Abstract
To describe the clinical manifestations, treatments, prognosis, and prevalence of autoimmune diseases (ADs) in human immunodeficiency virus (HIV)-infected patients.
All HIV-infected patients managed in the Infectious Diseases Department of the Lyon University Hospitals, France, between January 2003 and December 2013 and presenting an AD were retrospectively included.
Thirty-six ADs were found among 5186 HIV-infected patients which represents a prevalence of 0.69% including immune thrombocytopenic purpura (n = 15), inflammatory myositis (IM) (n = 4), sarcoidosis (n = 4), Guillain–Barré syndrome (GBS) (n = 4), myasthenia gravis (n = 2), Graves’ disease (n = 2), and 1 case of each following conditions: systemic lupus erythematosus, rheumatoid arthritis, autoimmune hepatitis, Hashimoto thyroiditis and autoimmune hemolytic anemia. One patient presented 2 ADs. Thirty patients were known to be HIV-infected when they developed an AD. The AD preceded HIV infection in 2 patients. GBS and HIV infection were diagnosed simultaneously in 3 cases. At AD diagnosis, CD4 T lymphocytes count were higher than 350/mm3 in 63% of patients, between 200 and 350/mm3 in 19% and less than 200/mm3 in 19%. Twenty patients benefited from immunosuppressant treatments, with a good tolerance.
ADs during HIV infection are uncommon in this large French cohort. Immune thrombocytopenic purpura, sarcoidosis, IM, and GBS appear to be more frequent than in the general population. Immunosuppressant treatments seem to be effective and well tolerated.
Keywords: acquired immunodeficiency syndrome (AIDS), autoimmune disease, highly active antiretroviral therapy (HAART), human immunodeficiency virus (HIV), immune restoration inflammatory syndrome (IRIS), immune thrombocytopenic purpura (ITP), immunosuppressant drugs
1. Introduction
With studies focusing on the development of autoimmunity in human immunodeficiency virus (HIV) infected patients, many authors have shown that HIV is not only causing a state of immunodeficiency in infected patients but is also responsible for several serum abnormalities.[1–3] The most common serum abnormality remains the polyclonal hypergammaglobulinemia.[2,3] HIV also causes an immune dysregulation (with more or less clinical symptoms); this immune dysregulation (depending on the CD4 and CD8 levels) facilitates the overall pathogenic process and can lead to the development of autoimmune and systemic diseases.[1,3] The main autoimmune diseases (ADs) are HIV-related immune thrombocytopenia, which can be the first manifestation of the infection[4,5] and sarcoidosis which is described as a delayed immune reconstitution inflammatory syndrome (IRIS).[6] The frequency of rheumatological diseases in HIV patients was mostly described before the highly active antiretroviral treatment (HAART) era, and varies from less than 1% to 60%.[7–10]
Since the era of HAART, HIV-infected patients present a rise in the CD4 lymphocyte count, which enables ADs to emerge.[1] The type of ADs and their clinical manifestations, in HIV-infected patients, are poorly described. Only 2 studies have examined this issue, for rheumatic ADs. In a longitudinal analysis of 395 HIV-infected patients seen at their institution from 1989 to 2000, Calabrese et al[7] reported a remarkable drop in the rate of new rheumatic complications such as reactive arthritis, psoriatic arthritis, and various forms of connective tissue diseases. Yang et al[8] confirmed this in their analysis of 3623 HIV-infected patients and found 18 patients with ankylosing arthritis, 6 patients with rheumatoid arthritis (RA), 1 patient with psoriatic arthritis and 1 patient with primary Sjogren syndrome. On the other hand, several studies described HIV-related immune thrombocytopenia, in the HAART era.[11–15] Furthermore, several case-series reported sarcoidosis as a potential complication of immune restoration in patients receiving HAART for HIV infection.[6]
By surveying 14 medical departments in the Paris area, Iordache et al[16] reported 52 HIV-infected patients who presented an AD, including a wide range of disorders: vasculitis (n = 11), immune cytopenias (n = 8), rheumatic diseases (n = 7), sarcoidosis (n = 7), thyroid diseases (n = 6), hepatic diseases (n = 5) and antiphospholipid syndrome (n = 4).
Recently, Yen et al described the incidence of ADs in Taiwan between 2000 and 2012, using the Taiwan National Health Insurance Research Database. They found a higher incidence for Sjogren syndrome (standardized incidence rates (SIR) = 1.64), psoriasis (SIR = 2.05), systemic lupus erythematosus (SLE) (SIR = 2.59), autoimmune hemolytic anemia (SIR = 35.06) and uveitis (SIR = 2.50) than the general population.[17]
Another concern is the use of immunosuppressant treatments which is often avoided or delayed in this population, because of the lack of data and the potential risk of opportunistic infections.
The objectives of this study were to describe the clinical manifestations, treatments and prognosis of ADs from a large database of HIV-infected patients, managed in a University hospital. Moreover, we performed a cross sectional estimate of AD prevalence in the cohort and compared it to the prevalence in the general population as described in international studies.
2. Methods
2.1. Study population and design
We retrospectively reviewed the records of HIV-infected patients managed in the Department of Infectious Diseases of the Lyon University Hospitals, France, between January 2003 and December 2013. NADIS (Fedialis Medica, Marly le Roi, France), which is an electronic medical record, is prospectively used in our hospital since 2003 by physicians for the clinical practice and the management of HIV-, hepatitis B virus (HBV)- or hepatitis C virus (HCV)-infected adults. The patient provides written informed consent for the data collection, and for its use in anonymized observational studies. All patient data are prospectively recorded in a structured database, which can be used for observational, epidemiological, or therapeutic studies after anonymization.[18] This hospital cohort is part of the French Hospital Database on HIV and follows the protocols previously described.[19] We selected all patients having an AD, organ specific or no, and followed for an HIV infection, using codes of the International Classification of Diseases (ICD-10) 10th revision from hospital stays or consultations. The codes of ICD-10 used for HIV infection were: B20, B21, B22, B23, B24, R75, or Z21. The codes used to query for ADs were M314, D690, M300, M301, M310, M313, M319, D590, D591, D693, M05∗, M06∗, M353, M32∗, D686, D86∗, E06.3, E050, K754, K743, G610, G700, M331, M332, M339, M342, M348, M349, M35∗, or M36∗. Among them, we only included patients with a verified HIV infection and whose AD answered to the international classifications, after reading of the medical files and performing an extraction from the NADIS database.
2.2. Patients data
Clinical and biological data were retrospectively collected with a standardized form, by the same clinician (EV). General patient characteristics (age, gender, origin) were collected. HIV infection history was characterized by the date of diagnosis, HIV type (1 or 2) and HIV stage (Center for Diseases Control (CDC) stage). HBV and HCV coinfections were collected. Immunovirological evolution, opportunistic infections, neoplasia occurrence, duration of HAART, and type of HAART at AD diagnosis were recorded.
AD history was characterized by date of diagnosis, immunovirological context at diagnosis, clinical manifestations, treatments, complications of these treatments and outcome.
Concomitant HIV and AD was defined as less than 1 year between the 2 diagnoses.
The ACR and EULAR 2010 criteria were used to define cases of RA.[20] Cases of IM were defined according the ENMC criteria.[21] The SLICC SLE criteria were used to define cases of SLE.[22]
HIV-induced immune thrombocytopenic purpura (ITP) was defined as thrombocytopenia <100 G/L excluding others causes of thrombocytopenia (drug-induction, others infections including HBV, HCV, myelodysplasia, splenic sequestration, platelet consumption, autoimmune disease).[23]
Complete remission of AD was defined as the absence of any sign of disease activity (clinical and biological); partial remission was defined as a significant improvement in diseases signs with persistence of clinical or biological signs of disease activity. For ITP, complete remission was defined as a platelet count >100 G/L and absence of bleeding, partial remission was defined as a platelet count between 30 and 100 G/L or at least a 2-fold increase from baseline and absence of bleeding, treatment resistance was defined as a platelet count staying <30 G/L or less than a 2-fold increase from baseline or bleeding.[23]
Prevalence estimations of ADs were compared to international data on general population. Because prevalence studies are uncommon, we used data from an American study[24] or data from the latest Prevalence Journal of Rare Diseases, published by ORPHANET in 2016.[25]
2.3. Statistical analysis
Quantitative variables were reported as medians with interquartile range (IQR). Discrete variables were reported as numbers and percentages. For the percentage calculations, missing values were excluded from the denominator. Confidence intervals for prevalence estimations were set at 95% (95% CI), using an exact method based on the cumulative binomial distribution.[26] Confidence intervals were computed using the CRITBINOM function of MS Excel. Assuming a final sample size greater than 5000 subjects we were expecting accuracy for confidence interval of ±0.2% around an observed prevalence of 0.5%.
3. Results
3.1. Characteristics of the study population and prevalence estimations
Five thousand one hundred eighty-six HIV-infected patients were followed over a period of 10 years (2003–2013). Among them, 176 patients were selected by the codes of the ICD-10; but after reading the medical files, only 35 had evidence of ADs. There were 23 men and 12 women. Twenty of the 35 patients were Caucasian (57%), 8 originated from sub-Saharan Africa (23%), 4 from Maghreb (11%), 2 from the Caribbean (6%), and 1 from Asia (3%). Population characteristics are described in Table 1.
Table 1.
Demographic characteristics of the population.
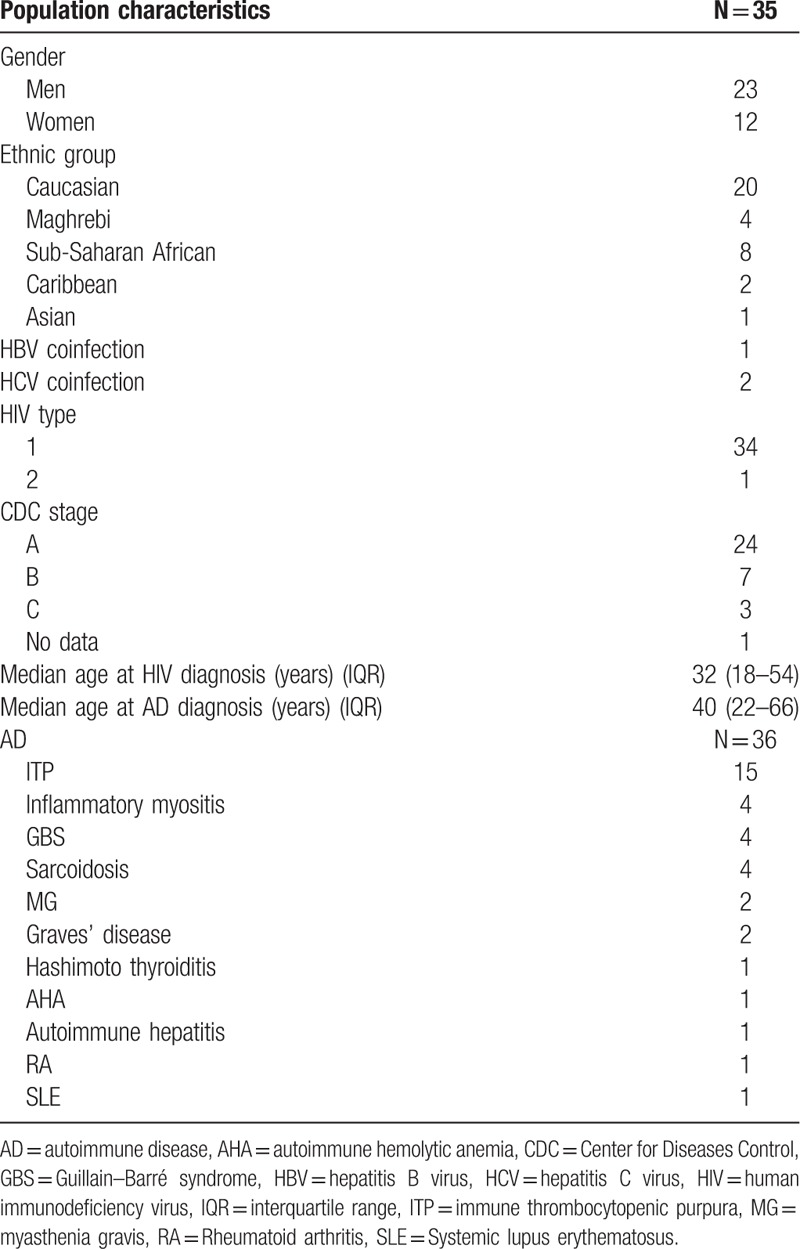
Median age at HIV diagnosis was 32 years (IQR 18–54) and 40 years (IQR 22–66) at AD diagnosis.
Thirty-six ADs were found (0.69%; 95% CI, 0.48–0.93). One patient presented 2 ADs. We found fifteen HIV-associated ITP, which represent a prevalence of 0.29% (95% CI, 0.15–0.44), 4 IM (3 polymyositis (PM) and 1 antisynthetase syndrome), 4 sarcoidosis and 4 Guillain–Barré syndromes (GBS), which represent a prevalence of 0.08% (95% CI, 0.02–0.15) for each of them. We found 2 myasthenia gravis (MG) and 2 Graves’ diseases (prevalence of 0.04%, 95% CI, 0.0–0.10 for each of them); and 1 case of each of the following conditions: SLE, RA, autoimmune hepatitis, Hashimoto thyroiditis, and autoimmune hemolytic anemia (AHA). Only 1 patient had a family history of AD. Clinical and paraclinical AD manifestations are described in Table 2 .
Table 2.
Clinical, paraclinical ADs manifestations, and treatment of the ADs.
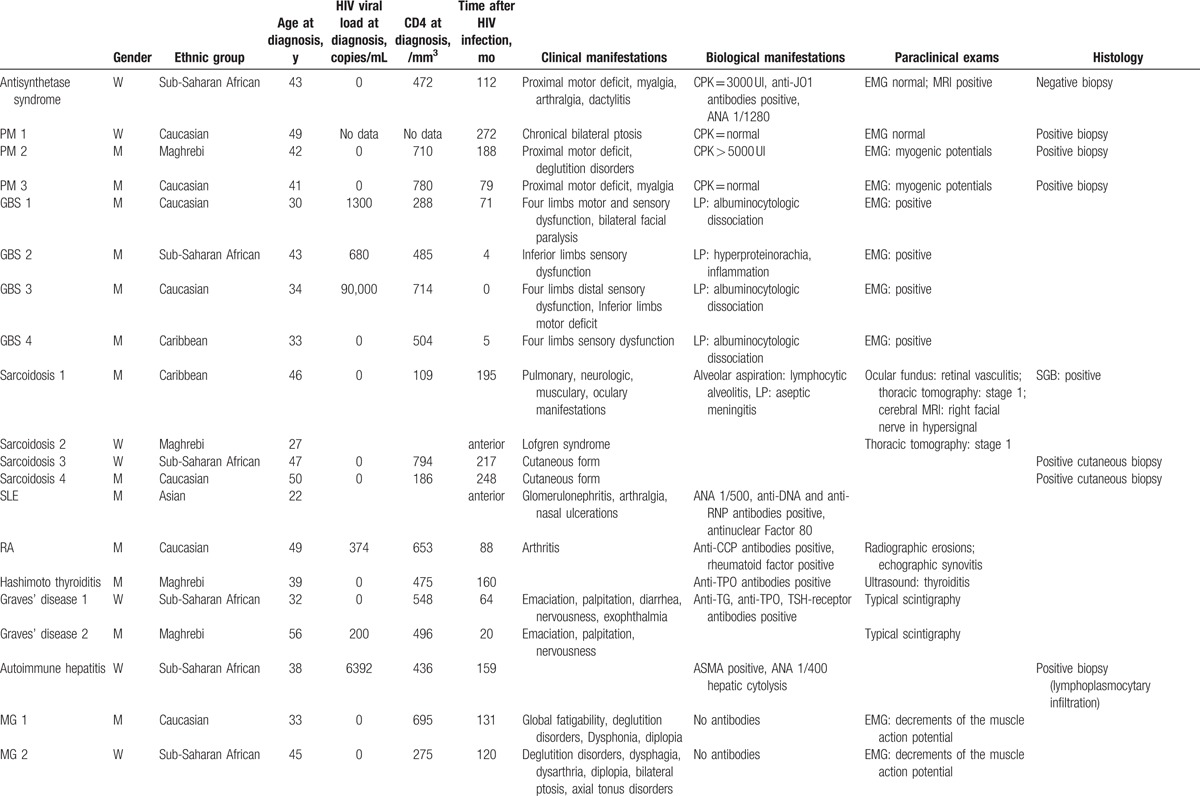
3.2. HIV infection
Only 1 patient was infected with type 2 HIV (1 ITP), the other patients[34] were infected with type 1 HIV. One patient was coinfected with HBV. Two patients had a positive HCV serology, 1 was cured and 1 had chronic hepatitis with HCV viremia. These 3 patients developed sarcoidosis (cutaneous form).
CDC stage at HIV infection was A for 24 patients, B for 7 patients, C for 3 patients (no data for 1 patient). Opportunistic diseases declared for these last patients were 1 pneumocystosis, 1 HIV encephalitis, and 1 Kaposi sarcoma.
Sixteen patients received antiretroviral therapy at HIV diagnosis and 18 had a deferred therapy (no data for 1 patient). Median viral load at HIV diagnosis was 87,600 copies/mL (IQR 100–6,500,000). Median CD4 T lymphocyte count, percentage, and CD4/CD8 T lymphocytes ratio at HIV diagnosis were 298.5/mm3 (IQR 17–1042), 19% (IQR 5–47%), 0.405 (IQR 0.23–1.31), respectively. Median viral load at HAART introduction was 32,800 copies/mL (IQR 100–6,500,000). Median CD4 T lymphocyte count, percentage, and CD4/CD8 T lymphocytes ratio at HAART introduction were 316/mm3 (IQR 4–714), 19% (IQR 1–32%), 0.38 (IQR 0.01–0.71), respectively.
3.3. Analysis of the chronology of AD occurrence in HIV-infected patients
Thirty patients were HIV-infected before the AD. Median time between HIV infection and AD was 113.5 months (IQR 0–306).
Fifteen patients were HIV-infected and HAART treated when they developed an AD. Median time between HAART introduction and AD was 88 months (IQR 0–195) for these patients. Five patients were HIV-infected and treated by biantiretroviral therapy. Ten patients were HIV-infected but without any antiretroviral treatment.
In 2 patients, the AD preceded HIV infection diagnosis (1 SLE and 1 sarcoidosis). For 3 patients, HIV infection was diagnosed concomitantly with the AD (less than 1 year between the diagnosis of the 2 disorders) (3 GBS).
For the 15 patients developing ITP, median viral load at ITP diagnosis was 8750 copies/mL (IQR 85–282,723). Median CD4 T lymphocyte count, percentage and CD4/CD8 T lymphocytes ratio at ITP diagnosis were 279.5/mm3 (IQR 53–975), 22% (IQR 4–48%), 0.4 (0.05–0.61).
For the other 15 patients who were HIV infected before the AD, median viral load at AD diagnosis was undetectable (<50 copies/mL) (IQR 0–6392). Median CD4 T lymphocyte count, percentage and CD4/CD8 T lymphocytes ratio at AD diagnosis were 475/mm3 (IQR 109–794), 30% (IQR 16–39%), 0.89 (IQR 0.29–1.32), respectively.
For the 3 patients with concomitant AD and HIV infection (3 GBS), median viral load, median CD4 T lymphocyte count, percentage, and CD4/CD8 T lymphocytes ratio at AD diagnosis were 680 copies/mL (IQR 0–90,000), 504/mm3 (IQR 485–714), 17% (only 1 data), 0.29 (only 1 data).
3.4. Treatments used in the management of the ADs
Regarding ADs except ITP, 2 of them were treated with steroids alone (1 sarcoidosis, 1 AHA). Two patients received steroids and hydroxychloroquine (1 SLE, 1 RA) and 7 patients were treated with intravenous immunoglobulins (2 GBS, 2 MG, 3 IM). One patient received salazopyrine, then methotrexate (RA); 1 patient was treated with several immunosuppressant drugs (methotrexate, azathioprine, rituximab) for refractory IM, which was an antisynthetase syndrome (anti-JO1 antibodies positive). In 7 patients the AD was controlled by using solely nonimmunosuppressant drugs and HAART.
Regarding ITP, 6 patients benefited from HAART introduction to control the disease, whereas the others (9/15) received intravenous immunoglobulins, and sometimes platelet transfusions or other immunosuppressant drugs (dapsone, steroids, and splenectomy).
These treatments were relatively well tolerated since no patient developed opportunistic infection or cancer during the follow-up. Six drug-induced cytopenias and 1 severe osteoporosis were reported (Table 3).
Table 2 (Continued).
Clinical, paraclinical ADs manifestations, and treatment of the ADs.

Table 3.
ADs, HAART at diagnosis, AD treatment, and AD evolution.
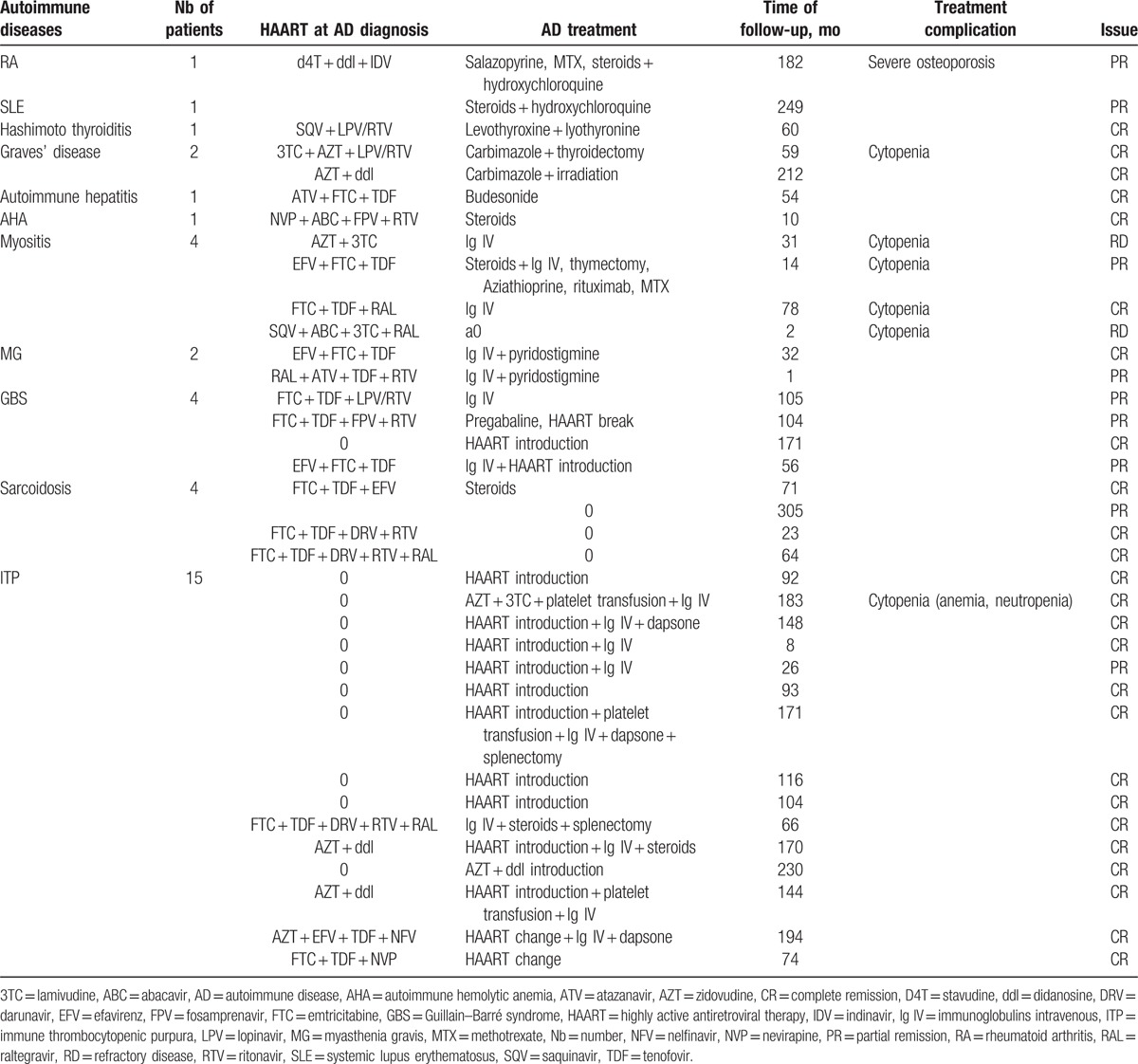
3.5. Evolution of the ADs and of the HIV infection at last follow-up
The median follow-up duration starting from the diagnosis of AD was 78 months (IQR 1–305).
For ITP, at last follow-up, median viral load was undetectable (<50 copies) (IQR 0–96,928). All of them, except one were HAART treated. Median CD4 T lymphocyte count, percentage and CD4/CD8 T lymphocytes ratio were 577/mm3 (IQR 94–1700), 30.2% (IQR 7.2–62%), 0.81 (IQR 0.07–2.84), respectively.
For the other ADs, at last follow-up, median viral load was undetectable (IQR 0–1056). All of them were HAART treated. Median CD4 T lymphocyte count, percentage and CD4/CD8 T lymphocytes ratio were 752/mm3 (IQR 262–1270), 35% (IQR 19–48%), 1 (IQR 0.36–1.59), respectively.
At last follow-up, all the ITP were in complete remission except one, which was in partial remission, with an uncontrolled HIV infection. For the other ADs, 11 were in complete remission, 8 were in partial remission, and 2 were refractory to the treatment (Table 3).
4. Discussion
4.1. General features
The present retrospective study in a large database of HIV-infected patients allowed us to determine the characteristics of ADs, to make a cross sectional estimate of prevalence and to define their prognosis over a long median follow-up of 5 years. Moreover, we provided a comprehensive review of the clinical features, pathogenesis, and treatments of these ADs reported in the literature.
Women were not overrepresented in our study, which is unexpected regarding general HIV-negative population,[24] but which is concordant with the literature in HIV-infected patients.[7,8] By focusing on the frequency of specific disorders, we found a proportion of sarcoidosis, GB, and IM of 0.08% (CI 0.02–0.15). International values are 0.0125% for sarcoidosis,[25] 0.0035% for GBS,[25] and 0.0051% for myositis.[24] Thus, these diseases seem to be more frequent in our cohort, suggesting a higher frequency in HIV population.
We found 0.04% of MG in our study (CI 0–0.1%) which is not significantly different from international prevalence, estimated at 0.0051%.[24]
Regarding Graves’ disease, we found only 2 cases (0.04%) (CI 0–0.1%), which is less than international values (1.151%).[24]
For ITP, we found fifteen cases (0.29%) (CI, 0.15–0.44), which is more than international values (0.025%),[25] but less than described in other case series and cohort studies.[11–15]
4.2. From serological markers of autoimmunity to clinical manifestations
The chronic infection by HIV results in production of cytokines, including interleukin 1[27] and interleukin 6,[28] which leads to activation of the CD4+ population and polyclonal B cell stimulation (in addition, production of interferon-γ will further activate the immune system). Such a mechanism may contribute to the immunopathogenesis of HIV and many other abnormalities.[29,30] The immune system is thus maintained in an activated state which maximizes viral expression and further induces CD4 receptors thereby increasing the number of cells which are susceptible to HIV infection.[31,32] Some authors suggested that clinical manifestations are the result of loss of regulatory CD8 T cells and the production of autoantibodies.[33] An infectious trigger for immune activation is one of the discussed mechanisms in autoimmunity and would derive from molecular mimicry.[34,35] This emerging concept of molecular mimicry was suggested as a way for retroviruses to escape the innate immune surveillance.[34] As a result, autoimmune diseases may only occur when there is no immunosuppression: the clinical latency with high viral load and still high CD4 count; and the immune restoration with still high CD4 count and low viral load. In between those 2 stages, there would be more immune complex or vasculitis and spondyloarthritis.[1]
4.3. HIV-associated immune thrombocytopenic purpura
Thrombocytopenia is commonly observed among HIV-infected patients (5–30%) and may be the first manifestation of HIV infection. HIV-associated ITP is more prevalent in advanced HIV infection, meaning clinical acquired immunodeficiency syndrome (AIDS) or a CD4-lymphocyte count <200/mm3, with high viral load.[5] We observed a predominance of ITP in men, which is consistent with the literature (25 men, 5 women in Ambler study,[11] 34 men, 21 women in Nascimento study).[12] Even if platelet count can be very low (<10 G/L), major bleeding is rare[36] and only a few cases of severe bleeding, like intracranial hemorrhage, have been documented.[5]
There are 2 mechanisms to explain HIV-related ITP. First, there is an immune-mediated peripheral platelet destruction, like in classic ITP, with anti-GpIIIa antibodies and the action of immune complexes.[37,38] This is more frequent early in HIV infection. The second mechanism is a defect of platelet production because of the infection of the megakaryocytes of the bone marrow, which usually occurs at an advanced stage of AIDS.[38] Of course, thrombocytopenia can also be secondary to drugs, opportunistic diseases, hypersplenism, lymphoma, or other infections. These causes of secondary thrombocytopenia were excluded in our study.
HIV-associated ITP is generally responsive to therapeutic interventions used in classical ITP: steroids, intravenous immunoglobulins, splenectomy,[39] associated with antiretroviral therapy, when it occurs early in HIV infection. These classical ITP drugs seem to be less effective in patients with advanced disease because of central infection of the megakaryocyte.[38] Thrombopoietic agents might be interesting in this case, improving T-regulatory cells function and restoring immune tolerance.[40] Only 2 cases are described in HIV-infected patients and were effective.[41,42]
4.4. Sarcoidosis and HIV
Sarcoidosis is an immune-mediated disease of unknown origin, in which the CD4 Th1 type immune response is thought to play an important role, with CD4 T lymphocytes accumulation in active granulomas. This pathogenesis might explain why sarcoidosis was rarely reported in AIDS patients.[43] As in 3 of our 4 patients, cases of sarcoidosis are usually described as a form of delayed IRIS in patients receiving HAART.[43–45] Interestingly, as for 2 of our patients (1 HCV coinfected and 1 HBV coinfected) sarcoidosis has been reported to occur after interferon-alpha therapy in both HIV-positive and HIV-negative patients.[6,46] Therefore, it appears that immune reconstitution after T cell depletion resulting from a number of causes, including HIV infection, is associated with an increased susceptibility to immune dysregulation. This dysregulation induces the Th1 immune responses against unknown antigens, producing interferon-gamma, interleukin 2, tumor necrosis factor, that underlies the granulomatous inflammation of sarcoidosis.[47,48] The susceptibility to dysregulated Th1 immune responses is presumably increased further by the use of interferon-alpha therapy, which enhances Th1 responses. In this context, it is important to differentiate immune reconstitution-associated sarcoidosis from mycobacterial and fungal immune restoration diseases.[45] Clinical and radiological characteristics, laboratory values for bronchoalveolar fluid samples, and course of sarcoidosis are similar to that observed in HIV-seronegative patients.[6,49] One of our patients presented a disseminated form with neurologic involvement which required oral steroids.
4.5. HIV-associated myositis
HIV-associated PM was first described in 1986,[50] and several reports within the last years confirm this association.[51–54] Dermatomyositis is also seen in HIV infection, but is rarer.[55–57] The clinical course (progressive, proximal, and symmetric weakness), laboratory (elevated serum creatine phosphokinase which can be absent),[58] electromyography and myopathic muscle biopsy findings are similar to the idiopathic form.[52,53,58,59] Demography in previous studies is discordant, since in 1 American series, all except 1 were men, while in 1 South African series all were women.[53,58]
Physiopathology of PM during HIV infection remains poorly understood. It can occur in all stages of HIV infection, from asymptomatic individuals to those with advanced AIDS. There is no correlation with the degree of immunodeficiency.[52,53,58] The loss of CD4 T lymphocytes during the course of HIV infection may contribute to the immune dysregulation and the generation of autoreactive CD8 T lymphocytes, which might increase CD8 T lymphocyte mediated AD such as PM.[1,52]
Corticosteroids are the most common treatment used in HIV-associated myositis, while second-line agents include immunoglobulins, methotrexate, and azathioprine.[52,53] Some cases can improve without any immunosuppressive therapy[53] or, as one of our patient, under antiretroviral therapy alone.[58] Some patients can keep significant weakness for many years despite these treatments, as 2 of our patients at last follow-up.[52]
One of our treatment refractory patient was a woman with anti-JO1 antibodies; this case is, to our knowledge, the first description of this type of inflammatory myopathy associated in an HIV patient. She presented classical clinical manifestations of myositis (proximal weakness, myalgia), associated with mechanic's hands, dactylitis, and interstitial lung disease. She has been treated with steroids, immunoglobulins, azathioprine, and methotrexate without any effectiveness and now receives rituximab.
It is important to differentiate HIV-associated myositis from toxic myopathy (zidovudine, stavudine) where symptoms recede at the end of antiretroviral treatment and muscle biopsy reveals ragged red fibers.[60]
4.6. Neurologic disorders associated with HIV infection
Neurologic complications are present at all stages of HIV infection.[61] They include HIV neurological diseases, treatment-related neurological diseases, and neoplasic or opportunistic neurological disorders.[62] As in our study, GBS usually occurs either as an acute neurologic manifestation in a primary HIV infection, at the seroconversion time, or within the first 3 to 6 months after the initiation of antiretroviral therapy.[63,64] It can also occur later during HIV infection but generally in the presence of high CD4+ T lymphocytes counts.[65]
The mechanisms proposed include a direct action of HIV-1 on the nerves, by neurotropic strains, or autoimmune mechanisms, with the formation of antibodies against myelin secondary to the immune dysregulation by HIV infection.[66]
The outcome of GBS in HIV-infected subjects is often favorable with complete remission or mild aftereffects (as in our series), although fast clinical deterioration, as respiratory failure, or deaths have been reported.[67] One study showed similar outcomes in HIV-positive and HIV-negative patients with GBS.[68]
Among treatments, plasmapheresis or immunoglobulins can be used, but the later are preferred because of their easier use.[65,67]
MG is rare during HIV infection. There are a few case reports in the literature but a causal relationship has not been shown.[52,69] Pyridostigmine is the most common treatment but use of steroids, azathioprine, cyclosporine, immunoglobulins, and rituximab has been reported.[70–73]
4.7. Autoimmune thyroid diseases and HIV
Autoimmune thyroid diseases are the most common autoimmune disease in the world. More and more authors have shown the association between HIV and autoimmune thyroid disorders. Small studies report a prevalence rate of Hashimoto thyroiditis to be up to 2.6% in HIV positive patients.[74] Some authors described Graves’ disease as a manifestation of delayed IRIS and suggested that CD4 T lymphocytes could play a role in its occurrence.[75,76] Other authors found no association between HIV infection and thyroid dysfunction.[77–79]
4.8. Rheumatoid arthritis associated with HIV infection
We found 1 patient with RA in our case series. RA associated with HIV infection is quite rare.[80–82] Stein found only 1 case of RA with erosive arthritis in his 58 HIV infected-patient cohort with arthritis.[81] In Cunha review of the literature, 9 of 198 HIV patients with arthritis presented with a rheumatoid pattern.[82] Clinical presentation, biologic features, and radiologic erosions are similar to that observed in HIV-negative patients.[80] RA usually occurs when immunity is reconstituted by HAART. The role of CD4 T cells in RA pathogenesis is debated but some cases of RA improvement after HIV infection, mediated by a decrease in CD4 T cells, have been described.[83]
However, rheumatoid factor and anticyclic citrullinated peptide antibodies can be detected in HIV-infected patients, without any rheumatic complaint.[84]
4.9. Autoimmune hepatitis and HIV
Autoimmune hepatitis during HIV infection is extremely rare and we found, in addition to our case, only 14 cases reported in the literature.[16,85–89] It has been reported during immune reconstitution,[89] or during a good immunovirological control with HAART.[16] A favorable impact of HAART is suggested by some authors.[87] Standard immunosuppressive therapy, even in HIV positive patients, is described as the optimal treatment by other authors.[86]
4.10. Autoimmune hemolytic anemia and HIV
AHA is rare in HIV-infected people, and the hemoglobin level is lower in patients at CDC stage B or C.[90] AHA usually occurs at an advanced stage of HIV disease. The response to steroids is often excellent,[91] as for our patient. Direct antiglobulin test can give false-positive results in HIV-infected patients.[90] Some cases of AHA during HIV infection are described, during angiotropic large cell lymphoma, Burkitt lymphoma or diffuse large B-cell lymphoma, and during Castleman disease.[92–95]
4.11. Systemic lupus erythematosus and HIV
SLE during HIV infection is rare. Fifty-five cases have been identified by Carugati in the literature between 1981 and 2012.[96] SLE diagnosis during HIV infection is difficult, because there are many clinical and laboratory similarities between the 2 diseases (oral ulcerations, sicca syndrome, arthritis, fever, neuropathies, cytopenias, hypergammaglobulinemia).[97] Further, antinuclear antibodies and antiphospholipid antibodies can be positive during HIV infection.[98] Because CD4 T lymphocytes play an important part in SLE pathogenesis, SLE generally improves during the course of untreated HIV infection.[99] Our case had an initial glomerulonephritis, which had been treated by cyclophosphamide. After HIV infection, he only presented cutaneous and articular manifestations, controlled by methotrexate and steroids.
4.12. Vasculitis and HIV
We did not identify any patient with vasculitis associated with HIV infection in our study, although these disorders have been reported at all stages of HIV infection.[100] Incidence is estimated less than 1%.[101] Vasculitis mostly affects small, or medium-sized vessels and is not related to antineutrophil cytoplasm antibodies (ANCA).[101] Various pathogenic mechanisms have been proposed in this setting, including cell-mediated inflammation, immune complex-mediated inflammation and autoantibody-mediated inflammation.[101] Relapse is unusual.[101] ANCA positivity by indirect immunofluorescence is reported in 13% to 42% of HIV patients, but antigen-specific ELISA is usually negative.[102] Several cases of Behcet disease during HIV infection have been described.[103–106] Diagnosis is challenging, because the 2 diseases share many clinical features: oral and genital ulcers, arthritis, uveitis, and skin lesions.[104,105] The course of the disease seems to be improved by HAART.[105,106]
4.13. Use of immunosuppressant treatment during HIV infection
Immunosuppressant treatments used in our case series are classical, according to reference treatments in non-HIV population, and to literature in HIV-infected subjects, as we have already detailed for each AD.
For a few patients, HAART alone is enough; autoimmune manifestations are directly HIV-related and improve with restauration of immunity, as during GBS at time of seroconversion or for ITP, at any stage of the infection.[38,39] The most frequent and the most effective seem to be immunosuppressant treatments, in association with HAART. These treatments are well tolerated in our case series, with a few of complications (only 1 severe osteoporosis and 5 cytopenias in our study and no severe side effects in the literature).
Our work has some limits and potential bias. First, it is a retrospective, monocentric study, led from informatical coding in a hospital information system. Because the diagnoses and data have all been checked against patient medical record with a standardized form, we believe our specificity for case selection is good. On the other hand, our screening methodology may have underestimated the prevalence of ADs due to unknown sensitivity of our extraction algorithms and potential loss of follow-up. As person-time of observation was not available, we could not estimate the incidence rates. Then, we did not compare AD cases with non-AD cases in our HIV-infected patients database.
Furthermore, we compared a cross sectional estimate of prevalence in our study with international studies which are heterogeneous: American data[24] for MG, myositis and Graves’ disease, European data for GBS and ITP[25] and global data for sarcoidosis.[25] Those estimations would deserve more accurate measurement based on multicentric incidence studies. Finally the good tolerance of immunosuppressant drug in our series should be confirmed with a large number of patients.
5. Conclusion
AD during HIV infection is a rare event. Sarcoidosis, ITP, IM, and neurologic manifestations seem to be more frequent in our cohort than in the general population. Except ITP which is more prevalent in advanced HIV infection, they occur most often in a context of effective HAART with good immunological response or during IRIS. GBS often occur at the time of HIV infection diagnosis. Their clinical manifestations are quite similar to the general population. HAART allows immune modulations, with immune restoration and development of autoimmune manifestations. Immunosuppressant drugs in this context seem to be effective, often well tolerated and not associated with new opportunistic infection.
Acknowledgments
The authors thank Soifya Daoud, Olivier Loria, and Anthony Heredia for their technical help for this study.
Footnotes
Abbreviations: 95% CI = confidence intervals at 95%, AD = autoimmune disease, AHA = autoimmune hemolytic anemia, AIDS = acquired immunodeficiency syndrome, ANCA = antineutrophil cytoplasm antibodies, CDC = Center for Diseases Control, GBS = Guillain–Barré syndrome, HAART = highly active antiretroviral therapy, HBV = hepatitis B virus, HCV = hepatitis C virus, HIV = human immunodeficiency virus, ICD-10 = International Classification of Diseases 10th, IM = inflammatory myositis, IQR = interquartile range, IRIS = immune restoration inflammatory syndrome, ITP = immune thrombocytopenic purpura, MG = myasthenia gravis, PM = polymyositis, RA = rheumatoid arthritis, SIR = standardized incidence rates, SLE = systemic lupus erythematosus.
Study performed at Hospices Civils de Lyon, Quai des Célestins, 69288 Lyon Cedex 02, France.
Preliminary results of this study were presented during the 68th Congress of the French Society of Internal Medicine (Besançon, June 2015).
Authors’ contributions: EV collected the data. EV, AD, and PS performed the statistical analyses and interpreted the data. EV, AD, LA, PM, TF, AH, and PS drafted and revised the manuscript. All authors have read and approved the final version.
The authors have no conflicts of interest to disclose.
References
- [1].Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev 2002;1:329–37. [DOI] [PubMed] [Google Scholar]
- [2].Kaku Y, Kodama S, Higuchi M, et al. Corticoid therapy for overlapping syndromes in an HIV-positive patient. Intern Med 2015;54:223–30. [DOI] [PubMed] [Google Scholar]
- [3].Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol 2009;9:235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mientjes GH, van Ameijden EJ, Mulder JW, et al. Prevalence of thrombocytopenia in HIV-infected and non-HIV infected drug users and homosexual men. Br J Haematol 1992;82:615–9. [DOI] [PubMed] [Google Scholar]
- [5].Sloand EM, Klein HG, Banks SM, et al. Epidemiology of thrombocytopenia in HIV infection. Eur J Haematol 1992;48:168–72. [DOI] [PubMed] [Google Scholar]
- [6].Foulon G, Wislez M, Naccache J-M, et al. Sarcoidosis in HIV-infected patients in the era of highly active antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am 2004;38:418–25. [DOI] [PubMed] [Google Scholar]
- [7].Calabrese LH, Kirchner E, Shrestha R. Rheumatic complications of human immunodeficiency virus infection in the era of highly active antiretroviral therapy: emergence of a new syndrome of immune reconstitution and changing patterns of disease. Semin Arthritis Rheum 2005;35:166–74. [DOI] [PubMed] [Google Scholar]
- [8].Yang J-J, Tsai M-S, Sun H-Y, et al. Autoimmune diseases-related arthritis in HIV-infected patients in the era of highly active antiretroviral therapy. J Microbiol Immunol Infect 2015;48:130–6. [DOI] [PubMed] [Google Scholar]
- [9].Berman A, Espinoza LR, Diaz JD, et al. Rheumatic manifestations of human immunodeficiency virus infection. Am J Med 1988;85:59–64. [DOI] [PubMed] [Google Scholar]
- [10].Cuellar ML, Espinoza LR. Rheumatic manifestations of HIV-AIDS. Baillières Best Pract Res Clin Rheumatol 2000;14:579–93. [DOI] [PubMed] [Google Scholar]
- [11].Ambler KL, Vickars LM, Leger CS, et al. Clinical features, treatment, and outcome of HIV-associated immune thrombocytopenia in the HAART era. Adv Hematol 2012;2012:910954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nascimento FG, Tanaka PY. Thrombocytopenia in HIV-infected patients. Indian J Haematol Blood Transfus 2012;28:109–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Franzetti M, Adorni F, Oreni L, et al. Changes in the incidence of severe thrombocytopenia and its predisposing conditions in HIV-infected patients since the introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2014;67:493–8. [DOI] [PubMed] [Google Scholar]
- [14].Taremwa IM, Muyindike WR, Muwanguzi E, et al. Prevalence of HIV-related thrombocytopenia among clients at Mbarara regional referral hospital, Mbarara, southwestern Uganda. J Blood Med 2015;6:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].O’Bryan TA, Okulicz JF, Bradley WP, et al. Impact of the highly active antiretroviral therapy era on the epidemiology of primary HIV-associated thrombocytopenia. BMC Res Notes 2015;8:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Iordache L, Launay O, Bouchaud O, et al. Autoimmune diseases in HIV-infected patients: 52 cases and literature review. Autoimmun Rev 2014;13:850–7. [DOI] [PubMed] [Google Scholar]
- [17].Yen YF, Chuang PH, Jen IA, et al. Incidence of autoimmune diseases in a nationwide HIV/AIDS patient cohort in Taiwan, 2000–2012. Ann Rheum Dis 2016. [DOI] [PubMed] [Google Scholar]
- [18].Pugliese P, Cuzin L, Cabié A, et al. A large French prospective cohort of HIV-infected patients: the Nadis Cohort. HIV Med 2009;10:504–11. [DOI] [PubMed] [Google Scholar]
- [19].Phillips AN, Grabar S, Tassie JM, et al. Use of observational databases to evaluate the effectiveness of antiretroviral therapy for HIV infection: comparison of cohort studies with randomized trials. EuroSIDA, the French Hospital Database on HIV and the Swiss HIV Cohort Study Groups. AIDS Lond Engl 1999;13:2075–82. [DOI] [PubMed] [Google Scholar]
- [20].Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- [21].Hoogendijk JE, Amato AA, Lecky BR, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, The Netherlands. Neuromuscul Disord NMD 2004;14:337–45. [DOI] [PubMed] [Google Scholar]
- [22].Petri M, Orbai A-M, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 2009;113:2386–93. [DOI] [PubMed] [Google Scholar]
- [24].Jacobson DL, Gange SJ, Rose NR, et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 1997;84:223–43. [DOI] [PubMed] [Google Scholar]
- [25].Les Cahiers d’Orphanet - Prevalence of rare diseases: Bibliographic data. 2016. [Google Scholar]
- [26].Altman DG. Practical Statistics for Medical Research, Chapter 4—Theoretical Distributions. London:Chapman and Hall; 1991. [Google Scholar]
- [27].Lepe-Zuniga JL, Mansell PW, Hersh EM. Idiopathic production of interleukin-1 in acquired immune deficiency syndrome. J Clin Microbiol 1987;25:1695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nakajima K, Martínez-Maza O, Hirano T, et al. Induction of IL-6 (B cell stimulatory factor-2/IFN-beta 2) production by HIV. J Immunol (Baltim Md 1950) 1989;142:531–6. [PubMed] [Google Scholar]
- [29].Malatzky-Goshen E, Shoenfeld Y. AIDS and autoimmunity. Autoimmunity 1989;3:201–12. [DOI] [PubMed] [Google Scholar]
- [30].Stricker RB, McHugh TM, Moody DJ, et al. An AIDS-related cytotoxic autoantibody reacts with a specific antigen on stimulated CD4+ T cells. Nature 1987;327:710–3. [DOI] [PubMed] [Google Scholar]
- [31].Ascher MS, Sheppard HW. AIDS as immune system activation: a model for pathogenesis. Clin Exp Immunol 1988;73:165–7. [PMC free article] [PubMed] [Google Scholar]
- [32].Rosenberg ZF, Fauci AS. Immunology of AIDS: approaches to understanding the immunopathogenesis of HIV infection. Ric Clin Lab 1989;19:189–209. [DOI] [PubMed] [Google Scholar]
- [33].Morrow WJ, Isenberg DA, Sobol RE, et al. AIDS virus infection and autoimmunity: a perspective of the clinical, immunological, and molecular origins of the autoallergic pathologies associated with HIV disease. Clin Immunol Immunopathol 1991;58:163–80. [DOI] [PubMed] [Google Scholar]
- [34].Cohen AD, Shoenfeld Y. The viral-autoimmunity relationship. Viral Immunol 1995;8:1–9. [DOI] [PubMed] [Google Scholar]
- [35].Shoenfeld Y. Common infections, idiotypic dysregulation, autoantibody spread and induction of autoimmune diseases. J Autoimmun 1996;9:235–9. [DOI] [PubMed] [Google Scholar]
- [36].Scaradavou A. HIV-related thrombocytopenia. Blood Rev 2002;16:73–6. [DOI] [PubMed] [Google Scholar]
- [37].Johnsen J. Pathogenesis in immune thrombocytopenia: new insights. Hematology Am Soc Hematol Educ Program 2012;2012:306–12. [DOI] [PubMed] [Google Scholar]
- [38].Passos AM, Treitinger A, Spada C. An overview of the mechanisms of HIV-related thrombocytopenia. Acta Haematol 2010;124:13–8. [DOI] [PubMed] [Google Scholar]
- [39].Liebman HA. Viral-associated immune thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program 2008;2008:212–8. [DOI] [PubMed] [Google Scholar]
- [40].Bao W, Bussel JB, Heck S, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood 2010;116:4639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gudbrandsdottir S, Frederiksen H, Hasselbalch H. Thrombopoietin-receptor agonists in haematological disorders: the Danish experience. Platelets 2012;23:423–9. [DOI] [PubMed] [Google Scholar]
- [42].Arumugaswamy A, He S, Quach H, et al. Thrombopoietin receptor agonists in immune thrombocytopenia of less than 6 months duration. Intern Med J 2014;44:519–21. [DOI] [PubMed] [Google Scholar]
- [43].Gomez V, Smith PR, Burack J, et al. Sarcoidosis after antiretroviral therapy in a patient with acquired immunodeficiency syndrome. Clin Infect Dis Off Publ Infect Dis Soc Am 2000;31:1278–80. [DOI] [PubMed] [Google Scholar]
- [44].Mirmirani P, Maurer TA, Herndier B, et al. Sarcoidosis in a patient with AIDS: a manifestation of immune restoration syndrome. J Am Acad Dermatol 1999;41(2 Pt 2):285–6. [DOI] [PubMed] [Google Scholar]
- [45].Lassalle S, Selva E, Hofman V, et al. Sarcoid-like lesions associated with the immune restoration inflammatory syndrome in AIDS: absence of polymerase chain reaction detection of Mycobacterium tuberculosis in granulomas isolated by laser capture microdissection. Virchows Arch Int J Pathol 2006;449:689–96. [DOI] [PubMed] [Google Scholar]
- [46].Faurie P, Broussolle C, Zoulim F, et al. Sarcoidosis and hepatitis C: clinical description of 11 cases. Eur J Gastroenterol Hepatol 2010;22:967–72. [DOI] [PubMed] [Google Scholar]
- [47].Trevenzoli M, Cattelan AM, Marino F, et al. Sarcoidosis and HIV infection: a case report and a review of the literature. Postgrad Med J 2003;79:535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Roustan G, Yebra M, Rodriguez-Braojos O, et al. Cutaneous and pulmonary sarcoidosis in a patient with HIV after highly active antiretroviral therapy. Int J Dermatol 2007;46:68–71. [DOI] [PubMed] [Google Scholar]
- [49].Lenner R, Bregman Z, Teirstein AS, et al. Recurrent pulmonary sarcoidosis in HIV-infected patients receiving highly active antiretroviral therapy. Chest 2001;119:978–81. [DOI] [PubMed] [Google Scholar]
- [50].Dalakas MC, Pezeshkpour GH, Gravell M, et al. Polymyositis associated with AIDS retrovirus. JAMA 1986;256:2381–3. [PubMed] [Google Scholar]
- [51].Espinoza LR, Aguilar JL, Espinoza CG, et al. Characteristics and pathogenesis of myositis in human immunodeficiency virus infection—distinction from azidothymidine-induced myopathy. Rheum Dis Clin North Am 1991;17:117–29. [PubMed] [Google Scholar]
- [52].Robinson-Papp J, Simpson DM. Neuromuscular diseases associated with HIV-1 infection. Muscle Nerve 2009;40:1043–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Johnson RW, Williams FM, Kazi S, et al. Human immunodeficiency virus-associated polymyositis: a longitudinal study of outcome. Arthritis Rheum 2003;49:172–8. [DOI] [PubMed] [Google Scholar]
- [54].Simpson DM, Bender AN. Human immunodeficiency virus-associated myopathy: analysis of 11 patients. Ann Neurol 1988;24:79–84. [DOI] [PubMed] [Google Scholar]
- [55].Gresh JP, Aquilar JL, Espinoza LR. Human immunodeficiency virus (HIV) infection-associated dermatomyositis. J Rheumatol 1989;16:1397–8. [PubMed] [Google Scholar]
- [56].Carroll MB, Holmes R. Dermatomyositis and HIV infection: case report and review of the literature. Rheumatol Int 2011;31:673–9. [DOI] [PubMed] [Google Scholar]
- [57].Ogoina D, Umar A, Obiako OR. Dermatomyositis associated with HIV-1 infection in a Nigerian adult female: a case report. Afr Health Sci 2012;12:74–6. [PMC free article] [PubMed] [Google Scholar]
- [58].Heckmann JM, Pillay K, Hearn AP, et al. Polymyositis in African HIV-infected subjects. Neuromuscul Disord NMD 2010;20:735–9. [DOI] [PubMed] [Google Scholar]
- [59].Illa I, Nath A, Dalakas M. Immunocytochemical and virological characteristics of HIV-associated inflammatory myopathies: similarities with seronegative polymyositis. Ann Neurol 1991;29:474–81. [DOI] [PubMed] [Google Scholar]
- [60].Cupler EJ, Danon MJ, Jay C, et al. Early features of zidovudine-associated myopathy: histopathological findings and clinical correlations. Acta Neuropathol (Berl) 1995;90:1–6. [DOI] [PubMed] [Google Scholar]
- [61].Geraci AP, Simpson DM. Neurological manifestations of HIV-1 infection in the HAART era. Compr Ther 2001;27:232–41. [DOI] [PubMed] [Google Scholar]
- [62].Singer EJ, Valdes-Sueiras M, Commins D, et al. Neurologic presentations of AIDS. Neurol Clin 2010;28:253–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Piliero PJ, Fish DG, Preston S, et al. Guillain-Barré syndrome associated with immune reconstitution. Clin Infect Dis Off Publ Infect Dis Soc Am 2003;36:e111–4. [DOI] [PubMed] [Google Scholar]
- [64].Brannagan TH, Zhou Y. HIV-associated Guillain-Barré syndrome. J Neurol Sci 2003;208:39–42. [DOI] [PubMed] [Google Scholar]
- [65].Fantauzzi A, Digiulio MA, Cavallari EN, et al. Guillain Barre syndrome in an HIV-1-infected patient after the beginning of combined antiretroviral therapy: an immune reconstitution inflammatory syndrome? New Microbiol 2014;37:103–7. [PubMed] [Google Scholar]
- [66].Dalakas MC, Pezeshkpour GH. Neuromuscular diseases associated with human immunodeficiency virus infection. Ann Neurol 1988;23(suppl):S38–48. [DOI] [PubMed] [Google Scholar]
- [67].Pontali E, Feasi M, Crisalli MP, et al. Guillain-Barré syndrome with fatal outcome during HIV-1-seroconversion: a case report. Case Rep Infect Dis 2011;2011:972096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Schleicher GK, Black A, Mochan A, et al. Effect of human immunodeficiency virus on intensive care unit outcome of patients with Guillain-Barré syndrome. Crit Care Med 2003;31:1848–50. [DOI] [PubMed] [Google Scholar]
- [69].Nath A, Kerman RH, Novak IS, et al. Immune studies in human immunodeficiency virus infection with myasthenia gravis: a case report. Neurology 1990;40:581–3. [DOI] [PubMed] [Google Scholar]
- [70].Kuntzer T, Carota A, Novy J, et al. Rituximab is successful in an HIV-positive patient with MuSK myasthenia gravis. Neurology 2011;76:757–8. [DOI] [PubMed] [Google Scholar]
- [71].Knopf L, Menkes DL. Comorbid HIV and myasthenia gravis: case report and review of the literature. J Clin Neuromuscul Dis 2010;12:80–4. [DOI] [PubMed] [Google Scholar]
- [72].Strong J, Zochodne DW. Seronegative myasthenia gravis and human immunodeficiency virus infection: response to intravenous gamma globulin and prednisone. Can J Neurol Sci J Can Sci Neurol 1998;25:254–6. [DOI] [PubMed] [Google Scholar]
- [73].Kurokawa T, Nishiyama T, Yamamoto R, et al. Anti-MuSK antibody positive myasthenia gravis with HIV infection successfully treated with cyclosporin: a case report. Rinshō Shinkeigaku Clin Neurol 2008;48:666–9. [DOI] [PubMed] [Google Scholar]
- [74].Parsa AA, Bhangoo A. HIV and thyroid dysfunction. Rev Endocr Metab Disord 2013;14:127–31. [DOI] [PubMed] [Google Scholar]
- [75].Chen F, Day SL, Metcalfe RA, et al. Characteristics of autoimmune thyroid disease occurring as a late complication of immune reconstitution in patients with advanced human immunodeficiency virus (HIV) disease. Medicine (Baltimore) 2005;84:98–106. [DOI] [PubMed] [Google Scholar]
- [76].Jubault V, Penfornis A, Schillo F, et al. Sequential occurrence of thyroid autoantibodies and Graves’ disease after immune restoration in severely immunocompromised human immunodeficiency virus-1-infected patients. J Clin Endocrinol Metab 2000;85:4254–7. [DOI] [PubMed] [Google Scholar]
- [77].Hoffmann CJ, Brown TT. Thyroid function abnormalities in HIV-infected patients. Clin Infect Dis Off Publ Infect Dis Soc Am 2007;45:488–94. [DOI] [PubMed] [Google Scholar]
- [78].Madge S, Smith CJ, Lampe FC, et al. No association between HIV disease and its treatment and thyroid function. HIV Med 2007;8:22–7. [DOI] [PubMed] [Google Scholar]
- [79].Afhami S, Haghpanah V, Heshmat R, et al. Assessment of the factors involving in the development of hypothyroidism in HIV-infected patients: a case-control study. Infection 2007;35:334–8. [DOI] [PubMed] [Google Scholar]
- [80].Azeroual A, Harmouche H, Benjilali L, et al. Rheumatoid arthritis associated to HIV infection. Eur J Intern Med 2008;19:e34–5. [DOI] [PubMed] [Google Scholar]
- [81].Stein CM, Davis P. Arthritis associated with HIV infection in Zimbabwe. J Rheumatol 1996;23:506–11. [PubMed] [Google Scholar]
- [82].Cunha BM, Mota LMH, Pileggi GS, et al. HIV/AIDS and rheumatoid arthritis. Autoimmun Rev 2015;14:396–400. [DOI] [PubMed] [Google Scholar]
- [83].Lapadula G, Iannone F, Zuccaro C, et al. Recovery of erosive rheumatoid arthritis after human immunodeficiency virus-1 infection and hemiplegia. J Rheumatol 1997;24:747–51. [PubMed] [Google Scholar]
- [84].du Toit R, Whitelaw D, Taljaard JJ, et al. Lack of specificity of anticyclic citrullinated peptide antibodies in advanced human immunodeficiency virus infection. J Rheumatol 2011;38:1055–60. [DOI] [PubMed] [Google Scholar]
- [85].Wan DW, Marks K, Yantiss RK, et al. Autoimmune hepatitis in the HIV-infected patient: a therapeutic dilemma. AIDS Patient Care STDs 2009;23:407–13. [DOI] [PubMed] [Google Scholar]
- [86].Puius YA, Dove LM, Brust DG, et al. Three cases of autoimmune hepatitis in HIV-infected patients. J Clin Gastroenterol 2008;42:425–9. [DOI] [PubMed] [Google Scholar]
- [87].German V, Vassiloyanakopoulos A, Sampaziotis D, et al. Autoimmune hepatitis in an HIV infected patient that responded to antiretroviral therapy. Scand J Infect Dis 2005;37:148–51. [DOI] [PubMed] [Google Scholar]
- [88].Hagel S, Bruns T, Herrmann A, et al. Autoimmune hepatitis in an HIV-infected patient: an intriguing association. Int J STD AIDS 2012;23:448–50. [DOI] [PubMed] [Google Scholar]
- [89].O’Leary JG, Zachary K, Misdraji J, et al. De novo autoimmune hepatitis during immune reconstitution in an HIV-infected patient receiving highly active antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am 2008;46:e12–4. [DOI] [PubMed] [Google Scholar]
- [90].Lai M, Visconti E, D’Onofrio G, et al. Lower hemoglobin levels in human immunodeficiency virus-infected patients with a positive direct antiglobulin test (DAT): relationship with DAT strength and clinical stages. Transfusion (Paris) 2006;46:1237–43. [DOI] [PubMed] [Google Scholar]
- [91].Koduri PR, Singa P, Nikolinakos P. Autoimmune hemolytic anemia in patients infected with human immunodeficiency virus-1. Am J Hematol 2002;70:174–6. [DOI] [PubMed] [Google Scholar]
- [92].Sukthankar AD, Bowman CA, Carey M, et al. HIV infection with haemolytic anaemia. Genitourin Med 1997;73:66–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chen M, Abedi M. Atypical lymphocytosis, cold agglutinin hemolytic anemia, and monoclonal gammopathy in an HIV patient with marrow involvement by diffuse large B-cell lymphoma. Blood 2013;122:3711. [DOI] [PubMed] [Google Scholar]
- [94].López Dupla JM, Rodríguez Pérez A, Martínez Martínez P, et al. Hemolytic anemia due to cold-reacting antibodies: association with human immunodeficiency virus infection and non-Hodgkin's lymphoma. Med Clin 1992;98:502–4. [PubMed] [Google Scholar]
- [95].Gopal S, Liomba NG, Montgomery ND, et al. Characteristics and survival for HIV-associated multicentric Castleman disease in Malawi. J Int AIDS Soc 2015;18:20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Carugati M, Franzetti M, Torre A, et al. Systemic lupus erythematosus and HIV infection: a whimsical relationship. Reports of two cases and review of the literature. Clin Rheumatol 2013;32:1399–405. [DOI] [PubMed] [Google Scholar]
- [97].Walker UA, Tyndall A, Daikeler T. Rheumatic conditions in human immunodeficiency virus infection. Rheumatol Oxf Engl 2008;47:952–9. [DOI] [PubMed] [Google Scholar]
- [98].Kopelman RG, Zolla-Pazner S. Association of human immunodeficiency virus infection and autoimmune phenomena. Am J Med 1988;84:82–8. [DOI] [PubMed] [Google Scholar]
- [99].Fox RA, Isenberg DA. Human immunodeficiency virus infection in systemic lupus erythematosus. Arthritis Rheum 1997;40:1168–72. [DOI] [PubMed] [Google Scholar]
- [100].Otedo AEO, Oyoo GO, Obondi JO, et al. Vasculitis in HIV: report of eight cases. East Afr Med J 2005;82:656–9. [DOI] [PubMed] [Google Scholar]
- [101].Guillevin L. Vasculitides in the context of HIV infection. AIDS Lond Engl 2008;22(suppl 3):S27–33. [DOI] [PubMed] [Google Scholar]
- [102].Evans R, Gueret-Wardle A, Edwards S, et al. ANCA-associated vasculitis and pauci-immune glomerulonephritis in HIV disease. BMJ Case Rep 2014. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Buskila D, Gladman DD, Gilmore J, et al. Behçet's disease in a patient with immunodeficiency virus infection. Ann Rheum Dis 1991;50:115–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Gómez-Puerta JA, Espinosa G, Miró JM, et al. Behçet's disease in an HIV-1-infected patient treated with highly active antiretroviral therapy. Isr Med Assoc J IMAJ 2006;8:513–4. [PubMed] [Google Scholar]
- [105].Mahajan VK, Sharma NL, Sharma VC, et al. Behcet's disease with HIV infection: response to antiretroviral therapy. Indian J Dermatol Venereol Leprol 2005;71:276–8. [DOI] [PubMed] [Google Scholar]
- [106].Roscoe C, Kinney R, Gilles R, et al. Behçet's disease diagnosed after acute HIV infection: viral replication activating underlying autoimmunity? Int J STD AIDS 2015;26:432–5. [DOI] [PubMed] [Google Scholar]


