Abstract
This multicenter clinical trial was conducted to examine current practice of benign epilepsy with centrotemporal spikes and especially address the question that in what circumstances 1 antiepileptic drug (AED) should be preferred.
Twenty-five medical centers participate in this clinical trial. The general information, clinical information, and treatment status were collected under the guidance of clinicians and then analyzed. Difference between different treatment groups was compared, and usefulness of the most commonly used AEDs was evaluated.
A total of 1817 subjects were collected. The average age of the subject was 8.81 years. The average age of onset is 6.85 years (1–14 years). Male-to-female ratio is 1.13:1. A total of 62.9% of the patients are receiving monotherapies, and 10.6% are receiving multidrug therapy. Both age and course of disease of treated rolandic epilepsy (RE) patients are significantly different from those of untreated patients. Bilateral findings on electroencephalography (EEG) are less seen in patients with monotherapy compared with patients with multidrug therapy. Except for 25.4% patients not taking any AEDs, oxcarbazepine (OXC), sodium valproate (VPA), and levetiracetam (LEV) are the most commonly used 3 AEDs. VPA and LEV are commonly used in add-on therapy. OXC and LEV are more effective as monotherapy than VPA.
Age of onset of Chinese RE patients is 6.85 years. Bilateral findings on EEG could be a risk factor to require multidrug therapy. In Chinese patients, OXC, VPA, and LEV are most commonly used AEDs as monotherapy and OXC and LEV are more effective than VPA.
Keywords: antiepileptic drugs, BECTS, epilepsy
1. Introduction
Benign epilepsy with centrotemporal spikes (BECTS) is inherited childhood idiopathic focal epilepsy characterized by focal onset seizures (mostly partial motor seizures) and subtle cerebral structural abnormalities. BECTS accounts for 8% to 23% of childhood onset epilepsy, and the male-to-female ratio is 6:4. The age of seizure onset is from 3 to 13 years, and the peak frequency occurs between 8 and 10 years. Seizure usually begins with unilateral tongue and perioral paresthesia, followed by facial tonic and/or clonic activity that may spread to the upper limb mostly during sleep. Age also significantly affects clinical course. Although some of the patients may exhibit minor cognitive and behavioral abnormalities, most of the abnormalities, including seizures, undergo long-lasting remission during adolescence, as revealed by electroencephalography (EEG) results. Thus, children with BECTS can avoid antiepileptic drug (AED) treatment. Small proportions of patients are resistant to AED treatment and may even progress to epileptic encephalopathy. “Idiopathic” indicates that the etiology of a condition is poorly understood; however, BECTS is highly related to inheritance. Approximately, 9% to 59% of patients with BECTS report a positive family history of seizures; chromosome 15q14 and N-acetylcholine receptor 27 subunit have been reported as suspicious causes.
There are widespread historical and geographical divergences in practice for BECTS patients.[1–4] Textbooks and expert suggestions advise no antiepileptic drugs in BECTS treatment.[5,6] Relationship between EEG spikes and cognitive disorders are still unresolved. Wolff et al[7] reported that cognitive deficits may be associated with spike location, but not with spike rate in 20 children with IFE. Overvliet et al[8] suggest the existence of relationship between cognitive deficits and nocturnal epileptiform discharges in a comprehensive review. The relationship could be explained by disturbance of functional connectivity responsible for the corresponding cognitive disorders.[9,10] However, Nicolai et al[11] reported that the correlation between cognitive deficits and locations of EEG is not conclusive. According to the above-mentioned evidence, the benign nature has been reconsidered allowing for the learning and memory impairment and regional function disruption that could be associated with focal EEG spikes.[12,13] Thus, the discrepancies of treatment versus nontreatment still exist and the evidence for favorable AEDs in practice is still unknown. Studies with large sample size of Chinese children with BECTS have yet to be performed. In our study, clinical information was collected from 2234 patients admitted to multiple medical centers, including 14 institutions in north China and 12 institutions in south China. We aim to examine current practice of BECTS and demonstrate what proportion of patients is not treated. We specially address the question in what circumstances 1 AED should be preferred to help establish comprehensive management of BECTS.
2. Methods
2.1. Study population
We collected information regarding the children diagnosed with BECTS from 25 pediatric neurological centers across China from September 2011 to January 2014. Inclusion criteria were as follows: patients diagnosed according to the International League Against Epilepsy (1989) diagnostic criteria of BECTS[14]; patients whose EEG results (ictal and interictal) showed positive- and negative-spike slow complex waves and sharp slow complex waves from unilateral or bilateral middle temporal, posterior temporal, and central regions with arrhythmic interictal activity; and patients whose EEG results revealed these waves sometimes accompanied with sharp spikes in other regions.[15] Complete clinical data were available. All of the patients completed the provided participant consent forms, and this study has been approved by the local ethics committee of PLA general hospital. Exclusion criteria were as follows: patients exhibiting nonepileptic seizure and patients suffering from other systematic diseases and receiving related medications.
2.2. Data collection
The following information must be provided by the patients: neurological examination, head magnetic resonance, long-range sleep EEG, and other pertinent data. The patients and their parents completed the corresponding general information forms and informed consent forms under the guidance of clinicians. The following general information was obtained: sex , age of data collection, age of onset, family history, diagnosis, and treatment status. Monotherapy was defined as treatment with 1 AED; add-on therapy was described as treatment with additional AEDs to enhance the therapeutic effects; multidrug therapy was defined as simultaneous treatment with >2 AEDs. Radiographic abnormalities were classified into intracranial cyst, subtle abnormalities in cerebral ventricle, and mild abnormalities in white matter on the basis of magnetic resonance imaging findings. EEG manifestation was classified into unilateral and bilateral epileptiform discharge on the basis of the results of long-range sleep EEG. Course of disease is defined as duration since diagnosis.
2.2.1. Consensus process for EEG and MR evaluation
Every patient should undergo MR and EEG examinations. MR images and EEG results are allocated to radiologists or neurologists selected from junior panel and experienced panel, both. They all have formal radiologists or neurologists’ qualification certificate and evaluate MR and EEG results based on medical records. Disagreements across radiologists and neurologists will be discussed and consensus achieved in all cases.
2.3. Data analysis
Population characteristics and clinical features were analyzed. The general information and clinical features of the treated patients were compared with those of the untreated patients. The general information and clinical features of the patients subjected to monotherapy were compared with those of the patients treated with multidrug therapy. The components of AEDs used for monotherapy and add-on therapy were analyzed. The commonly used AEDs for multidrug therapy were evaluated.
2.4. Statistical analysis
For continuous variables, results were described as mean ± standard deviation. Student t test and Kruskal–Wallis test were performed to assess the differences. For categorical variables, differences were evaluated through χ2 test. All analyses were conducted in SPSS 19.0 (SPSS Inc, Chicago, IL). The recorded P values were 2-sided, and P values of <0.05 were considered statistically significant.
3. Results
3.1. Population characteristics
Among the 2234 patients diagnosed with BECTS from 25 centers, 417 were excluded because of nonstandard information and 1817 were included for further analysis. Their general information is listed in Table 1. The age of data collection is 8.81 ± 2.7 years (2–19 years). The age of onset is 6.85 ± 2.45 years (1.92–14 years). Among the patients, 963 are males and 854 are females. The male-to-female ratio is 1.13:1. The age of data collection among male patients is 8.85 ± 2.69 years, and the age of data collection among female patients is 8.77 ± 2.7 years. The ages of onset in male and female patients are 6.89 ± 2.46 and 6.73 ± 2.77 years, respectively. The age of onset does not significantly differ between male and female patients (t = 1.017, P = 0.309). The family histories of 179 (9.9%) patients are positive. Mild radiographic abnormalities are found in 62 (3.4%) patients, but they are not related to BECTS. Of these patients, 20 exhibit intracranial cyst, 14 show mild abnormalities in the cerebral ventricle, and 20 manifest abnormalities in the white matter (Fig. 1). According to long-range sleep EEG reports from 1253 (69%) patients, unilateral discharge occurs in the Rolandic region in 889 (70.1%) patients, bilateral discharge exists in the Rolandic region in 364 (29.0%) patients, and electrical status epilepticus in sleep (ESES) manifests in 28 (2.23%) patients.
Table 1.
General information and population characteristics.
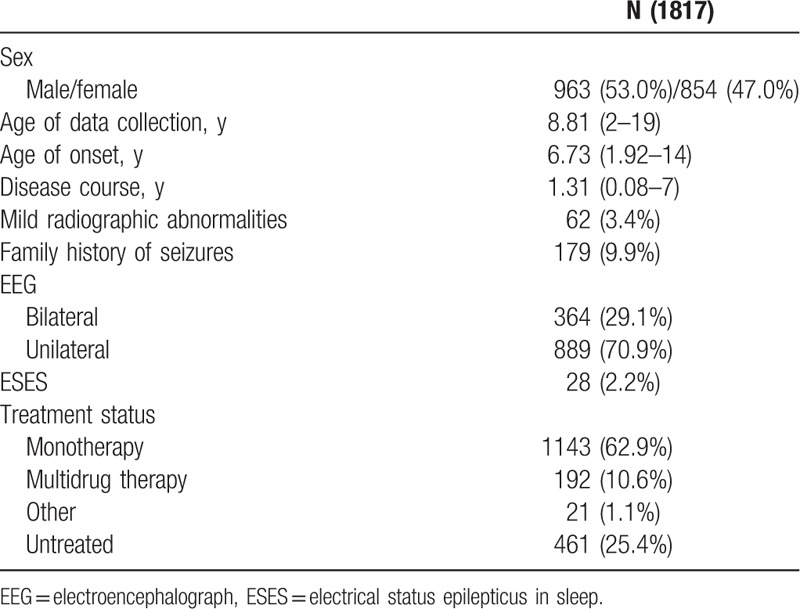
Figure 1.
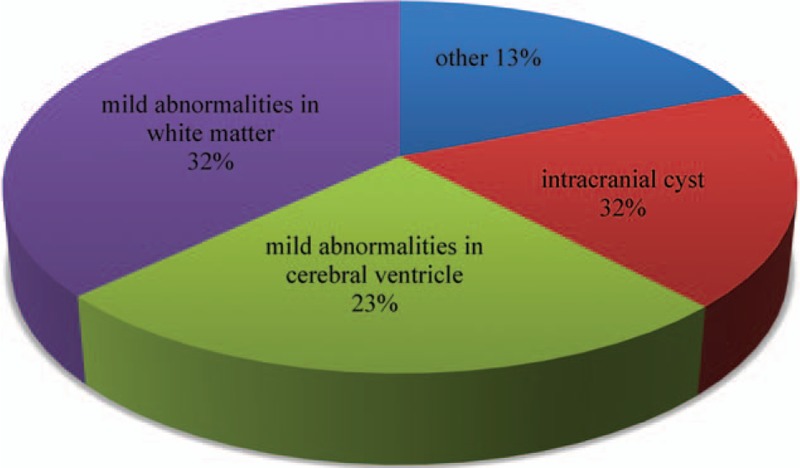
Component of radiographic abnormalities in 62 patients.
3.2. Treatment status
Among the 1817 patients, 416 (25.4%) did not receive any AED, 1143 (62.9%) received monotherapy, 192 (10.6%) underwent multidrug therapy, and 66 (3.63%) received irregular medication (Fig. 2).
Figure 2.
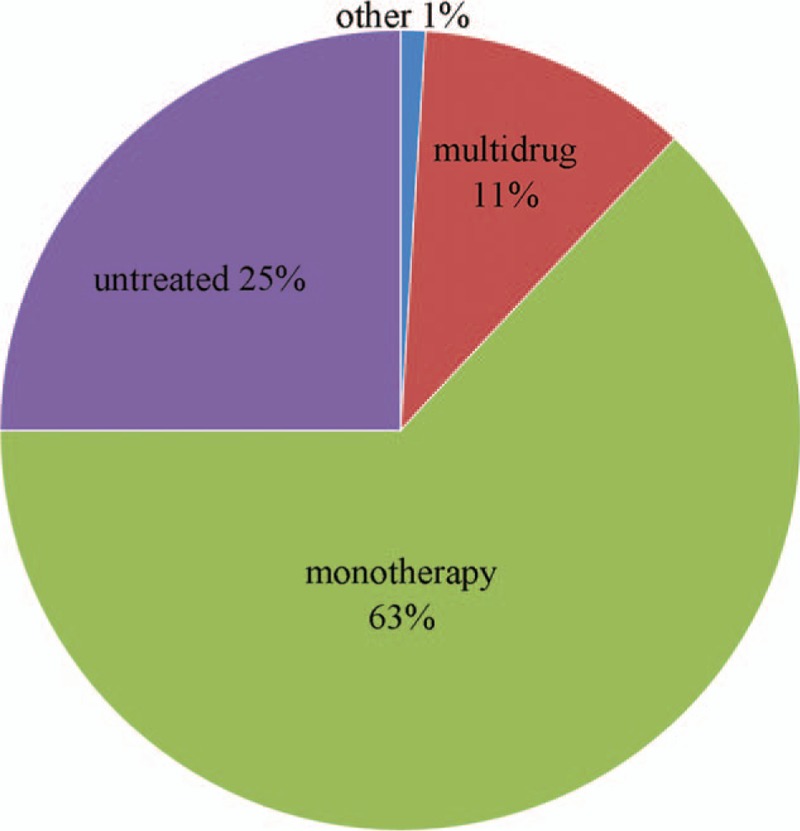
Treatment status of rolandic epilepsy patients.
3.3. Difference between our study and foreign studies on demographic features and treatment status
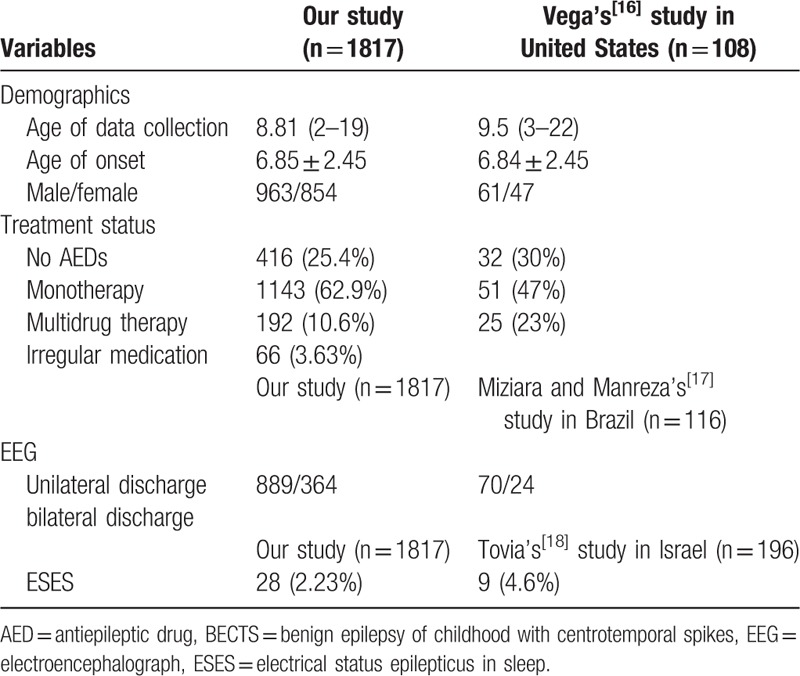
The above-mentioned results about demographic features and treatment status are compared with foreign studies (conducted in America,[16] Brazil,[17] and Israel[18]) to see whether our results support the previous studies or not, and the comparison is shown in Table 2.
Table 2.
Comparison of studies on BECTS in children conducted in China and foreign countries.
3.4. Difference in clinical features between treated and untreated patients
Of the 466 untreated (25.4%) patients, 252 are males and 209 are females. Of the 1356 (74.6%) treated patients, 711 are males and 645 are females. The treated patients significantly differed from the untreated patients (χ2 = 0.678, P = 0.407). Of the untreated patients, 50 reported a positive family history of seizures and 411 described a negative family history. Of the treated patients, 129 showed a positive family history and 1227 displayed a negative family history. The family history of seizures did not significantly differ between the 2 groups (χ2 = 0.688, P = 0.407). The numbers of the patients who manifest abnormalities in radiographic findings of the untreated and treated groups are 14 and 53, respectively, but these numbers did not significantly differ (χ2 = 0.736, P = 0.391). Of the 286 untreated patients with EEG results, 196 and 90, respectively, manifested unilateral and bilateral discharges, as revealed by ictal sleep EEG. Of the 967 treated patients with EEG results, 693 and 274, respectively, experienced unilateral and bilateral discharges. Despite these findings, the two groups did not significantly differ in terms of EEG results (χ2 = 1.051, P = 0.305). The ages of onset in the untreated and treated patients are 6.73 ± 2.38 and 6.80 ± 2.48 years, respectively, but this finding did not significantly differ between the 2 groups (t = 0.555, P = 0.579). The ages of data collection in the untreated and treated patients are 6.47 ± 2.47 and 9.58 ± 2.30 years, respectively, and these results significantly differed between the 2 groups (t = 24.461, P < 0.001). The courses of disease are 1.31 ± 1.82 and 2.24 ± 2.11 years in the untreated and treated patients, respectively. These findings also significantly differed between the 2 groups (t = 8.377, P < 0.001; Table 3).
Table 3.
Clinical features between treated and untreated patients.
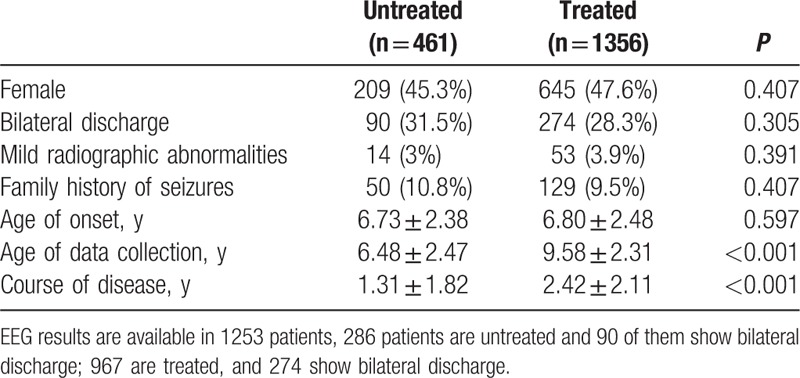
3.5. Difference between monotherapy and multidrug therapy
Of the total number of patients, 1143 received monotherapy and 192 underwent multidrug therapy. The monotherapy group comprises 602 male and 541 female patients. The multidrug therapy group includes 102 males and 90 females. The sex composition of the 2 groups did not significantly differ (χ2 = 0.014, P = 0.907). The family history of seizures is positive in 111 patients and negative in 1129 patients in the monotherapy group. This parameter is positive in 17 patients and negative in 175 patients in the multidrug therapy group. Nevertheless, the family history of seizures did not significantly differ between the 2 groups (χ2 = 0.139, P = 0.709). Of the patients with abnormalities in radiographic results, 39 and 14 belong to the untreated and treated groups, respectively, but this finding did not significantly differ between the 2 groups (χ2 = 6.49, P = 0.011). Of the 806 monotherapy patients whose EEG results are available, 588 show unilateral discharge and 218 exhibit bilateral discharges. Of the 146 multidrug therapy patients whose EEG results are available, 93 and 53 patients display unilateral and bilateral discharges, respectively, and there is significant difference between the 2 groups (χ2 = 5.199, P = 0.023). The rate of bilateral discharge from the Rolandic region in the multidrug therapy group is definitely higher than that in the monotherapy group.
The ages of onset in monotherapy and multidrug therapy groups are 6.84 ± 2.47 and 6.72 ± 2.43 years, respectively, but this parameter did not significantly differ between the 2 groups (t = 0.307, P = 0.759). The ages of data collection in monotherapy and multidrug therapy groups are 9.07 ± 2.66 and 8.80 ± 2.76 years, respectively. This parameter did not also significantly differ between the 2 groups (t = 1.327, P = 0.185). The disease courses in monotherapy and multidrug therapy groups are 2.12 ± 2.08 and 2.93 ± 2.15 years, respectively, and this finding significantly differed between the 2 groups (t = 5.199, P = 0.023). The disease course in the multidrug therapy group is significantly higher than that in the monotherapy group (Table 4).
Table 4.
Clinical features of patients in monotherapy and multidrug therapy group.
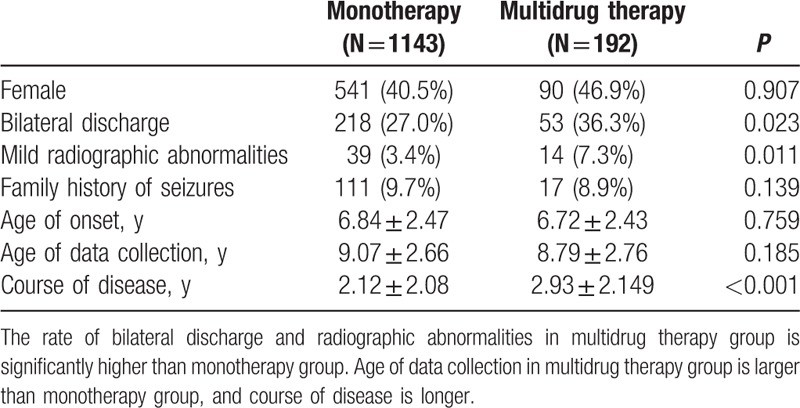
3.6. AEDs used in monotherapy
The AEDs used in monotherapy are oxcarbazepine (OXC), sodium valproate (VPA), levetiracetam (LEV), carbamazepine, lamotrigine, topiramate, and magnesium valproate. Among these AEDs (Fig. 3), the most commonly used are OXC 418 (37%), VPA 288 (25%), and LEV 216 (19%).
Figure 3.
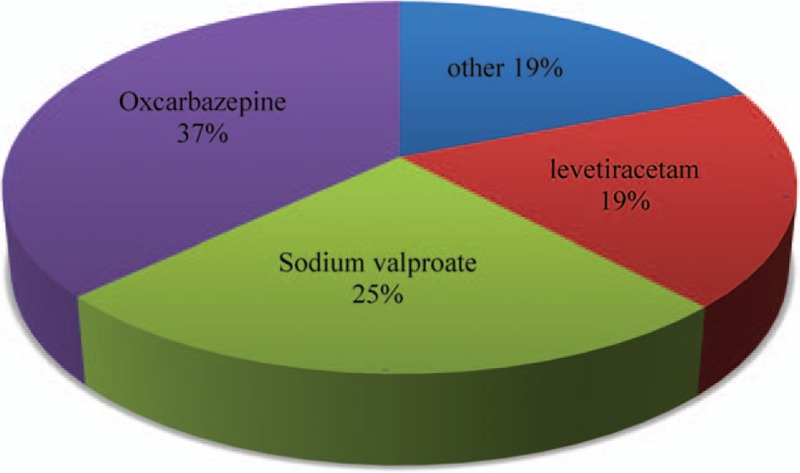
Antiepileptic drugs used in monotherapy for rolandic epilepsy patients.
3.7. Clinical features of patients receiving monotherapy and add-on therapy
The first AED administered to 418 patients is OXC, and 29 of these patients use add-on drugs when OXC fails. A total of 288 patients use VPA as the first AED, and 89 of these patients receive add-on drugs when VPA fails. The first AED given to 215 patients is LEV, and 35 of these patients take add-on drugs when LEV fails. The rates of add-on therapy significantly differed among the 3 groups existing (χ2 = 49.081, P < 0.001). After post-hoc multiple comparisons were performed (Fig. 4), the following results significantly differed: OXC vs. VPA (χ2 = 48.852, P < 0.001); VPA vs. LEV (χ2 = 8.745, P = 0.003); and OXC vs. LEV (χ2 = 10.850, P = 0.001).
Figure 4.
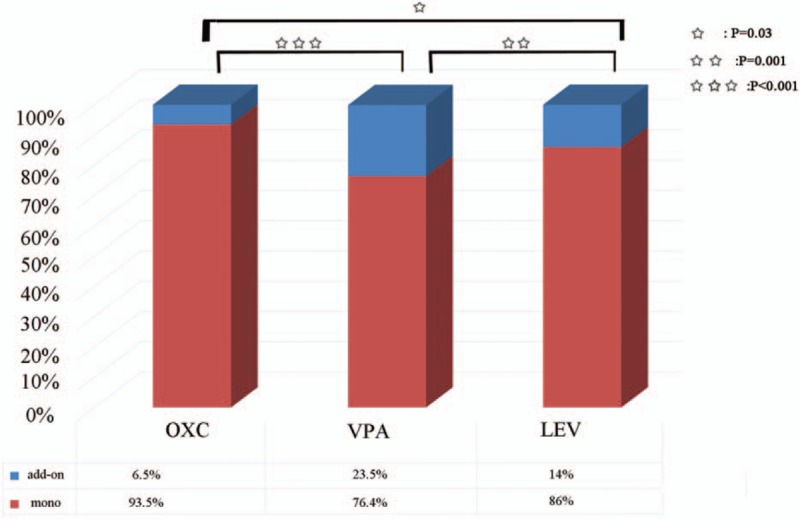
Add-on therapy/monotherapy ratio in the 3 most commonly used antiepileptic drugs. LEV = levetiracetam, OXC = oxcarbazepine, VPA = sodium valproate
3.8. Most commonly used AED combination
Among the multidrug therapies administered to 192 patients (Fig. 5), the most common AED combinations are LEV + VPA (46, 24.5%), OXC + VPA (26, 13.5%), VPA + lamotrigine (13, 6.8%), LEV + Topiramate (9, 4.7%), other 2-drug combinations (18, 4.7%), and 3-drug combinations (18, 4.7%). The most commonly used AEDs in the AED combinations are VPA (118, 61.5%), LEV (100, 52.1%), and OXC (60, 31.3%).
Figure 5.
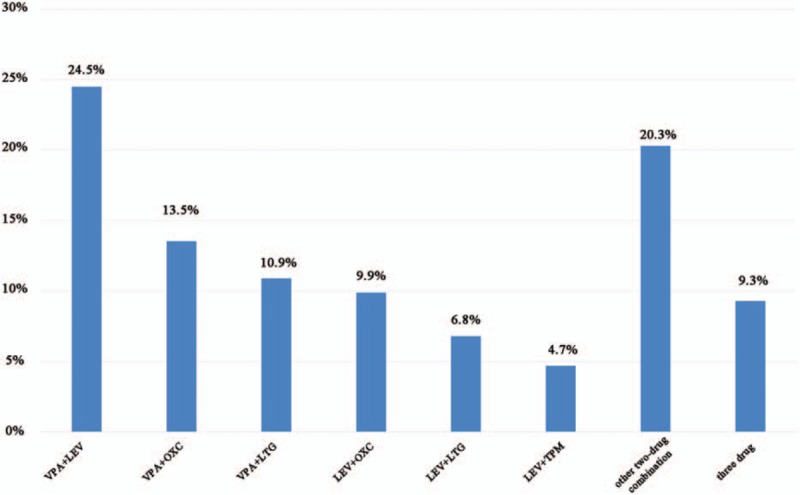
Antiepileptic drugs combinations in multidrug therapy.
4. Discussion
This multicenter study aims to analyze the treatment status and the following clinical features of Chinese patients with BECTS: vulnerable age, male/female ratio, radiographic manifestations, and EEG characteristics. Among the 2234 patients with BECTS collected from 25 neurologic centers, 1817 with complete information were included and further analyzed. The age of onset of the study population is 6.85 years (1.92–14 years). This finding is consistent with that in previous studies, which revealed that the peak incidence occurred between 7th and 8th year of life[19]; previous research also indicated that morbidity is related to age.[20] The male-to-female ratio is 963 (53.0%)/854 (47.0%), consistent with the previously reported male-to-female ratio of 6:4[21]; this result shows a male-dominating composition with an unknown cause. The ages of onset did not also significantly differ between the male and female patients. One of the BECTS diagnosis standards is the absence of neurological abnormalities. All of the subjects underwent imaging examination, which revealed that 62 (3.4%) patients suffered from mild radiographic abnormalities unrelated to the nervous system. Most of these abnormalities include intracranial cysts, white matter abnormalities, and cerebral ventricle abnormalities that are theoretically unrelated to seizures.[22] As demonstrated by other studies, clinical neuroimaging is typically negative. However, recent studies have made it clear that volume of the structures related to language and motor function and connections between these regions have changed to some extent.[23,24] Further analysis in our study should be done to investigate whether the abnormalities in neuroimaging also exist. The pathogenesis of BECTS has yet to be completely understood, but the age of onset, spontaneous remission, and genetic susceptibility indicate that this condition is caused by neurodevelopmental interference. The genetic analysis results of 22 families experiencing BECTS indicate that 70% of cases are closely related to chromosome 15q14 and N-acetylcholine receptor subunit.[25] In our study, 9.9% of the patients with BECTS report positive family histories of seizures.
The results of the long-range sleep EEG of 1253 patients show positive and negative spike slow complex waves or sharp slow complex waves located in the unilateral or bilateral central and middle and posterior temporal regions with arrhythmic activity; these waves are sometimes accompanied by sharp spike waves located in other regions. These results are consistent with the EEG features of previously reported BECTS.[26] Furthermore, 70.1% of the patients with sleep interictal activities exhibit unilateral spike slow waves or sharp slow waves and 29% display bilateral electrical activities. The frequency of bilateral epileptic discharges is consistent with previous studies. Bedoin et al[27] reported that 60% of the BECTS patients show unilateral discharge. Riva et al[26] reported that 16 of 24 patients (66.7%) show unilateral discharge. No definite relationship has been observed between the number of epileptic discharges and the frequency of clinical seizures. A frequent epileptic discharge unlikely corresponds to a high frequency of clinical seizures. Pavlou et al[28] reported that 30 patients with unilateral electrical activity elicit the same response to different AEDs, such as CBZ, VPA, and OXC, but 25 patients with bilateral electrical activity respond differently to these AEDs. Therefore, whether bilateral discharge can play an important role in the efficacy of AEDs remains unknown. In our study, 2.23% of the subjects show ESES, which has been shown to be related to age and could occur in many diseases, such as rolandic epilepsy, cerebral salt wasting syndrome, and Landau-Kleffner syndrome.[29] The frequency is reported to be 4.6% in another study, which is a little different from our study and this could be because of the different inclusion criteria.[18]
Considering the benign prognosis and spontaneous remission of BECTS, researchers have yet to obtain a consensus on whether AEDs can be administered to patients with BECTS. In this study, 25.4% (416) of the 1817 subjects did not receive AEDs. If clinical manifestations are subtle and neuropsychological abnormalities are nonexistent, treatment with AEDs can be postponed. In addition to the clinicians’ assessment of the patients, children's and their parents’ willingness should be considered when deciding whether AED therapy should be given. In theory, the treatment should not cause neurological sequela because of the benign prognosis of BECTS. However, the risk of neuropsychological disorder in patients with BECTS is higher than that of the general population. Neuropsychologists claim that patients with BECTS usually show normal IQ, but they manifest mild disorders in language, visual space capability, and social ability, which should also be considered.[30,31] The rate and pathogenesis of cognitive functions, such as language delay and learning disorder, have yet to be fully investigated. Furthermore, seizures and AEDs can be susceptible risk factors because some AEDs can induce cognitive dysfunction. Previous studies confirmed the relationship of seizures, their frequency, location, and other features with cognitive functions, but different studies have provided inconclusive findings.[32,33] The patients who did not receive AEDs in this study suffer from low-frequency subtle seizures, which unlikely affect their daily lives. However, some subtle cognitive dysfunctions may have been overlooked. Most of the subjects received AED treatment, but patients should receive AEDs under the following circumstances: early seizure onset; 6-month intervals between the first 3 seizures; secondarily generalized tonic–clonic seizure; and seizures during awake. After analyzing the difference between the treated and untreated groups, we found that most of the variables did not significantly differ, but the age of data collection and disease courses significantly differed. The age of data collection of the patients in the treated group was significantly larger and their disease course was longer than those of the patients in the untreated group. Considering that the age of data collection of the untreated group is close to the age of onset, we hypothesized that the patients of the untreated group are in early disease stages when these patients have not yet received their AED treatment. The average age of onset among patients with BECTS is 6 to 7 years. As children age and start schooling, seizures become a stressor, which prompts them to undergo AED treatment. This phenomenon could result in an older age of data collection in the treated group.
The rates of bilateral discharge and mild radiographic abnormalities were significantly higher and the disease course was significantly longer in the multidrug therapy group than those in the monotherapy group. Nevertheless, the differences in the other variables in this study were not significant. Considering that most of the BECTS patients remit during puberty, it is difficult to decide the effect of AEDs on epileptic discharge.[34] However, there has been sufficient evidence that neuropsychological disorders are related to epileptic discharges, and treatment of these discharges is beneficial to restoration of the related function. Multidrug group shows higher rate of bilateral discharge, this may be because of more serious neuropsychological disorders.[35–37] Bilateral discharge in the Rolandic region may adversely affect the efficacy of AEDs, and this finding is consistent with our result. Mild cerebral structural abnormalities in patients with BECTS have also been investigated. For example, Kim et al[38] found that the cortex of patients with BECTS is thicker than healthy individuals. However, researchers revealed discrepancies on whether these mild radiographic abnormalities show any clinical significance. The disease course in the multidrug therapy group was longer because add-on drugs were administered after the first drug failed. The need for multidrug therapy indicates that seizures cannot be easily controlled. Approximately 2% of patients with BECTS cannot easily control their seizures, and some of them progress to epileptic encephalopathy. We failed to determine and classify the seizure prognosis of the patients into intractable epilepsy because the information provided by the patients in the multidrug therapy group was incomplete. However, we predicted that these patients are more disposed to epileptic encephalopathy and should be taken good care of. Furthermore, some of the patients receiving monotherapy may have just started AED therapy and the efficacy of AED cannot be identified because this study is cross-sectional.
The most commonly used AED combination is VPA + LEV, and the most commonly used AEDs are VPA and LEV. VPA is a broad-spectrum and low-cost drug and thus suitable for long-term treatment.[39] LEV is commonly used because of its excellent pharmacokinetic characteristics, drug tolerance,[16,17] and low drug interaction.[40]
Among the most commonly used AEDs, namely, OXC, LEV, and VPA, additional AEDs are most commonly added to VPA monotherapy and are least frequently administered to OXC monotherapy. Although our study is cross-sectional and we failed to obtain information regarding the time when patients started receiving monotherapy and add-on therapy and their seizure prognosis, we can predict that the effectiveness of OXC and LEV as first-line therapy is better than that of VPA in Chinese BECTS patients.
The evidence base for specific AEDs in BECT patients is still unknown. We can see several studies supporting recommendations for specific AEDs treatment, such as VPA,[41,42] OXC,[43] LEV,[44] CBZ,[43] and we can also see studies against specific AEDs used in treatment for BECTS patients.[45,46] Nowadays, the first-line AED in different countries diverges, for example, Sulthiame in Germany, Austria, and Israel,[42,47] sodium valproate in France, and levetiracetam in United States. Whether the most commonly used AED in China is reasonable still needs further investigations.
In this study, 18 subjects received other treatments, but most of these subjects preferred to terminate their AED treatments. As a consequence, seizures recur. Hence, patients should be informed of the importance of treatment compliance.
We also compared the clinical features and treatment status of our patients with those from foreign countries. And we can see no significant difference between our studies with theirs. This suggests that the conclusions achieved in our study could also be applied to foreign patients.
In this multicenter cross-sectional study, clinical features, general information, and treatment status were analyzed. Nevertheless, we did not collect information on seizure prognosis and cognitive function because this study is cross-sectional. Most seizures happen during sleep, and the frequency of seizure is usually low, but these conditions may influence our results. This study provides a basis for future large-sample studies on Chinese patients with BECTS.
5. Conclusion
The average age of onset for Chinese children with BECTS is 6.85 years, and the age of 50% of vulnerable patients is between 5 and 9 years. The male-to-female ratio is 1.13:1. The ages of onset do not significantly differ between males and females. Furthermore, 25.4% of the patients did not receive AEDs, 62.9% received monotherapy, and 10.6% underwent multidrug therapy. The ages of data collection of the patients in the treated group are older and the disease courses are longer than those of the patients in the untreated group. Bilateral discharge in the Rolandic region and mild radiographic abnormalities may indicate the failure of monotherapy. The most commonly used AEDs for Chinese patients with BECTS are OXC, VPA, and LEV, and the effectiveness of OXC and LEV is better than that of VPA.
Acknowledgments
We gratefully acknowledge the assistance and cooperation of the staff of the following hospitals: Children's Medical Center of PLA General Hospital, Capital Institute of Pediatrics, Beijing Children's Hospital, Peking University First Hospital, Tianjin Children's Hospital, Shengjing Hospital of China Medical University, Tangshan Women's Children's Hospital, Harbin Children's Hospital, First Teaching Hospital of Jilin University, Qilu Hospital of Shandong University, Linyi People's Hospital, Henan Province People's Hospital, The Second Affiliated Hospital of Xi’an Jiaotong University, Children's Hospital of Soochow University, The Children's Hospital of Zhejiang University School of Medicine, Jiangxi Province Children's Hospital, Wuhan Women and Children Care Center, Children's Hospital of Fudan University, Xiangya Hospital Central South University, Guangdong General Hospital, Guangzhou Children's Hospital, West China Women's and Children's Hospital, Fuzhou General Hospital of Nanjing Military Command, Quanzhou Children's Hospital, and Affiliated Hospital of Zunyi Medical College.
Footnotes
Abbreviation: AED = antiepileptic drug; BECTS = benign epilepsy of childhood with centrotemporal spikes; CSWS = cerebral salt wasting syndrome; EEG = electroencephalography; ESES = electrical status epilepticus in sleep; LEV = levetiracetam; LKS = Landau-Kleffner syndrome; MR = magnetic resonance; OXC = Oxcarbazepine; RE = rolandic epilepsy; VPA = Sodium valproate.
M-JL and X-jS contributed equally to the manuscript.
The authors report no conflicts of interest.
This work was supported by grants from Capital of the public health program cultivation project (no. Z141100002114001), Major State Basic Research Development Program (973; no. 2012CB517903) and the National Natural Science Foundation of China (nos. 81471329, 81211140048, 81201013, 81200463).
References
- [1].Rating D, Wolf C, Bast T. Sulthiame as monotherapy in children with benign childhood epilepsy with centrotemporal spikes: a 6-month randomized, double-blind, placebo-controlled study. Sulthiame Study Group. Epilepsia 2000;41:1284–8. [DOI] [PubMed] [Google Scholar]
- [2].Gross-Selbeck G. Treatment of “benign” partial epilepsies of childhood, including atypical forms. Neuropediatrics 1995;26:45–50. [DOI] [PubMed] [Google Scholar]
- [3].Rating D. Treatment in typical and atypical rolandic epilepsy. Epileptic Disord 2000;2(Suppl 1):S69–72. [PubMed] [Google Scholar]
- [4].Wirrell E, Sherman EM, Vanmastrigt R, et al. Deterioration in cognitive function in children with benign epilepsy of childhood with central temporal spikes treated with sulthiame. J Child Neurol 2008;23:14–21. [DOI] [PubMed] [Google Scholar]
- [5].Ambrosetto G, Tassinari CA. Antiepileptic drug treatment of benign childhood epilepsy with rolandic spikes: is it necessary? Epilepsia 1990;31:802–5. [DOI] [PubMed] [Google Scholar]
- [6].Peters JM, Camfield CS, Camfield PR. Population study of benign rolandic epilepsy: is treatment needed? Neurology 2001;57:537–9. [DOI] [PubMed] [Google Scholar]
- [7].Wolff M, Weiskopf N, Serra E, et al. Benign partial epilepsy in childhood: selective cognitive deficits are related to the location of focal spikes determined by combined EEG/MEG. Epilepsia 2005;46:1661–7. [DOI] [PubMed] [Google Scholar]
- [8].Overvliet GM, Besseling RMH, Vles JSH, et al. Nocturnal epileptiform EEG discharges, nocturnal epileptic seizures, and language impairments in children: review of the literature. Epilepsy Behav 2010;19:550–8. [DOI] [PubMed] [Google Scholar]
- [9].Lillywhite LM, Saling MM, Harvey AS, et al. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia 2009;50:2276–84. [DOI] [PubMed] [Google Scholar]
- [10].Besseling RMH, Jansen JFA, Overvliet GM, et al. Reduced functional integration of the sensorimotor and language network in rolandic epilepsy. Neuroimage Clin 2013;2:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nicolai J, Aldenkamp AP, Arends J, et al. Cognitive and behavioral effects of nocturnal epileptiform discharges in children with benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav 2006;8:56–70. [DOI] [PubMed] [Google Scholar]
- [12].Aarts JH, Binnie CD, Smit AM, et al. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain 1984;107(Pt 1):293–308. [DOI] [PubMed] [Google Scholar]
- [13].Khan OI, Zhao Q, Miller F, et al. Interictal spikes in developing rats cause long-standing cognitive deficits. Neurobiol Dis 2010;39:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Proposal for revised classification of epilepsies, epileptic syndromes Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1989;30:389–99. [DOI] [PubMed] [Google Scholar]
- [15].Guerrini R, Pellacani S. Benign childhood focal epilepsies. Epilepsia 2012;53(Suppl 4):9–18. [DOI] [PubMed] [Google Scholar]
- [16].Vega YH, Smith A, Cockerill H, et al. Risk factors for reading disability in families with rolandic epilepsy. Epilepsy Behav 2015;53:174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Miziara CS, Manreza ML. Hemispheric lateralization in benign focal epilepsy in childhood with centrotemporal spikes (BECTS). Clinical Eeg Neurosci 2010;41:147–50. [DOI] [PubMed] [Google Scholar]
- [18].Tovia E, Goldberg-Stern H, Zeev BB, et al. The prevalence of atypical presentations and comorbidities of benign childhood epilepsy with centrotemporal spikes. Epilepsia 2011;52:1483–8. [DOI] [PubMed] [Google Scholar]
- [19].Kriz M, Gazdik M. [Epilepsy with centrotemporal (Rolandic) spikes. A peculiar seizure disorder of childhood]. Neurol Neurochir Pol 1978;12:413–9. [PubMed] [Google Scholar]
- [20].Kramer U, Ben-Zeev B, Harel S, et al. Transient oromotor deficits in children with benign childhood epilepsy with central temporal spikes. Epilepsia 2001;42:616–20. [DOI] [PubMed] [Google Scholar]
- [21].Watanabe K. Epilepsy in children. 1996;London:Chapman &Hall, 302. [Google Scholar]
- [22].Eeg-Olofsson O, Lundberg S, Raininko R. MRI in rolandic epilepsy. Epileptic Disord 2000;2(Suppl 1):S51–53. [PubMed] [Google Scholar]
- [23].Luo C, Zhang Y, Cao W, et al. Altered structural and functional feature of striato-cortical circuit in benign epilepsy with centrotemporal spikes. Int J Neural Syst 2015;25:1550027. [DOI] [PubMed] [Google Scholar]
- [24].Besseling RM, Overvliet GM, Jansen JF, et al. Aberrant functional connectivity between motor and language networks in rolandic epilepsy. Epilepsy Res 2013;107:253–62. [DOI] [PubMed] [Google Scholar]
- [25].Neubauer BA, Fiedler B, Himmelein B, et al. Centrotemporal spikes in families with rolandic epilepsy: linkage to chromosome 15q14. Neurology 1998;51:1608–12. [DOI] [PubMed] [Google Scholar]
- [26].Riva D, Vago C, Franceschetti S, et al. Intellectual and language findings and their relationship to EEG characteristics in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav 2007;10:278–85. [DOI] [PubMed] [Google Scholar]
- [27].Bedoin N, Herbillon V, Lamoury I, et al. Hemispheric lateralization of cognitive functions in children with centrotemporal spikes. Epilepsy Behav 2006;9:268–74. [DOI] [PubMed] [Google Scholar]
- [28].Pavlou E, Gkampeta A, Evangeliou A, et al. Benign epilepsy with centro-temporal spikes (BECTS): relationship between unilateral or bilateral localization of interictal stereotyped focal spikes on EEG and the effectiveness of anti-epileptic medication. Hippokratia 2012;16:221–4. [PMC free article] [PubMed] [Google Scholar]
- [29].Raha S, Shah U, Udani V. Neurocognitive and neurobehavioral disabilities in Epilepsy with Electrical Status Epilepticus in slow sleep (ESES) and related syndromes. Epilepsy Behav 2012;25:381–5. [DOI] [PubMed] [Google Scholar]
- [30].Kanemura H, Aihara M. Growth disturbance of frontal lobe in BCECTS presenting with frontal dysfunction. Brain Dev 2009;31:771–4. [DOI] [PubMed] [Google Scholar]
- [31].Overvliet GM, Besseling RM, Vles JS, et al. Nocturnal epileptiform EEG discharges, nocturnal epileptic seizures, and language impairments in children: review of the literature. Epilepsy Behav 2010;19:550–8. [DOI] [PubMed] [Google Scholar]
- [32].Metz-Lutz MN, Kleitz C, de Saint Martin A, et al. Cognitive development in benign focal epilepsies of childhood. Dev Neurosci 1999;21:182–90. [DOI] [PubMed] [Google Scholar]
- [33].D’Alessandro P, Piccirilli M, Tiacci C, et al. Neuropsychological features of benign partial epilepsy in children. Ital J Neurol Sci 1990;11:265–9. [DOI] [PubMed] [Google Scholar]
- [34].Vannest J, Tenney JR, Gelineau-Morel R, et al. Cognitive and behavioral outcomes in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav 2015;45:85–91. [DOI] [PubMed] [Google Scholar]
- [35].Binnie CD. Significance and management of transitory cognitive impairment due to subclinical EEG discharges in children. Brain Dev 1993;15:23–30. [DOI] [PubMed] [Google Scholar]
- [36].Binnie CD, Marston D. Cognitive correlates of interictal discharges. Epilepsia 1992;33(Suppl 6):S11–7. [PubMed] [Google Scholar]
- [37].Gordon N. Cognitive functions and epileptic activity. Seizure 2000;9:184–8. [DOI] [PubMed] [Google Scholar]
- [38].Kim EH, Yum MS, Shim WH, et al. Structural abnormalities in benign childhood epilepsy with centrotemporal spikes (BCECTS). Seizure 2015;27:40–6. [DOI] [PubMed] [Google Scholar]
- [39].Glauser T, Ben-Menachem E, Bourgeois B, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2013;54:551–63. [DOI] [PubMed] [Google Scholar]
- [40].Khurana DS, Kothare SV, Valencia I, et al. Levetiracetam monotherapy in children with epilepsy. Pediatr Neurol 2007;36:227–30. [DOI] [PubMed] [Google Scholar]
- [41].Yang ZX, Liu XY, Qin J, et al. [Clinical and electrophysiologic studies on epileptic negative myoclonus in atypical benign partial epilepsy of childhood]. Zhonghua Er Ke Za Zhi 2008;46:885–90. [PubMed] [Google Scholar]
- [42].Wheless JW, Clarke DF, Arzimanoglou A, et al. Treatment of pediatric epilepsy: European expert opinion, 2007. Epileptic Disord 2007;9:353–412. [DOI] [PubMed] [Google Scholar]
- [43].Wheless JW, Clarke DF, Carpenter D. Treatment of pediatric epilepsy: expert opinion, 2005. J Child Neurol 2005;20(Suppl 1):S1–56. quiz S59-60. [DOI] [PubMed] [Google Scholar]
- [44].Coppola G, Franzoni E, Verrotti A, et al. Levetiracetam or oxcarbazepine as monotherapy in newly diagnosed benign epilepsy of childhood with centrotemporal spikes (BECTS): an open-label, parallel group trial. Brain Dev 2007;29:281–4. [DOI] [PubMed] [Google Scholar]
- [45].Seidel WT, Mitchell WG. Cognitive and behavioral effects of carbamazepine in children: data from benign rolandic epilepsy. J Child Neurol 1999;14:716–23. [DOI] [PubMed] [Google Scholar]
- [46].Cerminara C, Montanaro ML, Curatolo P, et al. Lamotrigine-induced seizure aggravation and negative myoclonus in idiopathic rolandic epilepsy. Neurology 2004;63:373–5. [DOI] [PubMed] [Google Scholar]
- [47].Borggraefe I, Bonfert M, Bast T, et al. Levetiracetam vs. sulthiame in benign epilepsy with centrotemporal spikes in childhood: a double-blinded, randomized, controlled trial (German HEAD Study). Eur J Paediatr Neurol 2013;17:507–14. [DOI] [PubMed] [Google Scholar]


