Abstract
Background:
Hypoxia and high altitudes affect various organs, which impairs important physiological functions, such as a disruption of the intestinal barrier mediated by increased translocation of bacteria and increased circulating endotoxin levels. Physical exercise can alter endotoxin concentration in normoxia. The aim of this study is to evaluate the effects of moderate exercise on endotoxin concentration in normobaric hypoxia.
Methods:
Nine healthy male volunteers exercised on a treadmill for 60 minutes at an intensity of 50% VO2peak in normoxic or hypoxic conditions (4200 m). Blood was collected at rest, immediately after exercise and 1 hour after exercise to evaluate serum endotoxin levels.
Results:
Under hypoxic exercise conditions, SaO2% saturation was lower after exercise compared with resting levels (P < 0.05) and returned to the resting level during recovery in normoxia (P < 0.05). Endotoxin concentration increased after exercise in hypoxia (P < 0.05); it remained high 1 hour after exercise in hypoxia compared with normoxia (P < 0.05) and was higher after exercise and recovery compared with resting levels (P < 0.05). HR was higher during exercise in relation basal in both conditions (P < 0.05) and RPR increase after 60 minutes in comparison to 20 minutes in hypoxia (P < 0.05).
Conclusion:
Moderate exercise performed in hypoxia equivalent to 4200 m increased endotoxin plasma concentration after exercise. One hour of rest in normoxic conditions was insufficient for the recovery of circulating endotoxins.
Keywords: enterocytes, glutamine, high altitude, inflammation, intestinal barrier, moderate exercise
1. Introduction
Hypoxia and high altitudes affect various organs, impairing important physiological functions. A recent study showed that rodents exposed to hypoxia equivalent to 7000 m for 72 hours exhibit an intestinal barrier disruption mediated by an increased translocation of bacteria and increased circulating endotoxin levels;[1] this fact suggests that impaired gastrointestinal functions can contribute to inflammation observed in systemic hypoxia. However, little is known about the effects of hypoxia at approximately 4000 m, an altitude more often frequented by humans.
Chronic moderate physical exercise promotes a decrease in the concentration of endotoxin and regulates the pro/anti-inflammatory balance, partially reversing the inflammation that occurs in obesity.[2] Moreover, acute and strenuous exercise can disrupt the integrity of the intestinal epithelium[3,4] and increase intestinal permeability, that is, the endotoxin concentration that stimulates pro-inflammatory cytokine production.[5] However, the effects of acute moderate exercise on endotoxin concentration in hypoxic conditions are unknown.
The aim of this study is to evaluate the effects of moderate exercise on the concentration of endotoxin in hypoxia similar to an altitude of 4200 m. It has been hypothesized that exercise in hypoxia promotes a greater increase in endotoxin than exercise in normoxia because hypoxia adds additional stress to the body.
2. Materials and methods
2.1. Volunteers
The experimental protocol was approved by the ethics committee of the Federal University of São Paulo (#0620/09). All subjects were informed of the aims and risks of the study. The sample consisted of 9 healthy male volunteers with the following mean characteristics: 24.4 ± 3.8 years of age; 173.2 ± 45.8 cm height; 71.2 ± 12.7 kg weight; 23.5 ± 3.7 kg/m2 BMI; and VO2peak 46.9 ± 5.6 mL/kg/min. All volunteers were physically active and participated in aerobic training for at least 3 days a week for the previous 6 months. Noninclusion criteria included the presence of cardiovascular pathologies or other diseases diagnosed during the clinical evaluation that interfered with the response to exercise or study results. The sample size calculation was performed using the statistical G ∗ Power program, version 3.0, establishing a moderate effect size (d = 0.25), explanatory power (Power) of 0.80, and a level α equal 0.05. Based on this, a minimum sample size of 10 subjects was necessary.
2.2. Experimental design
All volunteers visited the laboratory 3 times with a 7-day interval between each visit. On the first visit, subjects were submitted to a progressive treadmill test to determine VO2peak in normoxia. On the second and third visits, after 4 hours fasting and 7 days from the incremental test, the subjects exercised on a treadmill for 60 minutes at an intensity of 50% VO2peak in normoxic or hypoxic conditions (simulating altitudes of up to 4200 m) in a CAT (Colorado Altitude Training/12 CAT-Air Unit) normobaric chamber with an FiO2 of 13.5% during exercise. The 9 volunteers were randomized and perform exercise in normoxic and hypoxic conditions. After exercising, the volunteers remained in normoxic conditions for 1 hour.
2.3. VO2peak determination
Peak oxygen consumption (VO2peak) was determined using an incremental exercise test on a treadmill and inclination of 1%. The initial velocity was 6.0 km/h, and the speed was increased by 1.0 km/h in 1-minute intervals to voluntary exhaustion. Respiratory and metabolic variables were obtained using a metabolic system (COSMED PFT4, Rome, Italy) to measure gaseous respiratory exchanges.
2.4. Blood collection and endotoxin concentration
Blood samples were collected from an antecubital vein at rest (normoxic condition), immediately after exercise (in a normoxic or hypoxic condition) and 1 hour after exercise. The recovery period after the exercise was performed under normoxia during 60 minutes. Five milliliters of blood were immediately allocated into two 5 mL Vacutainer tubes (Becton Dickinson, Franklin Lakes, New Jersey, USA) for serum separation. The tubes were centrifuged at 2500 × g for 12 minutes at 4°C, and serum samples were stored at −80°C until analysis. Endotoxin concentration was assayed using ELISA commercial kits (MyBioscience, San Diego, CA). Samples were analyzed in duplicate and coefficient of variation between the duplicate was 3.7%.
2.5. Hemoglobin O2 saturation (SaO2%)
This parameter was measured with a finger oximeter (FingerPulse®, Beijing, China).
2.6. Rating of perceived exertion (RPE)
The perceived exertion was assessed every 20 minutes during exercise and performed by a Subjective Scale of Perceived Exertion ranging from 6 to 20.[6]
2.7. Heart rate (HR)
HR was measured every 20 minutes during exercise and performed using the Frequencymeter Telemetry Apparatus (Polar, Advantage Model NV, Kempele, Finland).
2.8. Statistical analyses
The data are reported as the mean and standard deviation. Data distribution was evaluated using the Shapiro–Wilk test. The differences between moments were assessed by a 2-way ANOVA with repeated measures and a Tukey post hoc test. Statistical analysis was carried out using GraphPad Prism (version 5.0) software; the significance level was set at P <0.05.
3. Results
In relation to SaO2% there was a significant difference in interaction (F = 8.107 P = 0.001), and time point (F = 6.936 and P = 0.002). SaO2% saturation decreased after exercise in relation to rest (93.1 ± 3.8 vs 97.0 ± 1.0%). However, in hypoxic conditions, SaO2% saturation increased after a 1 hour recovery after exercise (97.3 ± 0.8 vs 93.1 ± 3.8%) (Fig. 1). About endotoxin there was a significant difference in the time point (F = 7.765 and P = 0.001) and trial condition (F = 21.7 and P < 0.001). In addition, endotoxin was higher after exercise in hypoxia compared with normoxia (7.7 ± 0.4 vs 5.3 ± 1.9 EU/mL); endotoxin concentration remained high 1 h after exercise in hypoxia compared with exercise in normoxia (7.0 ± 0.8 vs 5.1 ± 1.7 EU/mL). Additionally, endotoxin was high during exercise in hypoxia (7.7 ± 0.4 EU/mL) and recovery (7.0 ± 0.8 EU/mL) compared with base levels (5.2 ± 0.8 EU/mL) (Fig. 2). There was no significant correlation between SaO2% and endotoxin concentration at rest (r = 0.363, P = 0.51), after exercise (r = 0.115, P = 0.23) or 1 hour after exercise (r = 0.239, P = 0.51). HR during the exercise is shown in Fig. 3. There is not difference in interaction (F = 3.5 and P = 0.08) and difference in time point (F = 102.1 and P <0.001). We note increase during the exercise compared with resting values (P < 0.001), but there was no difference between normoxic and hypoxic conditions. The RPE is shown in Fig. 4. There is no difference in interaction (F = 3.565 and P = 0.07) and difference in time point (F = 4.177 and P = 0.02). We found increase on RPE at 60 minutes compared to 20 minutes (P = 0.02) in hypoxia condition, but there was no difference between normoxic and hypoxic conditions (Fig. 4).
Figure 1.
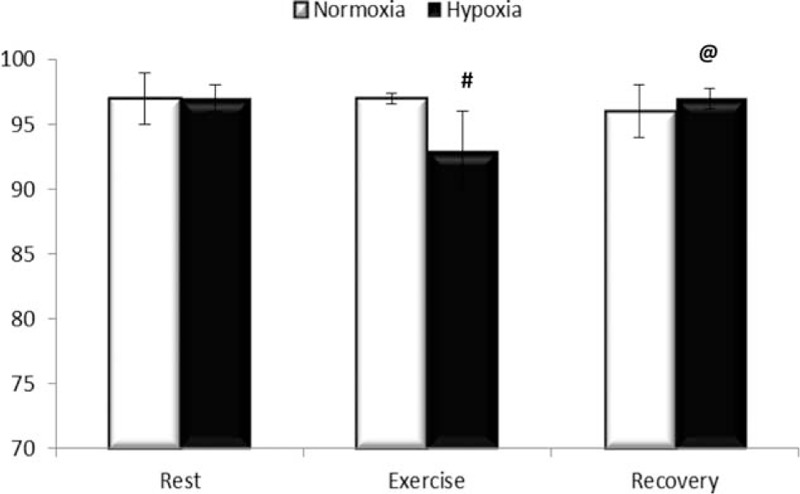
SaO2% in hemoglobin. Results are expressed in % saturation in normoxic condition (white bars) and hypoxia (black bar) at rest (Rest), immediately after exercise (Exercise), and 1 h after exercise (Recovery). Results are expressed in media ± standard deviation for 9 voluntaries. # Different in relation to rest and @ different compared with exercise, P <0.05.
Figure 2.
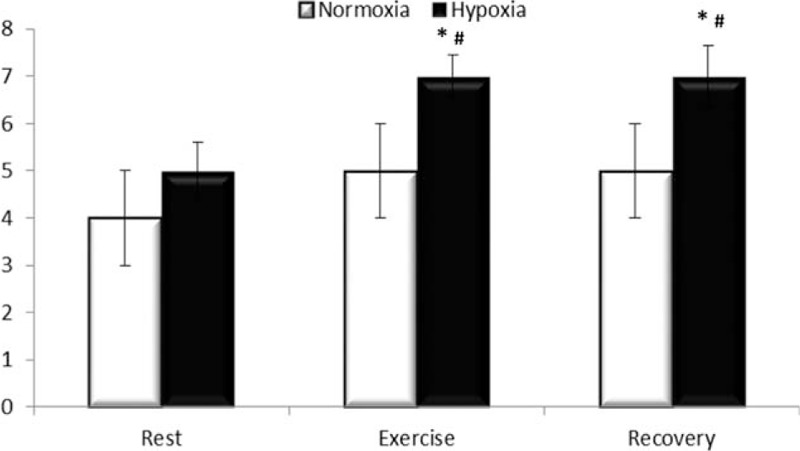
Endotoxin concentration. Results are expressed in EU/mL in normoxic condition (white bars) and hypoxia (black bar) at rest (Rest), immediately after exercise (Exercise) and 1 h after exercise (Recovery). Results are expressed in media ± standard deviation for 9 voluntaries. ∗ Different compared with normoxia, # different compared with rest, P <0.05.
Figure 3.
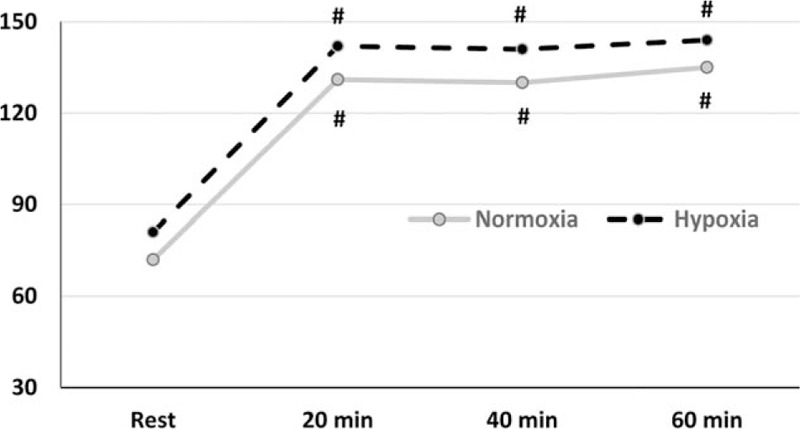
Heart rate. Results are expressed in beat per minute (BPM) in normoxic condition (gray line) and hypoxia (black line) at rest (Rest), 20, 40, and 60 min during exercise. Results are expressed in media for 9 voluntaries. # Different in relation to rest, P <0.05.
Figure 4.
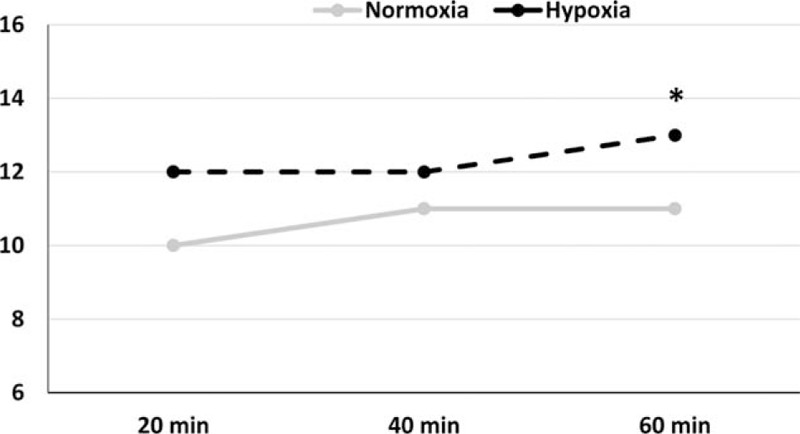
Rating of perceived exertion (RPE). Results are arbitrary units in normoxic condition (gray line) and hypoxia (black line) at 20, 40, and 60 min during exercise. Results are expressed in media for 9 voluntaries. ∗ Different in relation to 20 min, P <0.05.
4. Discussion
High altitude and hypoxia promote the increased translocation of bacteria and endotoxin to the blood.[1] Similarly, exercise can increase endotoxaemia depending on the intensity and duration of the task.[3,4] This is the first study to evaluate the effects of exercise in hypoxia on endotoxin concentration. The main finding is that moderate exercise in hypoxia increases endotoxin concentration, and 1 hour of rest is insufficient for recovery. Conversely, exercise in normoxia does not promote changes in endotoxin concentration.
Previous studies have suggested that exposure to 4200 m is sufficient to promote reduction of SaO2%.[7,8] In fact, there was no difference in SaO2% when exercise was performed in normoxic conditions; however, exercise in hypoxia decreased SaO2% in hemoglobin. This finding is in accordance with a previous study,[7] which informed the hypoxic condition used in this study. One hour of recovery in normoxia reversed the decline in SaO2% that occurred during exercise in hypoxia, suggesting that the impairment of O2 transport due to hypoxia was reversed in the presence of O2.
Exercise at 50% VO2peak for 1 hour can be considered moderate and was not sufficiently intense to promote any change in endotoxin concentration. In hypoxia, we found high endotoxin concentrations, confirming the hypothesis that hypoxia may be an additional stressor for those who perform exercise in high altitudes because of several mechanisms, including oxidative stress, tissue damage, and inflammation.[9,10] Interestingly, despite 1 hour being enough time to recover SaO2%, it was not enough time to reverse the higher endotoxin concentration, demonstrating the need for more time for full recovery. One could speculate that the increase in endotoxin concentration contributes to the increased migration of macrophages to the gastrointestinal tract, resulting in increased pro-inflammatory cytokines and GI disturbances including nausea, diarrhea, and bleeding.
The reasons for this increase in endotoxin are unknown. Recent studies have shown an increase of endotoxin concentration after marathon running, which is likely due to heat stress and mucosal ischemia[4] while others have suggested the influence of temperature change promote by exercise.[11,12] However, despite the effects of hypoxia on exercise intensity, 1 hour of exercise at 50% VO2peak cannot be characterized as exhaustive because it is not nearly as strenuous as exercise such as marathon running. Despite being subject to the same absolute intensity, it has been proposed that overload exercise in hypoxia would be greater than in normoxia condition. However, that was not demonstrated in our results about HR and RPE. They do not confirm this hypothesis because HR was not different between the two conditions during exercise, suggesting that the stress by the exercise in hypoxia and normoxia was similar. In addition, the RPE showed moderate values for the exercise in both conditions. The RPE is an important tool to indicate the feeling of tiredness or weakness because it relates individual physiological and psychological aspects within the same scale.[13]
On the other hand, exposure to a hypoxic environment can lead to damage to the intestinal barrier by increasing apoptosis of enterocytes and contributing to an increased bacterial translocation and the release of endotoxins into the blood stream. Therefore, it is possible that in our study, an increase in endotoxin concentration after exercise is mediated by gut wall damage.[11,14] Additionally, the correlation between endotoxin concentration and SaO2% saturation does not suggest a relationship between impaired O2 transport and endotoxins in our model, possibly because exposure time was only 1 hour.
5. Conclusion
We conclude that even moderate exercise performed in hypoxia that was equivalent to 4200 m increased endotoxin plasma concentration, and 1 hour of rest in normoxia was insufficient for the recovery of circulating endotoxin levels. Due to the important inflammatory role developed by endotoxin turther studies are needed to understand the significance of this increase and the time required for the recovery to resting values.
Footnotes
Abbreviations: BMI = body mass index, cm = centimeters, FiO2 = fractions of inspired oxygen, HR = heart rate, kg = kilogram, km = kilometer, m = meters, min = minutes, mL = milliliters, RPE = rating of perceived exertion, SaO2 = hemoglobin O2 saturation, VO2peak = peak oxygen consumption.
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), and Associação Fundo de Incentivo à Psicofarmacologia (AFIP). Financial support: FAPESP: 2013/01324-4.
The authors have no conflicts of interest to disclose.
References
- [1].Zhou QQ, Yang DZ, Luo YJ, et al. Over-starvation aggravates intestinal injury and promotes bacterial and endotoxin translocation under high-altitude hypoxic environment. World J Gastroenterol 2011;17:1584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lira FS, Rosa JC, Pimentel GD, et al. Long-term interdisciplinary therapy reduces endotoxin level and insulin resistance in obese adolescents. Nutr J 2012;18:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dokladny K, Zuhl MN, Moseley PL. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J Appl Physiol 2016;120:692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gill SK, Teixeira A, Rama L, et al. Circulatory endotoxin concentration and cytokine profile in response to exertional-heat stress during a multi-stage ultra-marathon competition. Exerc Immunol Rev 2015;21:114–28. [PubMed] [Google Scholar]
- [5].Mazzeo RS. Altitude, exercise and immune function. Exerc Immunol Rev 2005;11:6–16. [PubMed] [Google Scholar]
- [6].Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;5:377–81. [PubMed] [Google Scholar]
- [7].Santos SA, Silva ET, Caris AV, et al. Vitamin E supplementation inhibits muscle damage and inflammation after moderate exercise in hypoxia. J Hum Nutr Diet 2016;29:516–22. [DOI] [PubMed] [Google Scholar]
- [8].Chapman RF, Stager JM, Tanner DA, et al. Impairment of 3000-m run time at altitude is influenced by arterial oxyhemoglobin saturation. Med Sci Sports Exerc 2011;43:1649–56. [DOI] [PubMed] [Google Scholar]
- [9].Ermolao A, Travain G, Facco M, et al. Relationship between stress hormones and immune response during high-altitude exposure in women. J Endocrinol Invest 2009;32:889–94. [DOI] [PubMed] [Google Scholar]
- [10].Walsh NP, Gleeson M, Pyne DB, et al. A position statement. Part two: maintaining immune health. Exerc Immunol Rev 2011;17:64–103. [PubMed] [Google Scholar]
- [11].Sessions J, Bourbeau K, Rosinski M, et al. Carbohydrate gel ingestion during running in the heat on markers of gastrointestinal distress. Eur J Sport Sci 2016;16:1064–72. [DOI] [PubMed] [Google Scholar]
- [12].Gill SK, Allerton DM, Ansley-Robson P, et al. Does short-term high dose probiotic supplementation containing lactobacillus casei attenuate exertional-heat stress induced endotoxaemia and cytokinaemia? Int J Sport Nutr Exerc Metab 2016;26:268–75. [DOI] [PubMed] [Google Scholar]
- [13].Duke JW, Lane AR, Behr MB, et al. Exercise training biomarkers: influence of short-term diet modification on the blood lactate to rating of perceived exertion (La:RPE) ratio. Acta Physiol Hung 2011;98:128–36. [DOI] [PubMed] [Google Scholar]
- [14].Bosenberg AT, Brock-Utne JG, Gaffin SL, et al. Strenuous exercise causes systemic endotoxemia. J Appl Physiol 1998;65:106–8. [DOI] [PubMed] [Google Scholar]


