Abstract
Herpes simplex virus (HSV)-1 is a ubiquitous human infection, with increased prevalence in obese populations. Obesity has been linked to increased inflammation, susceptibility to infection, and higher rates of anxiety disorder and cognitive impairment. To determine how obesity alters neuroinflammation and behavior following infection, we infected weanling C57BL/6 or CCR2RFP/+/CX3CR1GFP/+ mice with a very low dose of HSV-1. Following viral latency (14 days post infection (d p.i.)), mice were randomly assigned to remain on the low fat (LF) diet or switched to a 45% high fat (HF) diet. Eight weeks post diet shift, latently infected mice on the HF diet (HSV-HF) had greater microglial activation and infiltration of inflammatory CCR2+ monocytes in the hypothalamus and dentate gyrus, in comparison to both HSV-LF mice and uninfected mice on LF and HF diets. VCAM staining was present in hypothalamus and hippocampus of the HSV-HF mice in the areas of monocyte infiltration. Infiltrating monocytes also produced proinflammatory cytokines demonstrating that, along with activated microglia, monocytes contribute to sustained neuroinflammation in latently infected obese mice. Utilizing a light-dark preference test, we found that HSV-HF mice had increased anxiety-like behavior. In the marble-burying test, HF diet and HSV infection resulted in increased numbers of buried marbles. Together, these mice provide a useful, testable model to study the biobehavioral effects of obesity and latent HSV-1 infection in regards to anxiety and may provide a tool for studying diet intervention programs in the future.
Keywords: HSV-1, obesity, neuroinflammation, anxiety, microglia and macrophage
1. Introduction
Herpes simplex virus (HSV)-1 is a ubiquitous human virus that predominately causes oral lesions, though there is an increase in the prevalence of genital HSV-1 infections (Gupta et al., 2007). During HSV-1 infection, microglia, the resident immune cells of the CNS, are activated and produce cytokines and chemokines in order to inhibit vital replication and to recruit other immune cells to the brain. The controlled and limited production of cytokines by microglia in response to insult or injury is necessary for clearing infections in the CNS and can be neuroprotective (Streit, 2002). However, excessive production of proinflammatory cytokines can result in neurotoxicity (Cunningham et al., 2009; Teeling and Perry). In response to microglial-derived chemokine, T Ly6Chi monocytes infiltrate the brain and differentiate into macrophages in conjunction with microglia, recruit T cells to the brain to resolve the infection (Marques et al., 2008; Terry et al., 2012). After a primary infection with HSV-1, the virus becomes latent and persists in neurons of the sensory and autonomic ganglia, including the trigeminal (TG). National Health and Nutrition Examination Survey (NHANES) data from 2005–2010 suggests that 30.1% of adolescents age 14–19 in the US are seropositive for HSV-1 (Bradley et al., 2014), while the prevalence of HSV-1 in children ages 6–16 is higher in children of lower socioeconomic status regardless of race (Dowd et al., 2009). The prevalence of HSV-1 infection continues to increase throughout life and by 49 years old (y.o.), 65% of all US residents are seropositive (Kasubi et al., 2006). In a small study of postmortem TG samples, HSV-1 DNA was present in 60–85% samples of people 0–20 y.o. (Hill et al., 2008). Together, these data indicate that most primary infections with HSV-1 occur early in life and that the presence of latent HSV-1 in the CNS is a lifelong condition for most humans.
Like HSV-1 infection, obesity is also a significant public health concern for both adults and children (Johnson et al., 2014). Infiltration of inflammatory macrophage into adipose tissue is a hallmark of obesity and is believed to play a role in the development of a number of comorbidities, including Type 2 diabetes. These infiltrating macrophage have an M1 or proinflammatory phenotype and as individuals become increasingly obese, macrophage phenotypes polarize to a proinflammatory/M1 state. This polarization occurs as the cells are activated by IFNγ, TNFα and IL-6, induced by saturated fatty acid stimulation via TLR 2 and 4 receptors (Lumeng et al., 2007a; Lumeng et al., 2007b; Nguyen et al., 2005; Suganami et al., 2007). While the infiltration of macrophage into adipose has been widely studied, the infiltration of these cells in to the brain of obese mice from the periphery was recently discovered (Buckman et al., 2013). The consequence of the macrophage infiltration has not been elucidated. High saturated fat diets also results in activated microglia in the hypothalamus and hippocampus of rodents (Huang et al., 2013; Hwang et al., 2009; Milanski et al., 2009) and in obese mice, these microglia are microglia are more proinflammatory (Aguzzi et al., 2013). Overall, high fat diets and obesity result in neuroinflammation.
Increasing epidemiological evidence has shown that early childhood events associated with poverty can affect health throughout life. Specifically, low socioeconomic status (SES) in childhood has been correlated with adult anxiety and major depressive disorders (McLaughlin et al., 2011). Two health factors often associated with lower SES are obesity and increased infectious burden, particularly with latent viruses, including HSV-1 (Beydoun et al., 2010; Frederick, 2014). Latent HSV-1 infection has been correlated with cognitive impairment during childhood, schizophrenia and dementia/Alzheimer’s disease (AD) in adulthood (Deleidi and Isacson, 2012; Tarter et al., 2013). Until recently, the rates of childhood/adolescent obesity had been increasing at the same rate across all SES levels. However, obesity rates have begun to level off in all but lower SES groups, where they continue to increase at a significant rate (Frederick, 2014). Obesity has been associated with increased risk for dementia/AD, mood disorders and anxiety. A positive association between obesity and HSV-1 seropositivity has been made (Fernandez-Real et al., 2007; Karjala et al., 2011). Brain regions associated with anxiety, such as the hypothalamus, hippocampus, medial amygdala and the bed nucleus of the stria terminalis are sites of HSV-1 antigen following infection (Armien et al., 2010; Mori et al., 2005; Mori et al., 2006). During HSV-1 infection, in conjunction with T cells, Ly6Chi monocytes infiltrate the brain and differentiate into macrophages in order to resolve the infection (Marques et al., 2008; Terry et al., 2012). A growing body of literature demonstrates that early life infection results in increases in social avoidance and anxiety in an animal model (Bilbo, 2010; Bilbo and Schwarz, 2009; Fernandez-Real et al., 2007; Karjala et al., 2011). In addition, HF diets and obesity can increase anxiety in both rodents and humans (Farr et al., 2008; Fitzpatrick et al., 2009; Holden et al., 2009; Kaczmarczyk et al., 2013). Microglial activation and the infiltration of macrophages into regions associated with anxiety may play a role in the development of anxiety in certain stress paradigms (Wohleb et al., 2014; Wohleb et al., 2013). Therefore, we sought to determine if HF diet following HSV-1 infection and latency results in increased neuroinflammation via microglia activation and proinflammatory monocyte/macrophage infiltration in regions associated with anxiety, as well as increased anxiety in mice.
2. Materials and methods
2.1. Mice and Infection
Weanling male, C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). All mice were housed at the University of North Carolina Animal Facility, which is fully accredited by the American Association for Laboratory Animal Care. Mice were maintained under protocols approved by the Institutional Animal Use and Care Committee. All mice were placed on the LF diet when they arrived and were allowed to acclimate for 1 week prior to infection. Cages of mice were randomized to either mock (saline) or HSV-1 infection via the intranasal route; this model of infection in mice best mimics infection in humans (Shivkumar et al., 2013). For infection, mice were lightly anesthetized with a ketamine (0.6mg/kg)/xylazine (0.35mg/kg) solution and infected intranasally (i.n.) with 2.5x105 pfu HSV-1 in 10μl in PBS. This dose of HSV-1 does not result in encephalitis or significant symptoms of sickness, but does result in HSV-1 latency in the TG. Following latency at 14 days post infection (p.i.) the cages were randomized again to remain on the LF diet or switched to the HF diet. Mice were housed 4/cage with free access to food and water. CCR2RFP/+/Cx3CR1GFP/+ mice were generated by crossing CCR2RFP/RFP (B6.129(Cg)-Ccr2tm2.1Ifc/J) mice with CX3CR1GFP/GFP (B6.129P-Cx3cr1tm1Litt/J, Jackson Labs, Bar Harbor, ME) (Mizutani et al., 2012; Saederup et al., 2010). CCR2RFP/+/Cx3CR1GFP/+ mice carry one functioning copy of CCR2 and CX3CR1 and one copy of CCR2-RFP and CX3CR1-GFP. CCR2RFP/+/CX3CR1GFP/+ mice have been used to differentiate microglia from infiltrating mononuclear cells as microglia express CX3CR1 throughout development and this can be monitored via GFP (and lack RFP expression) (Mizutani et al., 2012). The infiltrating CCR2+ monocytes in turn express RFP and are easily distinguishable from the microglia (Mizutani et al., 2012; Saederup et al., 2010). CCR2+ monocytes are proinflammatory, M1 precursors and the infiltration of these cells is associated with neuroinflammation (Shi and Pamer, 2011). After weaning at 23 d the procedures described above were followed.
2.2. HSV-1
HSV-1 McIntyre (ATCC, Manassas, VA) stocks were propagated in Vero cells (ATCC, Manassas, VA), collected, centrifuged and stored at −80°C.
2.3. Diets
We used D12451 (Research Diets, New Brunswick, NJ) a 45% fat by kcal diet with soybean oil and lard as the fat source. The LF control diet D12450B is a 10% fat diet with the same source of fat. These diets were chosen because the HF diet contains fat sources that yield a diet that is 16% saturated fat, 20% monounsaturated fat and 8% polyunsaturated fat. An analysis of the US diet conducted with NHANES data from 2005–6 demonstrates that the average American diet is approximately 33% fat, composed of 11% saturated fat, 12% monounsaturated fat and 7% polyunsaturated fat (U.S. Department of Agriculture, 2008).
2.4. Assessment of pathology and body weight measurement
During the course of infection, mice were observed 3 times daily for symptoms of HSV-induced illness and encephalitis as previously described (Sheridan and Beck, 2008). At 3 months p.i., mice were weighed on the day of sacrifice.
2.5. Brain mononuclear cell isolation
Microglia were isolated following a previously published method (Henry et al., 2009). Briefly, brains were removed and homogenized in HBSS. Single cells suspensions were filtered through 70uM nylon mesh and centrifuged at 500g for 5 min. The cells were resuspended in 70% Isotonic Percoll (Sigma-Aldrich, St. Louis, MO) and then layered with 50%, 35% and 0% isotonic Percoll. The Percoll gradients were centrifuged at 2000g for 20min at room temperature. The mononuclear layer located at the 70/50% interphase was carefully collected and washed 2 times with HBSS and finally resuspended in complete RPMI. Previous studies have demonstrated that this method results in the isolation of 6×105 viable cells that are 89–96% pure from uninfected mice. Importantly, this method does not cause the activation of microglia (Henry et al., 2009).
2.6. DNA isolation, RNA Isolation and qRT-PCR
Bilateral hippocampi (with subregions remaining intact) were dissected following a published protocol and rapidly frozen in liquid nitrogen (Sultan, 2013). Total RNA was isolated using the TRIzol method (Invitrogen, Carlsbad, CA). Reverse transcription was carried out using Superscript II First Strand Synthesis kit (Invitrogen) with oligo (dT) primers. qRT-PCR was performed using TaqMan Universal Master Mix with the Taqman Assays on Demand Primer Probe pairs (Applied Biosystems, Foster City, CA). In order to determine HSV-1 latency, DNA and RNA were extracted from TG following the TRIzol dual isolation method. Reverse transcription was carried out as described above for mRNA and qPCR was performed using ICP0 Taqman Assays on Demand Primer Probe pair. The levels of mRNA for GAPDH were determined for all samples and used to normalize gene expression. In our experience, levels of GAPDH are constant in lung, brain and spleen during influenza, HSV-1 and coxsackie infections (Sheridan and Beck, 2008; Karlsson et al., 2009; Smith et al., 2007).
2.7. Flow cytometry
For flow cytometry, Percoll isolated cells were washed then resuspended in DMEM-5% FBS and incubated with anti-CD16/32 to block non-specific binding to Fc receptors. Cells were then surface stained with anti-CD45, anti-CD11b, anti-CD86 (eBiosciences, San Diego, CA) following previously published methods. After gating on live cells, macrophage were identified as CD11b+ and CD45hi. After gating on the macrophage, CD86+ cells were identified (Wohleb et al., 2013). Appropriate isotype controls were used to determine positive staining. For flow cytometry on blood samples, whole blood was collected into EDTA-treated tubes. Cells were washed twice DMEM-5%HBS and then surface stained with anti-CD11b, Ly6c and Ly6G antibodies and examined by flow cytometry following previously published methods (Hettinger et al., 2013; Powell et al., 2013). Monocytes were identified as SSClo, CD11b+, Ly6G−, Ly6Chi monocytes are proinflammatory while Ly6Clo monocytes have a “patrolling/wound healing” phenotype (Shi and Pamer, 2011). Blood from CCR2RFP/+/CX3CR1GFP/+ mice was surface stained with anti-CD11b. CD11b+ cells were gated and the % of RFP (CCR2) and GFP (Cx3CR1) positive cells were determined by flow cytometry. Appropriate isotype controls were used to determine positive staining. Data were collected on the BD Accuri Flow Cytometer (Franklin Lakes, NJ) and analyzed by FlowJo Software (Tree Star, Ashland, OR).
2.8. Perfusion and immunohistochemical staining
Mice were anesthetized deeply with avertin and transcardially perfused with 0.1M sodium phosphate buffer (pH 7.4) at 4°C followed by 4% paraformaldehyde. Brains were removed and immediately postfixed in 4% paraformaldehyde, then cryopreserved by overnight incubations In 15% then 30% sucrose. The brains were in OCT until frozen sectioning. Tissue sections were fluorescently stained using standard immunohistochemical technique. Briefly, tissues were incubated in blocking buffer solution for one hour (5% Goat Serum (Sigma G6767), 1% BSA (Fisher BP1600-100), 0.05% Sodium Azide-20% (RPI, S24080-500), 0.5% Triton X-100 (Fisher BP151-100), 1X PBS-10X, dH2O). Tissues were incubated overnight with appropriate primary antibodies diluted in blocking buffer, washed with PBS, and subsequently incubated for one hour with appropriate secondary antibodies diluted in blocking buffer. Specifically, for ionized calcium-binding adapter molecule (IBA)-1 immunolabeling with antibody diluted 1:250 (BioCare Medical, Concord, CA Cat #290), sections were stained as described by Williamson et al (2012). VCAM immunolabeling was be preformed with anti-goat primary antibody diluted 1:50 from Santa Cruz (Cat # SC-1504), followed by secondary staining with Alexa Fluor 350 Donkey Anti-Goat IgG (H+L) (Life Technologies, Carlsbad, CA, Cat#A-21081). Sections incubated with secondary antibody served as controls for non-specific staining, while brain sections from mice with HSV-encephalitis from a separate study were used as positive controls as they have significant inflammation and cellular infiltrate. Tissues were mounted with aqueous mounting media and cover-slipped. Slides were imaged on a Nikon Eclipse 90i microscopy system (Nikon Instruments, Melville, NY) using Nikon NIS-Elements Advanced Research software with fluorescent objectives magnified by the objective + 1X making the objective magnification (20X/40X) the actual magnification. Due to the low magnification, specific subregions of the hypothalamus/amygdala were not specifically imaged. Rather, these areas were imaged across their broad regions to obtain an average intensity for the region as a whole. To ensure images came from approximately the same regions across many tissues, tissue architecture was compared against the Allen Brain Atlas, as well as across tissue sections to ensure consistency. For GFAP, IBA-1, and VCAM, pixel density/intensity was determined via threshold analysis using Nikon NIS-Elements Advanced Research software.
2.9. CCL2 ELISA
Serum was collected from whole trunk blood and the CCL2 ELISA was performed following the manufacturer’s protocol (eBioscience, San Diego, CA).
2.10. Behavior testing (light-dark preference and marble burying test)
Three months post infection mice (n=8/group) were tested for anxiety-like behaviors by the Mouse Behavioral Phenotyping Laboratory at UNC. In the light-dark preference test, mice were placed in the dark area of a two-chamber testing apparatus and activity was recorded for 10 min. Records were taken of the time spent in each side and entries into each side, measured by photobeam breaks by an automated system (Versamax, Accuscan Instruments, Columbus, OH). In the marble burying test, mice were placed in a Plexiglas cage located in a sound-attenuating chamber with ceiling light and fan. The cage contained 5 cm deep clean corncob bedding, with 20 black glass marbles (14 mm diameter) arranged in an equidistant 5 × 4 grid on top of the bedding. Animals were given access to the marbles for 30 min. Measures were taken of the number of buried marbles (2/3 of the marble covered by the bedding).
2.11. Statistics
Data were analyzed by 2-way ANOVA with the variables diet and infection using JMP statistical software (SAS, Cary, NC). A priori post hoc comparisons among groups were performed with Tukey-Kramer test and differences were considered significant if P<0.05. Data are presented as mean +/− SEM
3. Results
3.1. Intranasal infection results in HSV-1 latency in the trigeminal ganglion without symptoms of encephalitis
In order to determine if intranasal infection with HSV-1 would establish latency in the trigeminal ganglion, mice were either mock infected or infected with HSV-1. Mice were sacrificed at 14 d p.i. at this time point replicating virus was no longer detected in the TG of mice (no amplification of by 40 cycles of qRT-PCR), however, DNA for HSV-1 was present in the mice that had been infected (Table 1). These data confirm HSV-1 infection as well as latency by the time the mice are switched to the HF diet. No mice in these studies (C57Bl/6 or CCR2RFP/+/CX3CR1GFP/+) developed symptoms of encephalitis or any overt pathology.
Table 1.
Establishment of Latent HSV-1 in Mice via Intranasal Route
| Infection | Diet | HSV mRNA TG D14 p.i. | HSV DNA TG D14p.i. |
|---|---|---|---|
| HSV | LH | 0/6 | 6/6 |
| HSV | HF | 0/6 | 6/6 |
| Mock | LF | 0/6 | 0/6 |
| Mock | HF | 0/6 | 0/6 |
3.2. 45% HF diet results in significant weight gain
At 3 m p.i. (2.5 m of diet) mice on the HF diet were significantly heavier that mice on the LF diet, regardless of infection (Fig. 1).
Fig. 1.
45% diet results in significant weight gain in both uninfected and latently infected mice (Diet, P <0.001; Infection, P=0.11; Diet x Infection, P=0.50). Statistical analysis: two-way ANOVA. Letters above columns indicate statistical significance between groups by Tukey-Kramer post hoc test. Columns not sharing a letter differ significantly at P<0.05, n=12/group. Data are presented as mean +/− SEM.
3.3. HF diet following HSV-1 infection results increased IBA-1 expression and proinflammatory cytokine expression
Priming of microglia may occur by various means, but ultimately primed microglia upregulate many cell surface markers including TLR2, TLR4, MHCII, CD11b, CD80 and CD86. The production of proinflammatory cytokines by the primed microglia and a change to an amoeboid morphology defines them as being in an “activated” state (Perry et al., 2007).
At 3 months p.i., we examined IBA-1 immunolabeling in the hypothalamus, amygdala, hippocampus (total region) and dentate gyrus (Fig. 2). Among the groups, there were no significant differences in the hypothalamus, nor hippocampus (data not shown), however, HF-diet alone resulted in increased IBA-1 in the amygdala. A significant increase in IBA-1 immunolabeling and microglia with larger cell bodies and shorter processes was observed in the dentate gyrus region of the HSV-HF mice, an indication of significant microglial activation.
Fig. 2.
IBA-1 staining and quantification. (A) Representative sections from hypothalamus, amygdala and dentate gyrus at 20x magnification. Quantification of pixel density of IBA-1 in (B) hypothalamus (Diet, P=NS; Infection, P=NS; Diet x Infection, P=NS) (C) amygdala (Diet, P=0.044; Infection, P=0.041; Diet x Infection, P=0.11) and (D) dentate gyrus (Diet, P=0.001; Infection, P=0.040; Diet x Infection, P=0.04). Statistical analysis: two-way ANOVA. Letters above columns indicate statistical significance between groups by Tukey-Kramer post hoc test. Columns not sharing a letter differ significantly at P<0.05, n=4/group. Data are presented as mean +/− SEM.
In addition to its critical role in learning and memory, the hippocampus is also a primary relay center involved in anxiety-like behaviors (Burgess et al., 2002; Mineur et al., 2013). To further dissect the role of latent HSV infection and HF diet in activating microglia, we measured the upregulation of several cell surface markers and proinflammatory cytokines using qRT-PCR. Three months following the establishment of HSV latency, there were no differences in the expression of markers of glial priming and activation of HSV-LF mice compared to the LF uninfected controls (Table 2). While HF diet alone increased expression of MHCII and IL-10 (bolded black, P< 0.05 compared to LF and HSV-LF, but not HSV-HF) it is only in the latent HSV-HF fed group that significant changes were seen in the majority of markers of glial priming and activation (green, P< 0.05 compared to LF, HSV-LF and HF) (Table 2). It is important to note that we did not find mRNA for HSV-1 in the hippocampus nor TG in these mice, confirming that the virus had not reactivated and was not actively replicating.
Table 2. Expression of Glial Markers in the Hippocampus Presented as Fold Increase from LF mice.
(post-hoc test: Bolded black, P< 0.05 compared to LF and HSV-LF, but not HSV-HF; Green P< 0.05 compared to LF, HSV-LF, and HSV-HF; Blue P< 0.05 compared to LF, HSV-LF).
| Gene | LF | HSV-LF | HF | HSV-HF | Pdiet | Pinfection | Pdietxinfection |
|---|---|---|---|---|---|---|---|
| Markers of Primed Glia | |||||||
| TLR4 | 1.00±0.12 | 0.97±0.23 | 1.09±0.17 | 1.80±0.16 | 0.02 | 0.08 | 0.06 |
| MHCII | 1.00±0.08 | 1.12±0.14 | 1.74±0.30 | 1.80±0.20 | 0.00 | 0.58 | 0.95 |
| CD80 | 1.00±0.16 | 0.89±0.20 | 1.29±0.21 | 1.96±0.28 | 0.01 | 0.24 | 0.11 |
| CD86 | 1.00±0.18 | 0.85±0.25 | 1.26±0.21 | 2.08±0.34 | 0.02 | 0.24 | 0.10 |
| CD11b | 1.00±0.05 | 1.06±0.16 | 1.41±0.29 | 2.18±0.30 | 0.01 | 0.10 | 0.16 |
| Cytokines Released by Activated Glia | |||||||
| IL-1β | 1.00±0.15 | 1.18±0.18 | 1.40±0.24 | 2.50±0.46 | 0.02 | 0.06 | 0.17 |
| IL-6 | 1.00±0.26 | 1.45±0.65 | 1.26±0.40 | 3.62±1.67 | 0.31 | 0.25 | 0.04 |
| TNFα | 1.00±0.08 | 0.87±0.13 | 1.42±0.25 | 1.52±0.18 | 0.01 | 0.93 | 0.53 |
| IL-10 | 1.00±0.39 | 1.03±0.13 | 3.06±0.61 | 2.70±0.89 | 0.01 | 0.80 | 0.76 |
3.4. HSV-1 infection results in a significant increase in monocytes/macrophage in the brain 3 m p.i
Buckman et al. demonstrated that monocytes/macrophage from the periphery enter the brain of obese mice (Buckman et al., 2013). In order to determine if there was increased monocyte/macrophage infiltration in the brains of the HSV-HF mice we isolated mononuclear cells from the whole brain and determined the percentage of CD45hiCD11b+ macrophages by flow cytometry (Fig. 3). In these mice, HF diet alone did not significantly increase the percentage of macrophages in the brain, interestingly there was a significant increase in the percentage of CD45hiCD11b+ cells in the brains of the HSV-HF mice (Fig. 3A). CD86 expression is an established marker of M1/classically activated macrophages (Kadl et al., 2010; Kigerl et al., 2009; Vogel et al., 2013). The percentage of CD45hiCD11b+CD86+ cells was significantly increased in the HSV-HF mice compared to LF and HSV-LF mice (Fig. 3B). These data confirm that HF diet following HSV-1 infection results in M1-macrophage infiltration into the brain and that macrophages are maintained (or continuously recruited into the brain) of HSV-HF mice, however, macrophage that respond initially to the infection do not remain in HSV-LF mice. Together, these data indicate that the microenvironment created by both HSV and HF diet is required for the continued macrophage presence.
Fig. 3.
Increased CD45hiCD11b+ M1-like macrophage infiltrate in the brain of HSV-HF mice. (A) % of CD45hiCD11b+ macrophage (Diet, P=0.017; Infection, P=0.08; Diet x Infection, P=0.14) (B) % of CD45hiCD11b+ that are CD86+ (Diet, P=0.016; Infection, P=0.06; Diet x Infection, P=0.37). Statistical analysis: two-way ANOVA. Letters above columns indicate statistical significance between groups by Tukey-Kramer post hoc test. Columns not sharing a letter differ significantly at P<0.05, n=6/group. Data are presented as mean +/− SEM.
While we know that the HSV-HF mice have increased CD45hiCD11b+ M1/classically activated macrophage, identifying their precise neuroanatomical location is difficult, as both macrophage and monocytes are CD45+ and CD11b+ and express overlapping sets of cell surface markers. Therefore, it is very difficult to differentiate these cells from one another by IHC. To identify the locations of infiltrating monocytes/macrophage, we utilized an innovating strategy in which the CCR2RFP/+/CX3CR1GFP/+ mouse (Mizutani et al., 2012). The significant advantage of this system over the one described by Buckman et al is that it does not require bone marrow transplant using lethal irradiation that on its own can induce significant neuroinflammation (Buckman et al., 2014). In order to determine the location of the infiltrating cells, CCR2RFP/+/CX3CR1GFP/+ mice were sacrifice at 3m p.i., (Fig. 4 and 5). While single RFP+ cells were found scattered throughout the brain, there was a significant and consistent infiltration of RFP+ cells in the hypothalamus and dentate gyrus of the HSV-HF mice (Fig. 4D and Fig. 5D). RFP+ cells were not found in the amygdala nor other regions of the hippocampus.
Fig. 4.
CCR2+ (Red) cells infiltrate the hypothalamus of HSV-HF mice. Representative sections from the hypothalamus of CCR2RFP/+/CX3CR1GFP/+ (20X) n=4/group. (A) LF (B) HF (C) HSV-LF and (D) HSV-HF.
Fig. 5.
CCR2+ (red) cells infiltrate the hippocampus of HSV-HF mice. Representative sections from the hippocampus of CCR2RFP/+/CX3CR1GFP/+ (20X) n=4/group. (A) LF (B) HF (C) HSV-LF and (D) HSV-HF. In D, white arrows indicate the location of CCR2+ cells.
3.5. HSV-HF mice have significantly increased Ly6C+ and CCR2+ circulating monocytes
At 3m p.i. HSV-HF mice had significantly more circulating monocytes and the percentage of Ly6chi proinflammatory monocytes were significantly enriched with a corresponding decrease in Ly6Clo monocytes (Fig. 6A and B). Ly6Chi monocytes are CCR2+Cx3CR1− while Ly6Clo are CCR2+Cx3CR1− (Shi and Pamer, 2011). We also examined the expression of RFP (CCR2) and GFP (Cx3CR1) on CD11b+ cells from the blood of CCR2RFP/+/CX3CR1GFP/+ mice. As with Ly6C, there was a significant increase in the percentage of monocytes that were CCR2+Cx3CR1− proinflammatory monocytes in the HSV-HF mice (Fig. 6C).
Fig. 6.
Increased Ly6Chi monocytes and CCR2+ monocytes in the blood of HSV-HF mice. (A) Percent circulating monocytes (SSCloCD11b+Ly6G−) (Diet, P=0.048; Infection, P=0.070; Diet x Infection, P=0.07) (B) Ly6Clo monocytes (black bars) (Diet, P=0.17; Infection, P=0.29; Diet x Infection, P=0.08) and Ly6Chi monocytes (white bars) (Diet, P=0.27; Infection, P=0.12; Diet x Infection, P=0.20) and (C) CD11b+CCR2+ monocytes (Diet, P=0.10; Infection, P=0.26; Diet x Infection, P=0.04). Statistical analysis: two-way ANOVA. *indicates a significant difference at P<0.05 from all other groups by Tukey-Kramer post hoc test, n=6/group for A and B and n=4/group for C. Data are presented as mean +/− SEM.
3.6. HF diet following HSV infection increased VCAM expression in areas with RFP+ cell infiltration
HF diet following HSV infection results in a unique microenvironment that promotes monocyte infiltration into the brain. During HSV-1 infection upregulation of the endothelial cell adhesion molecule, VCAM occurs allowing macrophages and T cells to enter the brain pyranchema (Terry et al., 2012). VCAM is upregulated by the production of IL-1β and TNFα, because HSV-HF mice had increased mRNA expression of these cytokines in the hippocampus we hypothesized that they would have increased VCAM expression (Skelly et al., 2013). Unlike HSV-LF mice, HSV-HF brains have sustained macrophage infiltration/retention and this would suggest that chemokines and adhesion molecules (VCAM) continue to be upregulated in these mice. To determine if VCAM was upregulated, we immunolabeled sections with DAPI (a nuclear marker) and goat anti-mouse VCAM antibody. Consistent with previous findings, VCAM was not present in the hippocampus or hypothalamus of the LF mice, nor was it present in the HSV-LF or HF mice (Sawicki et al., 2015). However, VCAM was present in the hippocampus and hypothalamus of the HSV-HF mice (Fig. 7A–D; Additional images of VCAM immunolabeling Supp Fig. 1 A–F). Importantly, RFP+ cells co-localized or were in close proximity to VCAM-positive brain endothelia and suggest that regional VCAM expression allowed for extravasation of CCR2+ cells in to the parenchyma (popouts Fig. 7 D and H).
Fig. 7.
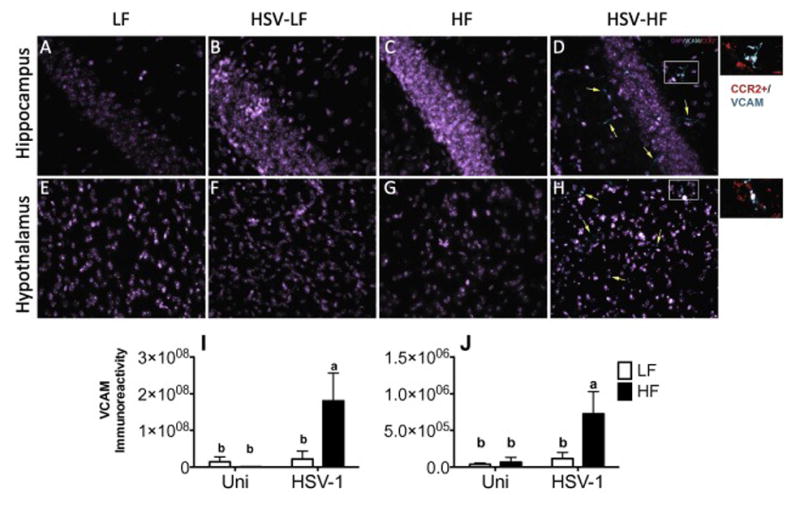
VCAM is upregulated in HSV-HF mice and co-localizes with CCR2+ cells. (A–H) Representative sections from Hypothalamus and Hippocampus at 20x magnification. Insets are from D and H. Quantification of VCAM pixel density in (I) hippocampus (Diet, P=0.13; Infection, P=0.05; Diet x Infection, P=0.07) and (J) hypothalamus (Diet, P=0.056; Infection, P=0.032; Diet x Infection, P=0.78). Statistical analysis: two-way ANOVA. Letters above columns indicate statistical significance between groups by Tukey-Kramer post hoc test. Columns not sharing a letter differ significantly at P<0.05, n=4/group. Data are presented as mean +/− SEM.
3.7. HSV-HF mice have increased CCL2 in the hippocampus and serum
Several models of neuroinflammation suggest that CCL2 (MCP-1) produced by microglia attract the M1-type, LY6Chi monocytes into the brain parenchyma. CCL2 is increased during inflammatory events including infection and obesity (Shi and Pamer, 2011; Takahashi et al., 2003). Relevant to our model, saturated fatty acids induce CCL2 expression in microglia of the hypothalamus (Valdearcos et al., 2014) and CCL2 is the main signal that recruits CCR2+/Ly6Chi monocytes from the bone marrow. We found that compared to LF controls, HSV-HF mice had significantly increased CCL2 expression in the hippocampus (Fig. 8A) and circulating serum CCL2 (Fig. 8B) at 3m p.i. Together, these data indicate that there is a unique microenvironment where increased circulating CCL2 recruits CCR2+LY6Chi monocytes from the bone marrow into circulation. The increase of CCL2 in the hippocampus, in conjunction with VCAM upregulation, allows for these cells to infiltrate the brain parenchyma.
Fig. 8.
Hippocampal CCL2 mRNA expression and serum CCL2 are increased in HSV-HF mice compared to LF controls. (A) Fold increase of CCL2 mRNA in the hippocampus (Diet, P=0.02; Infection, P=0.12; Diet x Infection, P=0.70) and (B) CCL2 protein in the serum of LF, HF, HSV-LF and HSV-HF mice (Diet, P=0.12; Infection, P=0.049; Diet x Infection, P=0.11). Statistical analysis: two-way ANOVA. Letters above columns indicate statistical significance between groups by Tukey-Kramer post hoc test. Columns not sharing a letter differ significantly at P<0.05, n=6/group. Data are presented as mean +/− SEM.
3.8. Obesity and latent HSV-1 increase anxiety-like and repetitive behavior
Collectively, these data show that latent HSV and a HF diet increased microglial activation, CCR2+ macrophage infiltration and proinflammatory cytokine production in regions of the brain associated with anxiety-like behaviors. Therefore, we determined whether HSV-HF mice exhibited increased anxiety-like behaviors in a light-dark preference test and a marble bury assay. Other researchers have reported decreased exploration and increased marble-burying in mouse models of immune system activation and obesity (Andre et al., 2014; Bachis et al., 2016; Malkova et al., 2012; Paris et al., 2014; Pascual et al., 2015). At 3 m p.i., mice were tested by two robust behavior tests for anxiety: light-dark preference and marble burying. In the light-dark preference test anxious mice will spend less time in the lighted side and make fewer crosses between the dark to light side (Bailey and Crawley, 2009). HSV-HF mice spent significantly less time in the light-side. There was a main effect of diet (F(1, 31)F=5.33, p=0.03), but not infection (F(1, 31) F=0.0, p=0.99) and there was no interaction (F(1, 31)F=0.67, p=.41). Post hoc analysis revealed that HSV-HF mice spent less total time on the lighted-side than HSV-LF mice (Fig. 9A). There were differences in the number of transitions made between the dark and light sides. There was a main effect of diet (F(1, 31)F=4.03, p=0.05), but not infection (F(1, 31) F=3.31, p=0.07) and there was no interaction (F(1, 31)F=0.0, p=1.0). Post hoc analysis revealed that only HSV-HF mice had significantly fewer transitions than LF mice (Fig. 9B). Marble burying is a test of both anxiety-like and repetitive behaviors (Thomas et al., 2009). The LF mice buried on average 12 marbles in 30 min, the expected number for mice of the C57Bl/6 strain (Thomas et al., 2009). There was a main effect of infection, but not diet (infection: F(1, 31) F=0.6.9, p=0.014; diet: F(1, 31) F=2.9, p=0.09 and no interaction: F(1, 31) F=2.48, p=0.13). HF diet alone resulted in a trend towards increased marble burying, while, compared to LF mice, HSV-LF and HSV-HF mice buried more marbles, however, there was not a significant difference between the infection groups (Fig. 9C).
Fig. 9.
Alterations in Anxiety behavior in HSV-HF mice compared to LF controls. A–C: Light-Dark Test D: Open Field Test. (A) Total time spent on light side (Diet, P=0.03; Infection, P=0.99; Diet x Infection, P=0.41) (B) # of transitions between dark and light sides (Diet, P=0.05; Infection, P=0.07; Diet x Infection, P=1.0) (C) Number of marbles buried (Diet, P=0.014; Infection, P=0.09; Diet x Infection, P=0.13). Statistical analysis: two-way ANOVA. Letters above columns indicate statistical significance between groups. Columns not sharing a letter differ significantly at P<0.05, n=8/group. Data are presented as mean +/− SEM.
4. Discussion
Our study demonstrates that obesity following HSV-1 latency lead to the potentiation of neuroinflammation including sustained microglial activation and the recruitment of inflammatory CCR2+ monocytes to the hypothalamus and hippocampus. The activated microglia and macrophages are likely the two sources of proinflammatory cytokines that are upregulated in the hippocampus of the HSV-HF mice. The CCR2+ monocytes/macrophages that were present in brain parenchyma of HSV-HF mice are of the M1-phenotype and come from CCR2+/Ly6Chi monocytes in the periphery. HF diet following HSV latency clearing resulted in a unique microenvironment in the brain; VCAM was upregulated and inflammatory monocytes colocalized with the adhesion molecule and CCL2 mRNA is upregulated in the hippocampus. These changes correlated with increased anxiety-like behavior as measured by the Light-Dark test in HSV-HF mice, however HSV-HF, HSV-LF and HF mice exhibited increased anxiety-like/repetitive behaviors as measured by marble burying. For the HSV-LF and HF mice, increased repetitive behavior was seen in the absence of neuroinflammation or cellular infiltration in the hippocampus or hypothalamus (though, there was increase IBA-1 staining in the amygdala of HF mice).
Microglial activation and cytokine production are associated with anxiety behaviors in mice (Block et al., 2007). In this study we found that HSV latency was not sufficient to maintain microglial activation, and while obesity resulted in increased IBA-1 in the amygdala and MHCII and IL-10 expression in the hippocampus, only the combinatorial effects of HSV and HF diet lead to sustained microglial activation and proinflammatory cytokine and cell surface marker expression. Previous studies using the 45% fat lard-based diet have demonstrated that saturated fatty acids can stimulate TLR4 signaling in the hypothalamus and that this upregulation is predominantly found on activated microglia (Milanski et al., 2009). However, in these studies, unpurified chow diet was used as the low fat control diet. The utilization of chow diets as “control” diets are problematic as these diets vary in the amount and type of fat, as well as other nutrients, compared to purified defined diets.
In addition to microglial activation, the infiltration of macrophages in to regions associated with anxiety may play a role in the development of anxiety. This has been shown to occur in some experimental stress paradigms (Wohleb et al., 2014; Wohleb et al., 2013). During HSV-1 infection, Ly6Chi monocytes infiltrate the brain and differentiate into macrophages in order to resolve the infection (Marques et al., 2008; Terry et al., 2012). These cells were not present in the HSV-LF mice, indicating that HF diet results in the retention of the cells or their continued recruitment in HSV-HF mice. While we can conclude that inflammatory bone marrow derived mononuculear cells infiltrate regions associated with anxiety behavior in HSV-HF mice, our current study does not allow us to determine if the HF diet results in the persistence of the macrophages that entered the brain during infection to stay or if it leads to the continual recruitment of CCR2+ cells. The increased circulating monocytes, as well as increased serum and brain CCL2 would suggest continual recruitment of CCR2+ cells to the brain. Additionally, other studies have suggested that macrophages recruited to the brain reduced CD45 expression and become more microglia-like (Ponomarev et al., 2011; Ritzel et al., 2015), again, an advantage of the CCR2RFP/+/CX3CR1GFP/+ mice as CCR2/RFP expression is maintained. In addition, the flow cytometry data suggests that the HSV-HF brain contains a large proportion of CD11b+ cells are CD45hi, suggesting newly recruited cells.
In the absence of latent infection, Buckman et al., demonstrated that HF diet is sufficient to allow macrophages to enter the brain (Buckman et al., 2014). In our study there were a few, sporadic CCR2+ cells in the HF mouse brain, but significantly fewer than in HSV-HF mice. The discrepancy between these studies is likely a result of the need for irradiation of the brain prior to the bone marrow transplant. By utilizing the CCR2RFP/+/CX3CR1GFP/+ mouse model, we were able to avoid manipulation that could potentially alter macrophage infiltration or the inflammatory state of the brain beyond that induced by infection or the HF diet. Several studies have demonstrated that CCR2+ cell infiltration is responsible for damage following ischemia (Hsieh et al., 2014) and experimental autoimmune encephaliomyelitis (Moreno et al., 2014) and of most consequence to our study, lack of CCR2 results in a failure of monocytes/macrophages to traffic to the brain (Wohleb et al., 2013). The inhibition of monocyte/macrophage trafficking to the CNS reverses stress-induced anxiety behaviors (Wohleb et al., 2013). Interestingly, obese CCR2 knockout mice have fewer adipose infiltrating macrophages and are resistant to the metabolic consequences of obesity (Weisberg et al., 2006).
A large body of literature exists that has examined the importance of the CCR2+Ly6Chi monocytes infiltration into the brain during inflammatory events. In general, the infiltration of these cells leads to increased and prolonged inflammation. During neuroinflammatory events, the chemokine CCL2 recruits CCR2+ monocytes that will become proinflammatory macrophages in the brain. In addition to being a significant source of proinflammatory cytokines, M1-like macrophages (from CCR2/Ly6Chi monocytes) produce the enzyme indoleamine-2,3-dioxygenase (IDO) (Murray et al., 2014). IDO diverts tryptophan from serotonin production into a pathway that produces kynurenine and quinolinic acid, both potentially neurotoxic agents (Schwarcz et al., 2012). IDO is expressed by several cells of the immune system, including macrophage and microglia, and neurons. IFN-γ, IL-1, IL-6 and TNF-α induce IDO production. Importantly, macrophages that have been activated to an M1-like/proinflammatory state are significant producers of IDO (Murray et al., 2014). High levels of IDO and the subsequent decrease in tryptophan, serotonin and/or increase in kynureinine have been linked to depression, anxiety and memory impairment in mouse models (Haroon et al., 2012; O’Connor et al., 2009; Salazar et al., 2012). In a model of HF diet with systemic immune activation by LPS, Andre et al. showed that the ratio of kynureinine to tryptophan in the brain was increased with an increase in IDO mRNA on the hippocampus and hypothalamus of obese mice exposed to LPS. There was also a concomitant increase in IFN-γ and TNFα. Finally, these mice exhibited anxiety-like behaviors. As proinflammatory cytokines and IFN-γ are increased in the obese state and during HSV-1 infection, and induce IDO expression, this is a potential target as a mechanism that links HSV-1, obesity and anxiety in our model. Further studies will elucidate the role of IDO in our model.
Independent of infection, HF diets and obesity have been shown to increase anxiety (Andre et al., 2014; Krishna et al., 2015). While HSV-HF diet resulted in increased anxiety-like behaviors in light-dark testing, marble burying was increased by latent infection and HF diet independently. Our data are consistent with other studies showing that high fat diets have increase marble burying in C57BL/6 mice (Krishna et al., 2015). It is possible that there is a ceiling effect in the marble bury test as C57BL/6 mice routinely bury approximately 75% of the marbles (Deacon and Rawlins, 2005; Thomas et al., 2009). This may mean that it is not sensitive enough to determine if the addition of obesity to HSV latency increases the burying behavior. In addition, Thomas et al suggest that marble burying is a better reflection of repetitive behaviors as it does not correlate with the number of transitions between the sides in the light-dark test (Thomas et al., 2009). The neural circuit responsible for repetitive behaviors does not include the hippocampus or hypothalamus, therefore, it is possible that there are changes associated with infection and obesity in areas of the that we did not measure, however, CCR2+ cell infiltration was not present in other areas of the brain (Langen et al., 2011). Future studies may determine the mechanism resulting in increased marble burying.
While there are many strengths of the current study, there are several limitations. While we have mRNA data form the hippocampus, we do not have comparable data from the hypothalamus and amygdala despite showing increased microglial activation in these regions. Additionally, we have not measured IDO nor tryptophan metabolism in this study, however, future studies will evaluate both. Finally, while there has been no direct link between HSV-1 and anxiety in humans, several studies have highlighted the role of HSV-1 in cognitive function, one in the elderly, and one in children. (Katan et al., 2013; Tarter et al., 2013). This epidemiological evidence suggests that HSV-1 infection has a significant impact on brain function throughout the lifespan. We anticipate that as the HSV-HF mice age we will see increased age-related cognitive impairment and an exaggeration of anxiety-like behaviors.
Supplementary Material
Highlights.
Neuroinflammation induced by obesity following HSV-1 infection is investigated
Obesity promotes microglial inflammation following HSV-1 latency
Obesity following HSV-1 latency results in recruitment of CCR2+ monocytes to the brain
Obesity and latent HSV result in increased anxiety-like behavior
Acknowledgments
The authors would like to thank Anais Monroy-Eklund and Betty Wanjiru for their excellent technical support.
This work was supported in part by the National Institutes of Health–supported Nutrition Obesity Research Center Grant (DK56350) and institutional support from University of North Carolina-Chapel Hill College of Arts and Sciences and the School of Medicine (PAS). The Mouse Behavioral Phenotyping Laboratory of the University of North Carolina Intellectual and Developmental Disabilites Research Center is funded by NICHD (U54HD079124). The UNC Confocal and Multiphoton Imaging Core is supported by NINDS Center Grant P30 NS045892 and NICHD Center Grant (U54 HD079124). Institutional support from Sanford Research and NIH support R01NS082283 (JMW).
Footnotes
Conflict of Interest
The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre C, Dinel AL, Ferreira G, Laye S, Castanon N. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav Immun. 2014;41:10–21. doi: 10.1016/j.bbi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Armien AG, Hu S, Little MR, Robinson N, Lokensgard JR, Low WC, Cheeran MC. Chronic cortical and subcortical pathology with associated neurological deficits ensuing experimental herpes encephalitis. Brain Pathol. 2010;20:738–750. doi: 10.1111/j.1750-3639.2009.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Forcelli P, Masliah E, Campbell L, Mocchetti I. Expression of gp120 in mice evokes anxiety behavior: Co-occurrence with increased dendritic spines and brain-derived neurotrophic factor in the amygdala. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KR, Crawley JN. Anxiety-Related Behaviors in Mice. Front Neurosci. 2009:77–101. [PubMed] [Google Scholar]
- Beydoun HA, Dail J, Ugwu B, Boueiz A, Beydoun MA. Socio-demographic and behavioral correlates of herpes simplex virus type 1 and 2 infections and co-infections among adults in the USA. Int J Infect Dis. 2010;14(Suppl 3):e154–160. doi: 10.1016/j.ijid.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD. Early-life infection is a vulnerability factor for aging-related glial alterations and cognitive decline. Neurobiol Learn Mem. 2010;94:57–64. doi: 10.1016/j.nlm.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2--United States, 1999–2010. J Infect Dis. 2014;209:325–333. doi: 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- Buckman LB, Hasty AH, Flaherty DK, Buckman CT, Thompson MM, Matlock BK, Weller K, Ellacott KL. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav Immun. 2014;35:33–42. doi: 10.1016/j.bbi.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RMJ, Rawlins JNP. Hippocampal lesions, species-typical behaviours and anxiety in mice. Behav Brain Res. 2005;156:241–249. doi: 10.1016/j.bbr.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Deleidi M, Isacson O. Viral and inflammatory triggers of neurodegenerative diseases. Sci Transl Med. 2012;4:121ps123. doi: 10.1126/scitranslmed.3003492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Zajacova A, Aiello A. Early origins of health disparities: burden of infection, health, and socioeconomic status in U.S. children. Soc Sci Med. 2009;68:699–707. doi: 10.1016/j.socscimed.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Real JM, Ferri MJ, Vendrell J, Ricart W. Burden of infection and fat mass in healthy middle-aged men. Obesity (Silver Spring) 2007;15:245–252. doi: 10.1038/oby.2007.541. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, Jr, Luchsinger JA. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick CB, Snellman K, Putnam RD. Increasing socioeconomic disparities in adolescent obesity. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1321355110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370:2127–2137. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nature immunology. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- Hill JM, Ball MJ, Neumann DM, Azcuy AM, Bhattacharjee PS, Bouhanik S, Clement C, Lukiw WJ, Foster TP, Kumar M, Kaufman HE, Thompson HW. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J Virol. 2008;82:8230–8234. doi: 10.1128/JVI.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol Aging. 2009;30:1483–1489. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL, Niemi EC, Wang SH, Lee CC, Bingham D, Zhang JS, Cozen ML, Charo I, Huang EJ, Liu JL, Nakamura MC. CCR2 Deficiency Impairs Macrophage Infiltration and Improves Cognitive Function after Traumatic Brain Injury. J Neurotraum. 2014;31:1677–1688. doi: 10.1089/neu.2013.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Burns AJ, Nonneman RJ, Baker LK, Riddick NV, Nikolova VD, Riday TT, Yashiro K, Philpot BD, Moy SS. Behavioral deficits in an Angelman syndrome model: effects of genetic background and age. Behav Brain Res. 2013;243:79–90. doi: 10.1016/j.bbr.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IK, Kim IY, Kim YN, Yi SS, Park IS, Min BH, Doo HK, Ahn SY, Kim YS, Lee IS, Yoon YS, Seong JK. Comparative study on high fat diet-induced 4-hydroxy-2E-nonenal adducts in the hippocampal CA1 region of C57BL/6N and C3H/HeN mice. Neurochem Res. 2009;34:964–972. doi: 10.1007/s11064-008-9846-y. [DOI] [PubMed] [Google Scholar]
- Johnson NB, Hayes LD, Brown K, Hoo EC, Ethier KA Centers for Disease C, Prevention. CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors--United States, 2005–2013. MMWR Suppl. 2014;63:3–27. [PubMed] [Google Scholar]
- Kaczmarczyk MM, Machaj AS, Chiu GS, Lawson MA, Gainey SJ, York JM, Meling DD, Martin SA, Kwakwa KA, Newman AF, Woods JA, Kelley KW, Wang Y, Miller MJ, Freund GG. Methylphenidate prevents high-fat diet (HFD)-induced learning/memory impairment in juvenile mice. Psychoneuroendocrinology. 2013;38:1553–1564. doi: 10.1016/j.psyneuen.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circulation research. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karjala Z, Neal D, Rohrer J. Association between HSV1 seropositivity and obesity: data from the National Health and Nutritional Examination Survey, 2007–2008. PLoS One. 2011;6:e19092. doi: 10.1371/journal.pone.0019092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson EA, Wang S, Shi Q, Coleman RA, Beck MA. Glycerol-3-phosphate acyltransferase 1 is essential for the immune response to infection with coxsackievirus B3 in mice. J Nutr. 2009;139:779–783. doi: 10.3945/jn.108.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasubi MJ, Nilsen A, Marsden HS, Bergstrom T, Langeland N, Haarr L. Prevalence of antibodies against herpes simplex virus types 1 and 2 in children and young people in an urban region in Tanzania. J Clin Microbiol. 2006;44:2801–2807. doi: 10.1128/JCM.00180-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan M, Moon YP, Paik MC, Sacco RL, Wright CB, Elkind MS. Infectious burden and cognitive function: the Northern Manhattan Study. Neurology. 2013;80:1209–1215. doi: 10.1212/WNL.0b013e3182896e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S, Keralapurath MM, Lin Z, Wagner JJ, De la Serre CB, Harn DA, Filipov NM. Neurochemical and Electrophysiological Deficits in the Ventral Hippocampus and Selective Behavioral Alterations Caused by High-Fat Diet in Female C57bl/6 Mice. Neuroscience. 2015;297:170–181. doi: 10.1016/j.neuroscience.2015.03.068. [DOI] [PubMed] [Google Scholar]
- Langen M, Kas MJ, Staal WG, van Engeland H, Durston S. The neurobiology of repetitive behavior: of mice. Neuroscience and biobehavioral reviews. 2011;35:345–355. doi: 10.1016/j.neubiorev.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of Clinical Investigation. 2007a;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007b;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques CP, Cheeran MC, Palmquist JM, Hu S, Urban SL, Lokensgard JR. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J Immunol. 2008;181:6417–6426. doi: 10.4049/jimmunol.181.9.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Breslau J, Green JG, Lakoma MD, Sampson NA, Zaslavsky AM, Kessler RC. Childhood socio-economic status and the onset, persistence, and severity of DSM-IV mental disorders in a US national sample. Soc Sci Med. 2011;73:1088–1096. doi: 10.1016/j.socscimed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A. 2013;110:3573–3578. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Pino PA, Saederup N, Charo IF, Ransohoff RM, Cardona AE. The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. Journal of immunology. 2012;188:29–36. doi: 10.4049/jimmunol.1100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Bannerman P, Ma J, Guo FZ, Miers L, Soulika AM, Pleasure D. Conditional Ablation of Astroglial CCL2 Suppresses CNS Accumulation of M1 Macrophages and Preserves Axons in Mice with MOG Peptide EAE. Journal of Neuroscience. 2014;34:8175–8185. doi: 10.1523/JNEUROSCI.1137-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I, Goshima F, Ito H, Koide N, Yoshida T, Yokochi T, Kimura Y, Nishiyama Y. The vomeronasal chemosensory system as a route of neuroinvasion by herpes simplex virus. Virology. 2005;334:51–58. doi: 10.1016/j.virol.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Mori I, Goshima F, Watanabe D, Ito H, Koide N, Yoshida T, Liu B, Kimura Y, Yokochi T, Nishiyama Y. Herpes simplex virus US3 protein kinase regulates virus-induced apoptosis in olfactory and vomeronasal chemosensory neurons in vivo. Microbes and infection / Institut Pasteur. 2006;8:1806–1812. doi: 10.1016/j.micinf.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MT, Satoh H, Favelyukis S, Babendure JL, Imamura T, Sbodio JI, Zalevsky J, Dahiyat BI, Chi NW, Olefsky JM. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. The Journal of biological chemistry. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology. 2014;231:2349–2360. doi: 10.1007/s00213-013-3385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Balino P, Aragon CMG, Guerri C. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: Role of TLR4 and TLR2. Neuropharmacology. 2015;89:352–359. doi: 10.1016/j.neuropharm.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat Med. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110:16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Patel AR, Grenier JM, Crapser J, Verma R, Jellison ER, McCullough LD. Functional differences between microglia and monocytes after ischemic stroke. Journal of neuroinflammation. 2015;12:106. doi: 10.1186/s12974-015-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL, Ransohoff RM, Charo IF. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PloS one. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar A, Gonzalez-Rivera BL, Redus L, Parrott JM, O’Connor JC. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm Behav. 2012;62:202–209. doi: 10.1016/j.yhbeh.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki CM, Mckim DB, Wohleb ES, Jarrett BL, Reader BF, Norden DM, Godbout JP, Sheridan JF. Social Defeat Promotes a Reactive Endothelium in a Brain Region-Dependent Manner with Increased Expression of Key Adhesion Molecules, Selectins and Chemokines Associated with the Recruitment of Myeloid Cells to the Brain. Neuroscience. 2015;302:151–164. doi: 10.1016/j.neuroscience.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan PA, Beck MA. The immune response to herpes simplex virus encephalitis in mice is modulated by dietary vitamin E. J Nutr. 2008;138:130–137. doi: 10.1093/jn/138.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivkumar M, Milho R, May JS, Nicoll MP, Efstathiou S, Stevenson PG. Herpes simplex virus 1 targets the murine olfactory neuroepithelium for host entry. J Virol. 2013;87:10477–10488. doi: 10.1128/JVI.01748-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly DT, Hennessy E, Dansereau MA, Cunningham C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1beta, [corrected] TNF- alpha and IL-6 challenges in C57BL/6 mice. PLoS One. 2013;8:e69123. doi: 10.1371/journal.pone.0069123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–1243. doi: 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, Kamei Y, Ogawa Y. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- Sultan FA. Dissection of Different Areas from Mouse Hippocampus. Bio-protocol. 2013;3:e955. doi: 10.21769/bioprotoc.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Mizuarai S, Araki H, Mashiko S, Ishihara A, Kanatani A, Itadani H, Kotani H. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J Biol Chem. 2003;278:46654–46660. doi: 10.1074/jbc.M309895200. [DOI] [PubMed] [Google Scholar]
- Tarter KD, Simanek AM, Dowd JB, Aiello AE. Persistent Viral Pathogens and Cognitive Impairment Across the Life Course in the Third National Health and Nutrition Examination Survey. J Infect Dis. 2014;209:837–44. doi: 10.1093/infdis/jit616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeling JL, Perry VH. Systemic Infection and Inflammation in Acute Cns Injury and Chronic Neurodegeneration: Underlying Mechanisms. Neuroscience. 2009;158:1062–1073. doi: 10.1016/j.neuroscience.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Terry RL, Getts DR, Deffrasnes C, van Vreden C, Campbell IL, King NJ. Inflammatory monocytes and the pathogenesis of viral encephalitis. Journal of neuroinflammation. 2012;9:270. doi: 10.1186/1742-2094-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Agriculture, A.R.S. Nutrient Intakes from Food: Mean Amounts and Percentages of Calories from Protein, Carbohydrate, Fat, and Alcohol, One Day, 2005–2006. 2008 http://www.ars.usda.gov/ba/bhnrc/fsrg.
- Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell reports. 2014;9:2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van der Valk P, Amor S, Teunissen CE, van Horssen J, Dijkstra CD. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. Journal of neuroinflammation. 2013;10:35. doi: 10.1186/1742-2094-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. Journal of Clinical Investigation. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LL, Chao A, Bilbo SD. Environmental enrichment alters glial antigen expression and neuroimmune function in the adult rat hippocampus. Brain Behav Immun. 2012;26:500–510. doi: 10.1016/j.bbi.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP. Re-establishment of Anxiety in Stress-Sensitized Mice Is Caused by Monocyte Trafficking from the Spleen to the Brain. Biol Psychiat. 2014;75:970–981. doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.










