Abstract
The aim of the study was to determine the prognostic role of systemic immune-inflammation index (SII) in patients with esophageal squamous cell carcinoma (ESCC).
A total of 298 ESCC patients were enrolled in the current retrospective study. The SII was calculated by the formula: neutrophil × platelet/lymphocyte. The optimal cut-off value was calculated by the Cutoff Finder. Univariate and multivariate analyses were evaluated for cancer-specific survival (CSS). Additional, we also established a nomogram model to predict the prognosis for patients with ESCC.
The optimal cut-off value was 410 × 109/L for SII. Patients with SII ≤ 410 (×109/L) had a significantly better 5-year CSS than patients with SII > 410 (×109/L) (51.9% vs 24.0%, P < 0.001). Multivariate analyses revealed that SII was a significant independent predictive indicator (P = 0.027). A nomogram could be more accuracy for CSS for patients with ESCC (c-index: 0.68).
The SII is a useful independent prognostic indicator for patients with resectable ESCC.
Keywords: cancer-specific survival (CSS), esophageal squamous cell carcinoma (ESCC), neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), prognosis
1. Introduction
Esophageal cancer (EC) is one of the most fatal types of cancer wordwide, with 455,800 new cases and 400,200 deaths in 2012.[1,2] Esophageal squamous cell carcinoma (ESCC) is the predominant type in China, in contrast to the predominance of esophageal adenocarcinoma in the western countries, which covers more than 90% of all EC cases.[3,4] Surgery remains the treatment of choice for localized cancer; however, the prognosis is still poor.[5]
In recent years, inflammation plays a key role in the prognosis of cancer.[6,7] Previous studies have shown that a number of inflammatory biomarkers, such as c-reactive protein (CRP), neutrophil to lymphocyte ratio (NLR), and platelet to lymphocyte ratio (PLR), were associated with prognosis in several types of cancer, including EC.[8–12] Recently, the systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with small cell lung cancer and hepatocellular carcinoma.[13,14] To the best of our knowledge, however, no studies regarding SII in patients with EC are available. In this study, therefore, we aimed to determine the prognostic value of SII in patients with resectable ESCC.
2. Materials and methods
A total of 298 patients with resectable ESCC were included in the current retrospective study from Jan 2005 to Dec 2008. The eligibility criteria were included: (1) ESCC diagnosed by histopathology; (2) curative surgery for localized disease (stage I–III); (3) without neoadjuvant treatment; (4) without any form of acute and/or chronic inflammatory disease; and (5) preoperative serum laboratory examination were obtained before surgery within 1 week. Patients who had received neoadjuvant therapy were excluded in the current study. The current study was approved by the Ethics Committees of Zhejiang Cancer Hospital.
Data on the preoperative laboratory examination were extracted in our medical records. The counts for neutrophil, lymphocyte, and platelet were taken within 1 week prior to surgery. The SII was defined as follows: SII = neutrophil × platelet/lymphocyte. In the current study, a cancer-specific survival (CSS) analysis was ascertained.[13,14] The last follow-up was June 2013. All patients in this study were staged according to the 7th edition Cancer Staging.[15]
2.1. Statistical analysis
Statistical analyses were conducted with SPSS 17.0 (SPSS Inc., Chicago, IL) and R 3.2.3 software (Institute for Statistics and Mathematics, Vienna, Austria). A web-based R software (Cutoff Finder) was used to determine the optimal cut-off value for SII. Cutoff Finder is a freely available web application that can be accessed using an arbitrary web browser (http://molpath.charite.de/cutoff).[16] Kaplan–Meier methods were used to estimate CSS. Univariate and multivariate analyses were used to evaluate the prognostic factors. A nomogram model was also established and the predictive accuracy was evaluated by Harrell's concordance index (c-index).[17] In the current study, P < 0.05 was considered statistically significant.
3. Results
Of the total number of patients, 38 (12.8%) were women and 260 (87.2%) were men. The mean values for neutrophil, platelet, lymphocyte, and SII were 4.54 ± 1.92 (×109/L), 225 ± 74 (×109/L) and 750 ± 482 (×109/L), respectively. Additionally, correlation analyses showed that SII was negatively correlated with lymphocyte (r = –0.362, P < 0.001) and positively correlated with neutrophil (r = 0.620, P < 0.001) and platelet (r = 0.607, P < 0.001) (Fig. 1).
Figure 1.
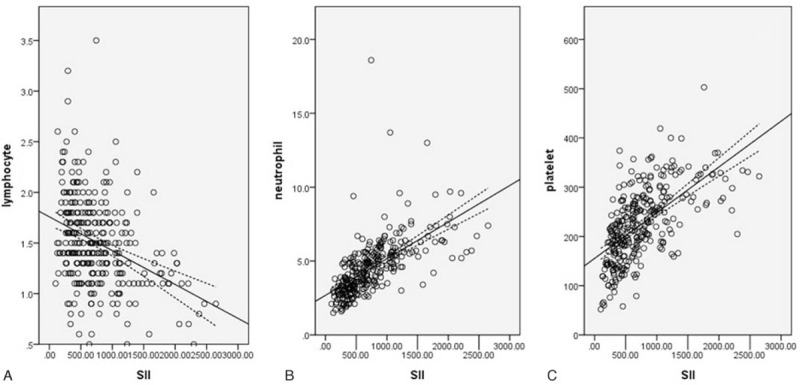
Pearson correlation. SII was negatively correlated with lymphocyte (r = –0.362, P < 0.001; A) and positively correlated with neutrophil (r = 0.620, P < 0.001; B) and platelet (r = 0.607, P < 0.001; C). SII = immune-inflammation index.
The optimal cut-off value for SII was 410 (×109/L) according to the Cutoff Finder (Fig. 2). Patients then were divided into 2 groups using the SII cut-off of 410 (×109/L). There were 81 (27.2%) patients with SII ≤ 410 (×109/L) and 217 (72.8%) patients with SII > 410 (×109/L). The relationships between SII and clinical characteristics were shown in Table 1. In the current study, the SII was significantly associated with tumor length (P = 0.006), TNM stage (P = 0.012), NLR (P < 0.001), PLR (P < 0.001), and CRP (P < 0.001).
Figure 2.
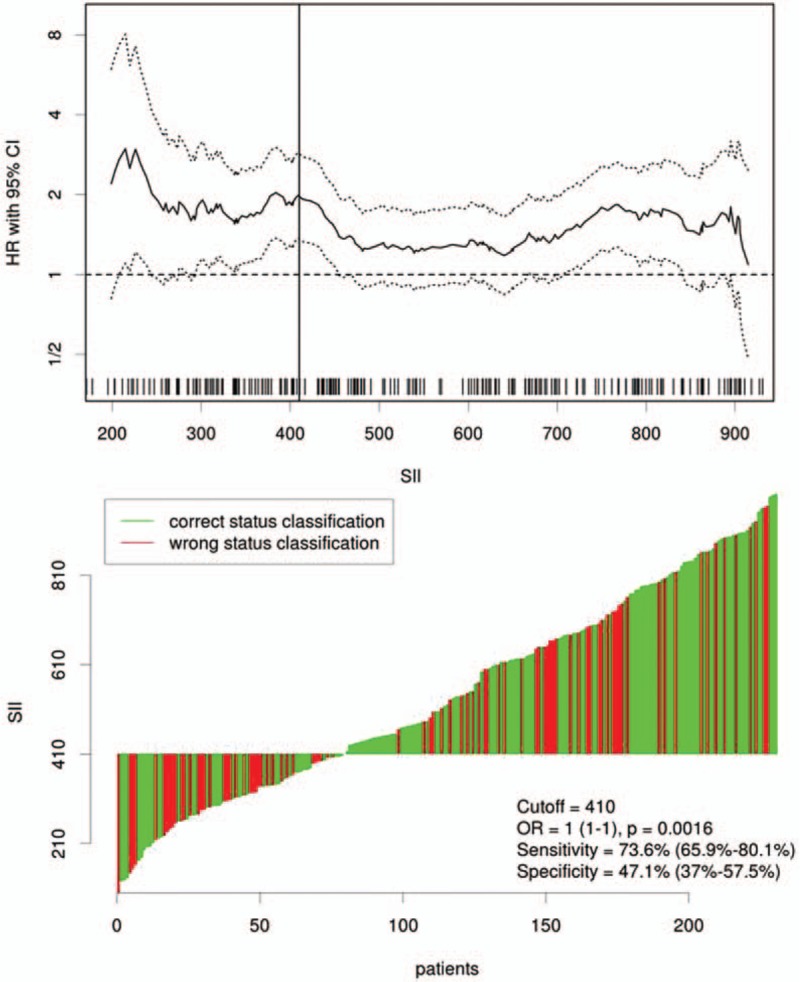
Cutoff Finder for optimal cut-off value of SII. The vertical line designates the optimal cut-off point with the most significant (log-rank test) split. The plots were generated using the biostatistical tool Cutoff Finder. The cut-off value was 410 with a sensitivity of 73.6% and a specificity of 47.1%. SII = immune-inflammation index.
Table 1.
The relationship between SII and clinical characteristics in ESCC patients.
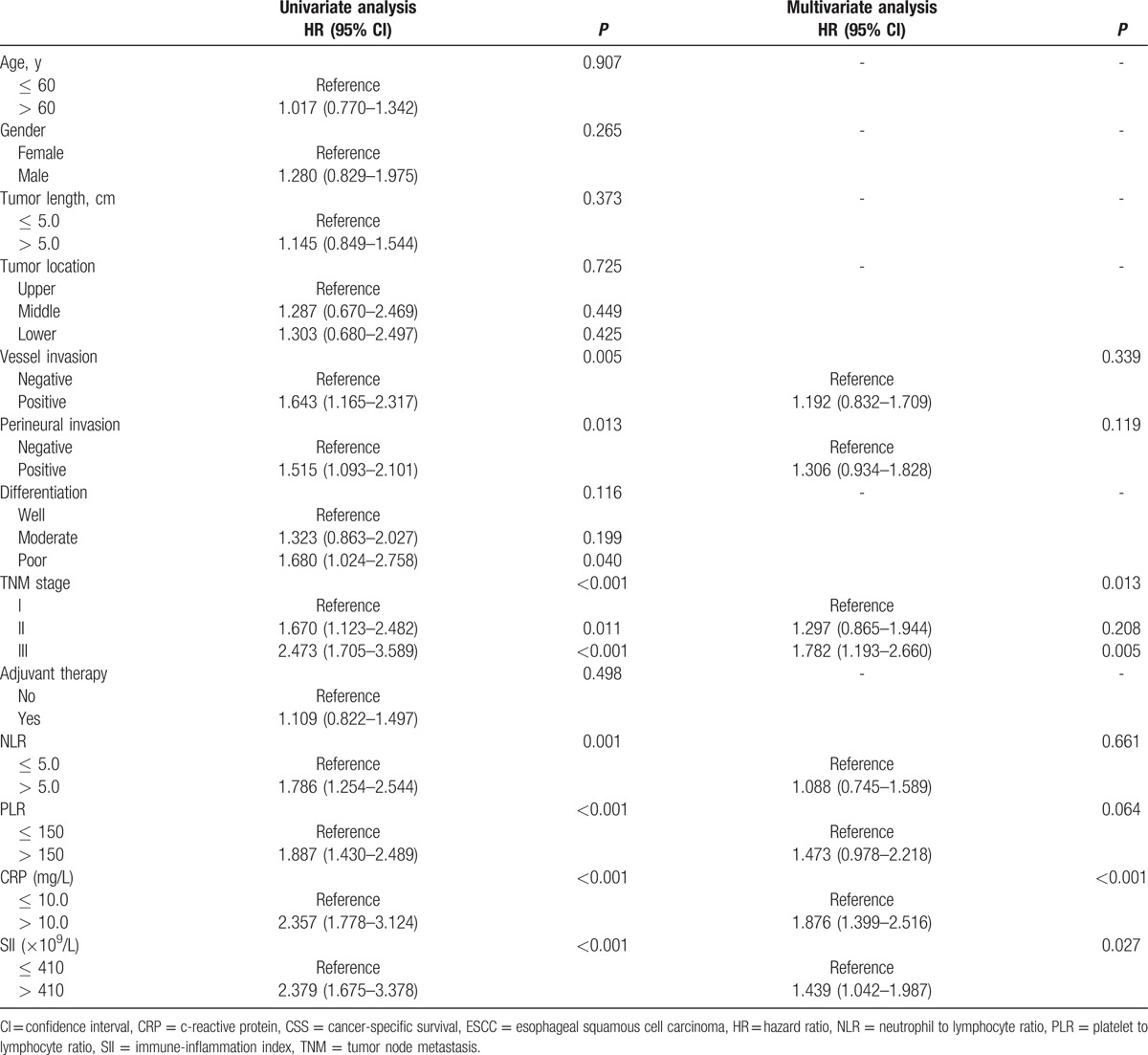
Patients with SII ≤ 410 (×109/L) had a significantly better 5-year CSS than patients with SII > 410 (×109/L) (51.9% vs 24.0%, P < 0.001) (Fig. 3A). In subgroup analyses, we revealed that SII was also significantly associated with CSS based on the TNM stage (Fig. 3B–D). The subgroup analyses based on SII for CSS were shown in Table 2. In multivariate analyses, SII was an independent prognostic factor in patients with resectable ESCC (P = 0.027). However, NLR (P = 0.661) or PLR (P = 0.064) were not significant independent predictors for CSS (Table 3). In the current study, we used the CRP, a well-known inflammatory indicator, in multivariate analyses. Our result revealed that CRP was still an independent prognostic factor in patients with ESCC (P < 0.001).
Figure 3.
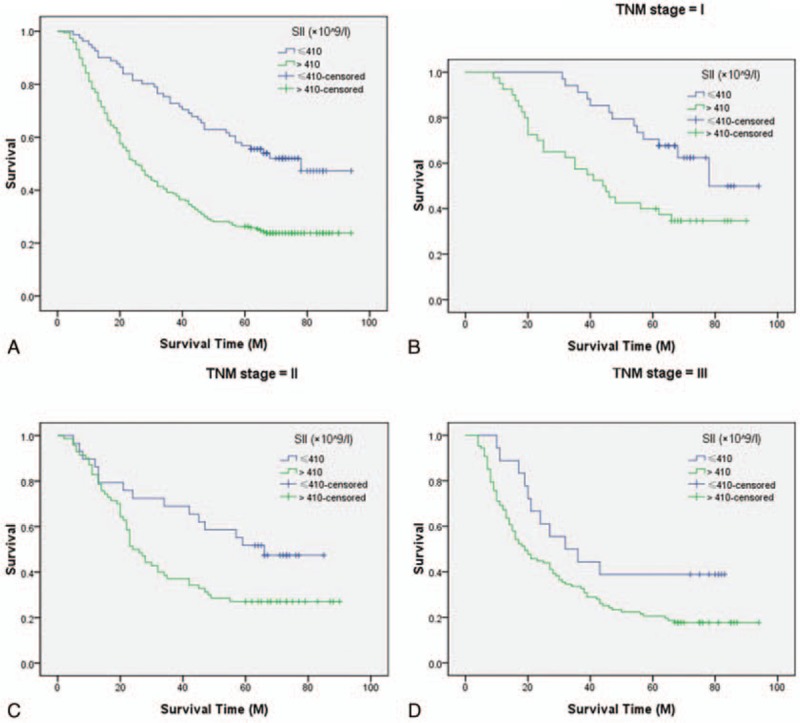
Kaplan–Meier CSS curves stratified by SII. Patients with SII ≤ 410 (×109/L) had a significantly better 5-year CSS than patients with SII > 410 (×109/L) (51.9% vs 24.0%, P < 0.001; A). The predictive value of SII was significant in patients based on the TNM stage (I stage: 61.8% vs 35.0%, P = 0.005; B), (II stage: 48.3% vs 27.1%, P = 0.028; C), and (III stage: 38.9% vs 17.8%, P = 0.045; D). CSS = cancer-specific survival, SII = immune-inflammation index, TNM = tumor node metastasis.
Table 2.
Subgroup analysis for CSS.
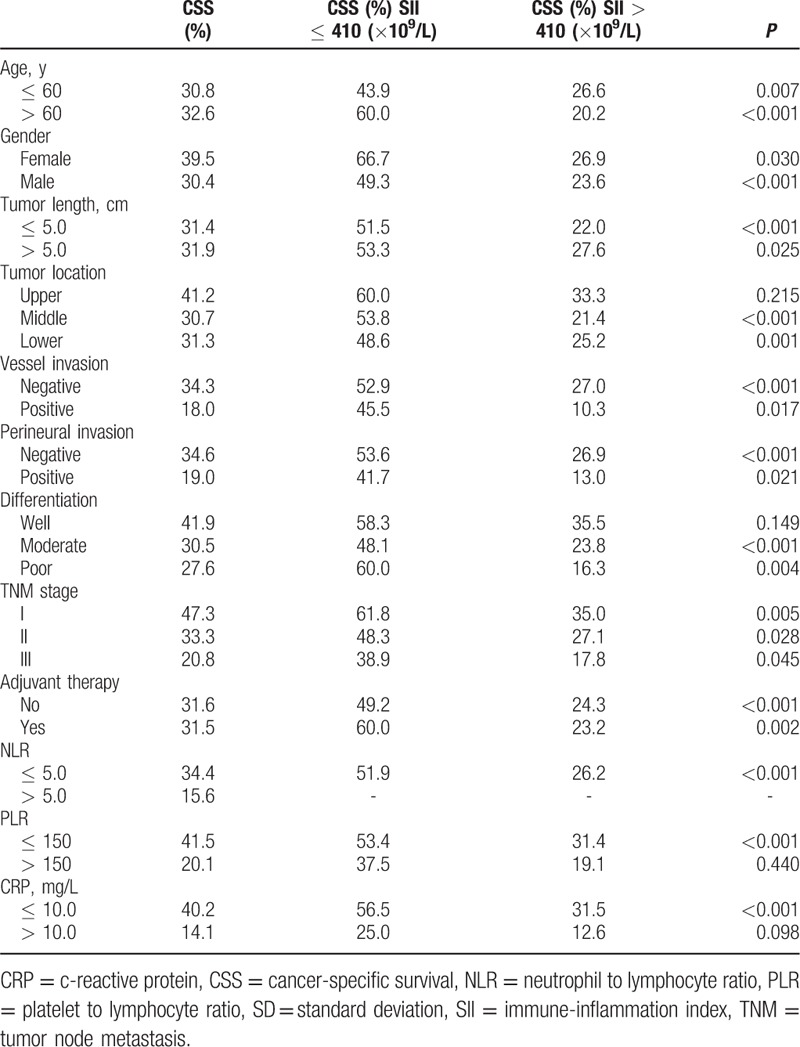
Table 3.
Univariate and multivariate analyses of CSS in ESCC patients.
To predict the risk for patients with ESCC, a novel nomogram model was established by SII, CRP, and TNM stage combined with age and sex (Fig. 4). It can predict the probability of death for patients with ESCC. In the current study, Harrell's c-index for CSS prediction was 0.68.
Figure 4.
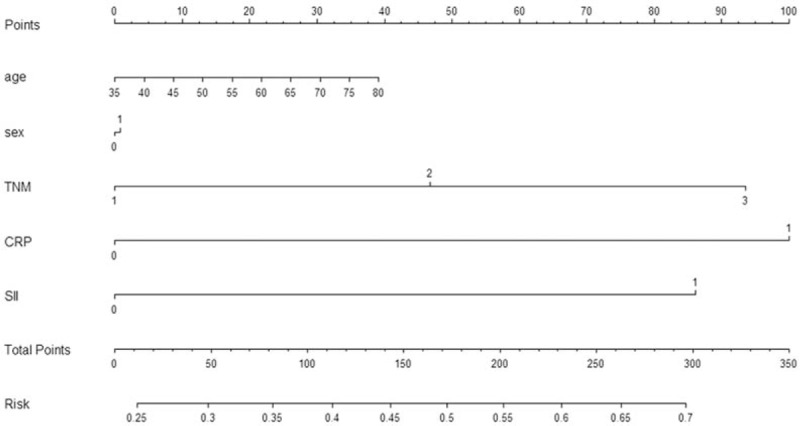
Nomogram model for death risk prediction. Harrell's c-index for CSS prediction was 0.68. CSS = cancer-specific survival.
4. Discussion
To the best of our knowledge, this is the first report to demonstrate the prognostic role of SII in patients with resectable ESCC. In this study, we revealed that SII was an independent significant predictive factor (P = 0.027). We performed a Cutoff Finder based on R software to verify to optimal cut-off value and conclude that 410 (×109/L) may be the optimal cut-off value for SII in predicting CSS in patients with resectable ESCC. In addition, the current study was also the first attempt to establish a predictive nomogram model to improve predictive accuracy based on SII.
There is strong linkage between cancer and inflammation. In our study, we analyzed the prognostic role of SII in ESCC patients without neoadjuvant treatment mainly because neoadjuvant therapy (chemotherapy and/or radiation) will have an important impact on the inflammation. Previous studies have shown that NLR and PLR were independent predictors of survival in various cancers, including EC.[8–12] However, due to the inconsistent results, the prognostic values of NLR and PLR in EC remain controversial.[18–20] Recently, we conducted a meta-analysis revealed that high levels of NLR were significantly correlated with poor survival in patients with EC.[21] In the current study, we revealed that preoperative NLR and PLR were all significantly associated with CSS. However, we demonstrated that PLR, but not NLR, was an independent prognostic factor in patients with resectable ESCC.
SII was initially performed to indicate the host inflammatory status in patients with resectable hepatocellular carcinoma.[13] They concluded that SII was a powerful prognostic indicator in patients with hepatocellular carcinoma. Then, another study confirmed that SII was superior to NLR and PLR as an independent factor for patients with small cell lung cancer.[14] In the current study, we revealed that SII was a significant predictive indicator in patients with resectable ESCC (P = 0.027). However, NLR (P = 0.661) or PLR (P = 0.064) were not significant independent predictors for CSS. Moreover, we used a Cutoff Finder based on R software to verify the optimal cut-off value, which was 410 (×109/L) compared to 330 (×109/L) and 1600 (×109/L) in previous studies.[13,14]
It is well know that nomogram could establish a simple graphic representation of a statistical predictive model.[22] In the current study, therefore, we attempt to establish a predictive nomogram model to predict the probability of the death risk for resectable ESCC patients based on SII, CRP, and TNM stage combined with age and sex. The nomogram performed well in predicting CSS by the c-index (0.68).
Several limitations should be acknowledged. First, the current study was a retrospective design with a small size population. Second, we excluded patients who had neoadjuvant chemotherapy and/or radiotherapy, which may have influenced our results. In addition, our study revealed that SII is an independent predictive factor in patients with ESCC; however, it should be kept in mind that the sensitivity and specificity looks like not very high. Moreover, the c-index showed that the nomogram model has a good accuracy but it is not perfect indicating that there is still room for improvement. Therefore, further studies are needed to illuminate the relationship between SII and prognosis in patients with resectable ESCC.
5. Conclusion
In summary, our study showed that SII is still a useful prognostic indicator for patients with resectable ESCC. We conclude that 410 (×109/L) may be the optimal cut-off value for SII.
Footnotes
Abbreviations: CI = confidence interval, CSS = cancer-specific survival, EAC = esophageal adenocarcinoma, EC = esophageal cancer, ESCC = esophageal squamous cell carcinoma, HR = hazard ratio, NLR = neutrophil to lymphocyte ratio, PLR = platelet to lymphocyte ratio, SII = immune-inflammation index, TNM = tumor node metastasis.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [3].Lin Y, Totsuka Y, He Y, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol 2013;23:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Napier KJ, Scheerer M, Misra S. Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014;6:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tachibana M, Kinugasa S, Hirahara N, et al. Lymph node classification of esophageal squamous cell carcinoma and adenocarcinoma. Eur J Cardiothorac Surg 2008;34:427–31. [DOI] [PubMed] [Google Scholar]
- [6].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [7].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- [8].Nakamura T, Matsumine A, Matsubara T, et al. The combined use of the neutrophil–lymphocyte ratio and C-reactive protein level as prognostic predictors in adult patients with soft tissue sarcoma. J Surg Oncol 2013;108:481–5. [DOI] [PubMed] [Google Scholar]
- [9].Karakiewicz PI, Hutterer GC, Trinh QD, et al. C-reactive protein is an informative predictor of renal cell carcinoma-specific mortality: a European study of 313 patients. Cancer 2007;110:1241–7. [DOI] [PubMed] [Google Scholar]
- [10].Jiang N, Deng JY, Liu Y, et al. The role of preoperative neutrophil–lymphocyte and platelet–lymphocyte ratio in patients after radical resection for gastric cancer. Biomarkers 2014;19:444–51. [DOI] [PubMed] [Google Scholar]
- [11].Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 2011;18:3362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg 2012;36:617–22. [DOI] [PubMed] [Google Scholar]
- [13].Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212–22. [DOI] [PubMed] [Google Scholar]
- [14].Hong X, Cui B, Wang M, et al. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med 2015;236:297–304. [DOI] [PubMed] [Google Scholar]
- [15].Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Staging Manuals. Cancer 2010;116:3763–73. [DOI] [PubMed] [Google Scholar]
- [16].Budczies J, Klauschen F, Sinn BV, et al. Cutoff finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One 2012;7:e51862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Antolini L, Boracchi P, Biganzoli E. A time-dependent discrimination index for survival data. Stat Med 2005;24:3927–44. [DOI] [PubMed] [Google Scholar]
- [18].Rashid F, Waraich N, Bhatti I, et al. A pre-operative elevated neutrophil: lymphocyte ratio does not predict survival from oesophageal cancer resection. World J Surg Oncol 2010;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dutta S, Crumley AB, Fullarton GM, et al. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancer. World J Surg 2011;35:1861–6. [DOI] [PubMed] [Google Scholar]
- [20].Miyata H, Yamasaki M, Kurokawa Y, et al. Prognostic value of an inflammation-based score in patients undergoing pre-operative chemotherapy followed by surgery for esophageal cancer. Exp Ther Med 2011;2:879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang X, Huang Y, Feng JF, et al. Prognostic significance of neutrophil-to- lymphocyte ratio in esophageal cancer: a meta-analysis. Onco Targets Ther 2015;8:789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364–70. [DOI] [PubMed] [Google Scholar]


