI] [PubMed] [Google Scholar]
- Gilbert S. P., Sloboda R. D. Identification of a MAP 2-like ATP-binding protein associated with axoplasmic vesicles that translocate on isolated microtubules. J Cell Biol. 1986 Sep;103(3):947–956. doi: 10.1083/jcb.103.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Pfister K. K., Yorifuji H., Wagner M. C., Brady S. T., Bloom G. S. Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration. Cell. 1989 Mar 10;56(5):867–878. doi: 10.1016/0092-8674(89)90691-0. [DOI] [PubMed] [Google Scholar]
- Hollenbeck P. J. The distribution, abundance and subcellular localization of kinesin. J Cell Biol. 1989 Jun;108(6):2335–2342. doi: 10.1083/jcb.108.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingold A. L., Cohn S. A., Scholey J. M. Inhibition of kinesin-driven microtubule motility by monoclonal antibodies to kinesin heavy chains. J Cell Biol. 1988 Dec;107(6 Pt 2):2657–2667. doi: 10.1083/jcb.107.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. P., Seow H. F., Holmes N., Drickamer K., Parham P. Clathrin light chains contain brain-specific insertion sequences and a region of homology with intermediate filaments. Nature. 1987 Mar 12;326(6109):154–159. doi: 10.1038/326154a0. [DOI] [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Schnapp B., Inouye H., Neve R. L. The primary structure and analysis of the squid kinesin heavy chain. J Biol Chem. 1990 Feb 25;265(6):3278–3283. [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Vaisberg E. A., Shanina N. A., Magretova N. N., Chernyak V. Y., Gelfand V. I. The quaternary structure of bovine brain kinesin. EMBO J. 1988 Feb;7(2):353–356. doi: 10.1002/j.1460-2075.1988.tb02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Vaisberg Y. A., Rothwell S. W., Murphy D. B., Gelfand V. I. Isolation of a 45-kDa fragment from the kinesin heavy chain with enhanced ATPase and microtubule-binding activities. J Biol Chem. 1989 Jan 5;264(1):589–595. [PubMed] [Google Scholar]
- Lasek R. J., Brady S. T. Attachment of transported vesicles to microtubules in axoplasm is facilitated by AMP-PNP. Nature. 1985 Aug 15;316(6029):645–647. doi: 10.1038/316645a0. [DOI] [PubMed] [Google Scholar]
- Leopold P. L., Snyder R., Bloom G. S., Brady S. T. Nucleotide specificity for the bidirectional transport of membrane-bounded organelles in isolated axoplasm. Cell Motil Cytoskeleton. 1990;15(4):210–219. doi: 10.1002/cm.970150404. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Karn J. Periodic features in the amino acid sequence of nematode myosin rod. J Mol Biol. 1983 Mar 15;164(4):605–626. doi: 10.1016/0022-2836(83)90053-0. [DOI] [PubMed] [Google Scholar]
- Paschal B. M., Vallee R. B. Retrograde transport by the microtubule-associated protein MAP 1C. Nature. 1987 Nov 12;330(6144):181–183. doi: 10.1038/330181a0. [DOI] [PubMed] [Google Scholar]
- Penningroth S. M., Rose P. M., Peterson D. D. Evidence that the 116 kDa component of kinesin binds and hydrolyzes ATP. FEBS Lett. 1987 Sep 28;222(1):204–210. doi: 10.1016/0014-5793(87)80220-x. [DOI] [PubMed] [Google Scholar]
- Pfister K. K., Wagner M. C., Stenoien D. L., Brady S. T., Bloom G. S. Monoclonal antibodies to kinesin heavy and light chains stain vesicle-like structures, but not microtubules, in cultured cells. J Cell Biol. 1989 Apr;108(4):1453–1463. doi: 10.1083/jcb.108.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J. L., Holmes W. P., Colman D. R. The amino acid sequences of the myelin-associated glycoproteins: homology to the immunoglobulin gene superfamily. J Cell Biol. 1987 Apr;104(4):957–965. doi: 10.1083/jcb.104.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey J. M., Heuser J., Yang J. T., Goldstein L. S. Identification of globular mechanochemical heads of kinesin. Nature. 1989 Mar 23;338(6213):355–357. doi: 10.1038/338355a0. [DOI] [PubMed] [Google Scholar]
- Schowalter D. B., Sommer S. S. The generation of radiolabeled DNA and RNA probes with polymerase chain reaction. Anal Biochem. 1989 Feb 15;177(1):90–94. doi: 10.1016/0003-2697(89)90019-5. [DOI] [PubMed] [Google Scholar]
- Tsou A. P., Lai C., Danielson P., Noonan D. J., Sutcliffe J. G. Structural characterization of a heterogeneous family of rat brain mRNAs. Mol Cell Biol. 1986 Mar;6(3):768–778. doi: 10.1128/mcb.6.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M. C., Pfister K. K., Bloom G. S., Brady S. T. Copurification of kinesin polypeptides with microtubule-stimulated Mg-ATPase activity and kinetic analysis of enzymatic properties. Cell Motil Cytoskeleton. 1989;12(4):195–215. doi: 10.1002/cm.970120403. [DOI] [PubMed] [Google Scholar]
- Warrick H. M., Spudich J. A. Myosin structure and function in cell motility. Annu Rev Cell Biol. 1987;3:379–421. doi: 10.1146/annurev.cb.03.110187.002115. [DOI] [PubMed] [Google Scholar]
- Yang J. T., Laymon R. A., Goldstein L. S. A three-domain structure of kinesin heavy chain revealed by DNA sequence and microtubule binding analyses. Cell. 1989 Mar 10;56(5):879–889. doi: 10.1016/0092-8674(89)90692-2. [DOI] [PubMed] [Google Scholar]
- Yang J. T., Saxton W. M., Stewart R. J., Raff E. C., Goldstein L. S. Evidence that the head of kinesin is sufficient for force generation and motility in vitro. Science. 1990 Jul 6;249(4964):42–47. doi: 10.1126/science.2142332. [DOI] [PubMed] [Google Scholar]