Abstract
This study aimed to investigate the effects of citicoline therapy on the network connectivity of the corpus callosum in patients with leukoaraiosis (LA) by diffusion tension imaging (DTI).
A total of 30 LA patients with Fazekas score of 2 to 3 were voluntarily assigned into citicoline group (n = 14) and control group (n = 16). In citicoline group, citicoline was administered at 0.6 g/d for 1 year. In control group, central nervous system drugs should not be used, except for sleeping pills and antidepressants. Interventions for pre-existing diseases should be conducted in both groups. During the periods of citicoline therapy and post-treatment follow-up, cranial magnetic resonance imaging and DTI were routinely performed in these patients, and the genu, body, and splenium of corpus callosum were selected as the regions of interest (ROIs). The fractional anisotropy (FA) and mean diffusivity (MD) of each ROI were determined with PANDA software.
On recruitment, there were no significant differences in the general characteristics, blood biochemical results, cognition function, and the FA and MD of the corpus callosum between 2 groups (P > 0.05). After 1-year treatment, the FA of the corpus callosum reduced gradually, but the MD of the corpus callosum tended to increased in both group, although significant differences were not observed. However, the reductions in FA of genu and splenium of corpus callosum in citicoline group were significantly lower than in control group (P < 0.05); the reductions in MD of genu, body, and splenium of corpus callosum in citicoline group were significantly lower than in control group (P < 0.05).
In LA patients, the disruption of the network connectivity of the corpus callosum deteriorates over time. Citicoline treatment may delay the reduction in FA of corpus callosum, which might be beneficial for the improvement of network connectivity of the corpus callosum.
Keywords: citicoline, corpus callosum, leukoaraiosis, network connectivity
1. Introduction
Leukoaraiosis (LA) is a term frequently used in the neuroimaging. It is rarely accompanied by the change in corpus callosum morphology and characterized by bilateral, patchy, or diffuse areas of hypodensity on computed tomography or hyperintensities on T2-weighted magnetic resonance imaging (MRI) scans. These changes involve the periventricular white matter, the corona radiata, and the centrum semiovale with different severity. LA is very common in the elderly and has been an important risk factor and a warning signal of cognition impairment and late-onset depression. The corpus callosum contains numerous neural fibers connecting the bilateral cerebral hemispheres, and LA displays symmetrical changes. Thus, our previous study attempted to investigate the relationship between the network connectivity of corpus callosum and LA, and results indicated that the abnormal conductivity of the corpus callosum was closely related to the pathogenesis of LA as well as the cognition impairment.[1] Citicoline is an essential intermediate in the synthesis of structural phospholipids of cell membranes. Exogenously administered citicoline can promote rapid repair of injured neuronal membrane by increasing the synthesis of phospholipids. In the present study, diffusion tension imaging (DTI) was employed to investigate the effects of citicoline therapy on the network connectivity of corpus callosum in LA patients, which may provide evidence for the therapy and prevention of LA.
2. Methods
1.1. Patients
In this prospective study, a total of 30 patients who were diagnosed with moderate to severe LA were recruited from the Department of Neurology of Tongji Hospital. The main symptoms included dizziness, headache, memory loss, insomnia, and anxiety. Inclusion criteria were as follows: aged 50 to 80 years; extensive LA by flair sequence of MRI. Exclusion criteria: stroke history; other central nervous system diseases such as infection, demyelinating diseases, neurodegenerative diseases, etc.; history of mental illnesses such as schizophrenia and major depression; dementia; severe physical illnesses; addictive to alcohol or drugs; unable to cooperate with treatment or follow-up; and contraindications to MRI examination. The risk factors of cerebrovascular diseases were recorded, and patients received neuropsychological assessment, blood biochemical examination, and routine cranial MRI examination (T1 + T2 + Flair) as well as DTI. Cognition function was evaluated with Montreal Cognitive Assessment and Mini-mental State Examination by corresponding experienced clinicians. Hypertensities in periventricular and deep white matter in Flair sequence were graded according to the Fazekas scale[2] (grade: 0–3). Periventricular hyperintensities (PVH): Grade 0: absence; Grade 1: capping or pencil thin lining; Grade 2: smooth halo; Grade 3: irregular PVH extending into the deep white matter. Deep white matter hyperintensities (DWMH): Grade 0: absence; Grade 1: punctate foci; Grade 2: beginning confluence of foci; Grade 3: large confluent areas. Grade 0 to 1 may be physiological, and thus not regarded as LA. Of these patients, Fazekas grade 2 and 3 were found in 18 and 12 patients, respectively.
1.2. Methods
1.2.1. Citicoline treatment
This study was supported by the grant from the Health and Family Planning Commission of Shanghai, and the study protocol was approved by the Ethics Committee of our hospital. The harm, outcome, and therapy of LA and the necessity of follow-up were explained to all patients, and informed consent was obtained before the study. Patients chose one of the treatments according to their own situations. Patients were voluntarily assigned into citicoline group (n = 14) and control group (n = 16). In citicoline group, patients received treatment with citicoline capsule (0.6 g/d; Shandong Qilu Pharmaceutical Co., Ltd. Jinan City, Shandong Province, China) for 1 year. In control group, central nervous system drugs should not be used, except for sleeping pills and antidepressants. The pre-existing diseases should be treated in 2 groups. After 1-year treatment, neuropsychological assessment, routine MRI, and DTI were performed in all these patients.
1.2.2. MRI examination
MRI scanning was performed with Siemens MAGNETOM Trio 3.0T MRI Equipment in the Department of Radiology of Tongji Hospital. Foam headrest was used to fix the head and the clinicians asked the patients to minimize the movement of the head and other parts of the body. Patients lied in a supine position and breathed smoothly. After the patients were accommodated to the environment, scanning was performed. First, routine MRI scanning was done with SE sequence (cross-sectional T1 weighted imaging (WI) and Flair) to exclude brain lesions rather than white matter lesions. The scanning parameters were as follows: T1WI [repetition time (TR): 1530 ms, echo time (TE): 9 ms, field of view (FOV): 220 × 20 mm, slice thickness: 5 mm, flip angle (FLIP): 150°, slices: 22]; Flair (TR: 8500 ms, TE: 90 ms, TI: 2438.9 ms, FOV: 235 × 35 mm, slice thickness: 3 mm, FLIP: 150°, slices: 40); DTI (TR: 3100 ms, TE: 92 ms, thickness/gap = 2 mm/0 mm, Matrix: 128 × 28, slices: 70, Nex: 2, Directions: 30, b value = 0, 1000 s/mm2).
1.2.3. Image processing after DTI
PANDA (http://www.nitrc.org/projects/panda) was used to process the data from DTI.[3] The format of images was transformed from DICOM into NifTI, and the b0 value was determined. Then, the nonbrain sections were removed, and the images were trimmed. After adjustment for eddy and head movement, the DTI parameters were calculated, followed by spatial standardization, Gaussian smoothing and calculation of fractional anisotropy (FA) and mean diffusivity (MD) of different brain regions based on Johns Hopkins University white matter templates. The genu, body, and splenium of corpus callosum were selected as the regions of interest (ROIs). On recruitment and during follow-up period after 1-year treatment, the FA and MD of ROIs were determined.
1.2.4. Statistical analysis
Statistical analysis was performed with SPSS version 18. Qualitative data were compared with chi-squared test. Quantitative data with normal distribution are expressed as mean ± standard deviation, and compared with t test between 2 groups. A value of P < 0.05 was considered statistically significant.
3. Results
Of 30 patients, 1 patient in each group was lost to follow-up, and finally 28 patients were included for analysis.
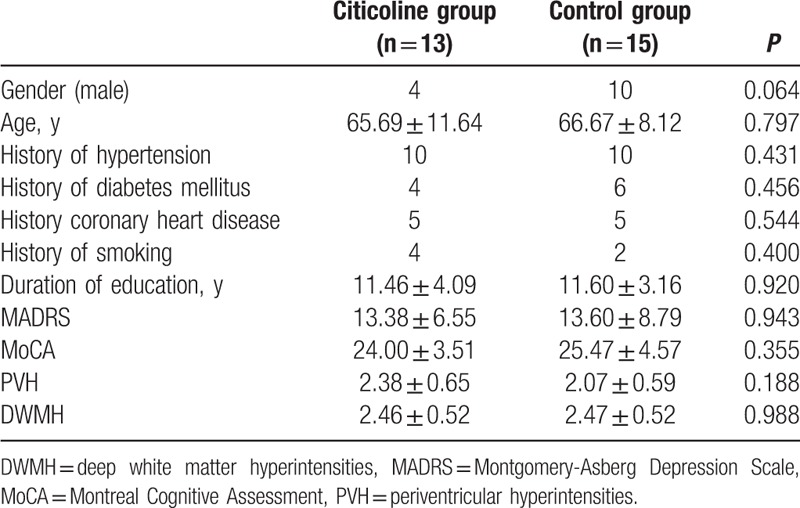
The demographics of patients in both groups are shown in Table 1. There were no significant differences in the gender, age, education level, risk factors of cerebrovascular diseases, and neuropsychological parameters (P > 0.05). In addition, significant differences were not observed in the routine blood biochemical parameters such as fasting glucose, glycated hemoglobin, liver function, kidney function, and homocysteine (P > 0.05).
Table 1.
Patients’ characteristics at baseline in citicoline group and control group.
The FA and MD of the genu, body, and splenium of corpus callosum in 2 groups are shown in Tables 2 and 3. Table 3 data were plotted in 3 before–after line graphs (Fig. 1), where the vertical axis is the MD value, and the horizontal axis has 2 conditions: baseline and 1 year later. There were no significant differences in the FA and MD at baseline between 2 groups (P > 0.05). After 1-year treatment, the FA and MD were comparable between 2 groups (P > 0.05). However, the changes in FA and MD from baseline to end of study were significantly different between 2 groups. As shown in Table 2, the reduction in FA of genu and splenium of corpus callosum in citicoline group was significantly lower than in control group (P = 0.005 and 0.011). As shown in Table 3, the MD of body and splenium of corpus callosum tended to reduce in citicoline group, but increased in control group after 1-year treatment, and the differences in the MD of genu body and splenium of corpus callosum were significantly different between 2 groups (P = 0.007, 0.002, and 0.002).
Table 2.
Change in FA of the corpus callosum in citicoline group and control group.
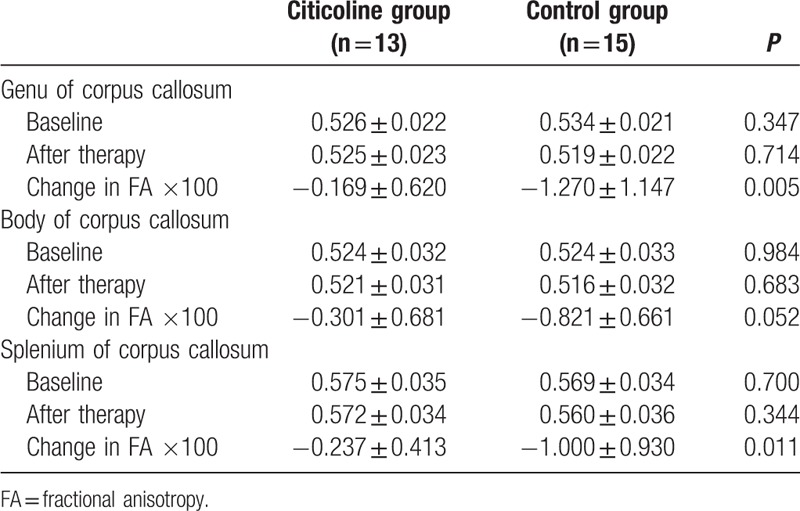
Table 3.
Change in MD of the corpus callosum in citicoline group and control group.
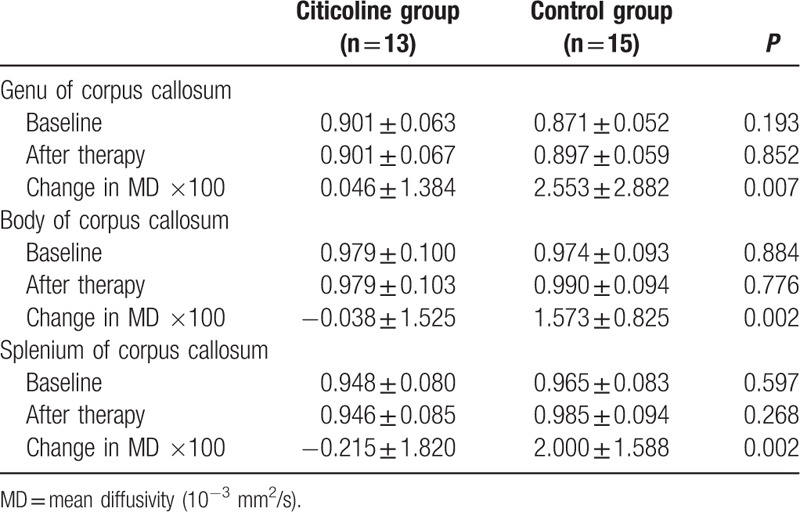
Figure 1.
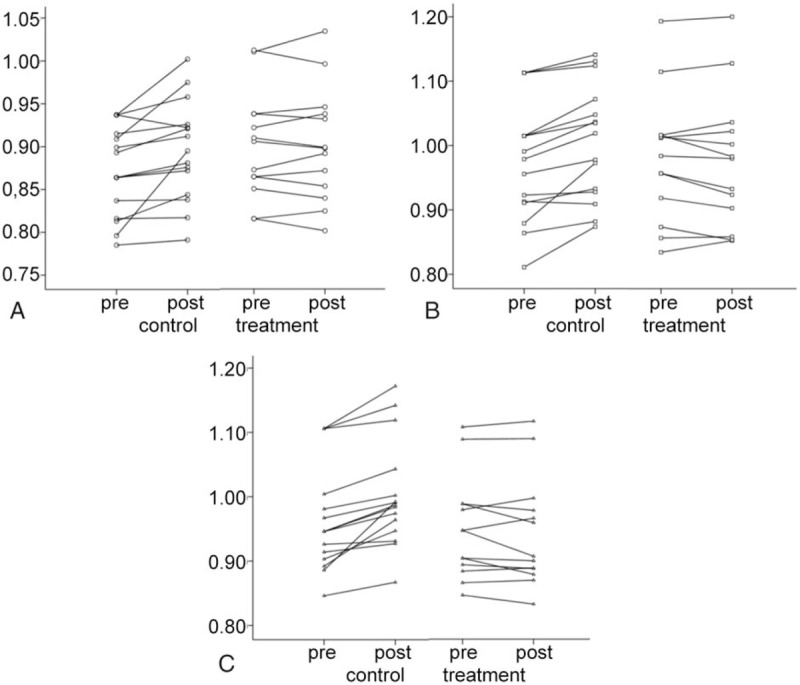
Change in MD of the corpus callosum in citicoline group and control group. (A) Change in MD of genu of corpus callosum in citicoline group and control group. (B) Change in MD of body of corpus callosum in citicoline group and control group. (C) Change in MD of splenium of corpus callosum in citicoline group and control group. MD = mean diffusivity.
Figures 2 and 3 show the severity of LA and the dynamic change in DTI of patients in 2 groups. In Fig. 2, patients received citicoline therapy for 1 year (Fazekas grade 3); in Fig. 3, patients did not receive citicoline therapy.
Figure 2.
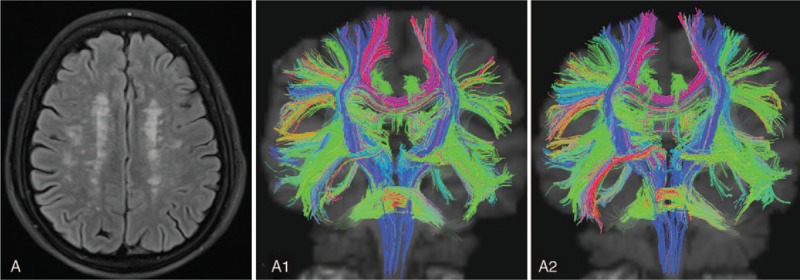
Patient A, female, 61 years old. She complained of dizziness and memory loss, but had no risk factors of cerebrovascular diseases. Citicoline therapy (0.6 g/d) was performed for 1 year. TrackVis software displayed the fasciculi in the white matter. A displays LA of Fazekas grade 3; A1 displays the DTI image at baseline; A2 displays the DTI image after 1-year treatment. The number of fasciculi in the white matter in A2 was slightly lower than in A1. Red: transverse fasciculi in the white matter; blue: longitudinal fasciculi in the white matter. DTI = diffusion tension imaging, LA = leukoaraiosis.
Figure 3.
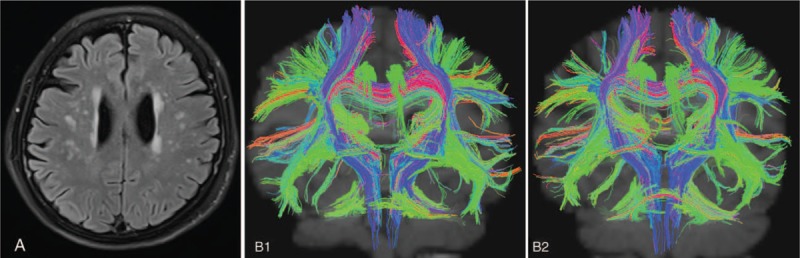
Patient B, female, 73 years old. She complained of headache, dizziness, and insomnia, but had no risk factors of cerebrovascular diseases. Sertraline therapy (50 mg/d) was performed for 1 year. TrackVis software displayed the fasciculi in the white matter. B displays LA of Fazekas grade 2; B1 displays the DTI image at baseline; and B2 displays the DTI image after 1-year treatment. The number of fasciculi in the white matter in B2 was significantly lower than in B1. DTI = diffusion tension imaging, LA = leukoaraiosis.
4. Discussion
LA is an age-related disease, and the incidence of LA increases with the increase in age. Patients with mild to moderate LA usually are asymptomatic and thus LA is often regarded as age related or physiological change. However, a large number of studies have shown that LA of a certain severity is pathological.[4–6] LADIS serial studies have revealed that the severity of LA is closely related to the risk for reduced implementation capacity, cognition impairment, late-onset depression, stroke, disability, and death.[7–10]
The pathogenesis of LA is still unclear and might be caused by chronic hypoperfusion secondary to cerebral small vessel disease or the degeneration of brain white matter. A 3-year follow-up study in LADIS serial studies showed the age-related white matter changes could cause the progressive loss of tissues in the corpus callosum, resulting in its atrophy.[11] Corpus callosum is composed of commissural fibers that bridge 2 hemispheres. Currently, the causal relationship between network change of the corpus callosum and LA is still unclear. The disruption of the network connectivity or structural atrophy of the corpus callosum may inevitably cause damage to the signal transmission and the signal integration between 2 hemispheres, leading to a series of clinical symptoms such as cognition impairment, reduced executive function, and dyskinesia. Thus, the abnormal network connectivity or structural atrophy of the corpus callosum may predict the possibility of future cognition impairment.[11–13] In our study, DTI showed the FA of genu, body, and splenium of corpus callosum reduced, but MD increased in LA patients, while FA reduction is related to cognition function.[1] After 1-year therapy, the FA of corpus callosum further reduced over time. FA may reflect the number of axons and myelins in the white matter, and FA reduction is suggestive of microstructural degeneration of white matter and disruption of the myelin integrity.[14]
Currently, no effective strategies have been developed for the treatment of LA. Our results showed that, although the FA of corpus callosum tended to reduce over time in LA patients, oral citicoline was able to significantly delay the reduction in the FA of corpus callosum; suggesting that citicoline may attenuate the damage to the axons and myelins in the corpus callosum and promote the repair of the corpus callosum. Citicoline is a precursor in the intrinsic synthesis of phosphatidylcholine and essential for the lecithin synthesis. It is involved in the biosynthesis of lecithin and an important component of biological membranes. After damage to the central nerves, citicoline may stabilize the cell membrane and inhibit the production of cytotoxic free fat acids in the brain. In addition, it is also involved in the repair and regeneration of neurons, exerting neuroprotective effects. Citicoline has been found to improve the cognition impairment in patients with chronic ischemic cerebrovascular diseases or dementia. Currently, citicoline is used in the therapy of brain injury, stroke, dementia, and brain atrophy.[15–18] There is evidence showing that citicoline is able to improve the permeability of blood–brain barrier, increase the superoxide dismutase activity, and inhibit calpain overactivation, exerting neuroprotective effects on closed brain injury.[19] MRS study showed citicoline therapy for 6 weeks in healthy volunteers could significantly increase creatine phosphate and triphosphate and markedly alter the phospholipids in membrane in the cortex of frontal anterior cingulate, suggesting that citicoline is able to improve the frontal bioenergy and the fluidity of phospholipids in the cell membrane.[20]
In summary, LA patients have abnormality in the network connectivity of the corpus callosum, which deteriorates over time. Citicoline therapy may delay the damage to the axons and myelins in the corpus callosum, exerting protective effects on the corpus callosum. Our findings may provide evidence for the early clinical intervention of LA.
Footnotes
Abbreviations: DTI = diffusion tension imaging, FA = fractional anisotropy, LA = leukoaraiosis, MD = mean diffusivity, ROI = region of interest.
This study was supported by the grant from the Health and Family Planning Commission of Shanghai.
The authors have no conflicts of interest to disclose.
References
- [1].He YS, Jiang H, Li YX, et al. Relationship between network connectivity of corpus callosum and cognitive impairment in leukoaraiosis. Int J Clin Exp Med 2016;9:14530–5. [Google Scholar]
- [2].Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351–6. [DOI] [PubMed] [Google Scholar]
- [3].Cui Z, Zhong S, Xu P, et al. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci 2013;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. Bmj 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Inzitari D. Leukoaraiosis: an independent risk factor for stroke? Stroke 2003;34:2067–71. [DOI] [PubMed] [Google Scholar]
- [6].Pantoni L. Leukoaraiosis: from an ancient term to an actual marker of poor prognosis. Stroke 2008;39:1401–3. [DOI] [PubMed] [Google Scholar]
- [7].Firbank MJ, O’Brien JT, Pakrasi S, et al. White matter hyperintensities and depression—preliminary results from the LADIS study. Int J Geriatr Psychiatry 2005;20:674–9. [DOI] [PubMed] [Google Scholar]
- [8].Inzitari D, Pracucci G, Poggesi A, et al. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. Bmj 2009;339:b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pantoni L, Poggesi A, Basile AM, et al. Leukoaraiosis predicts hidden global functioning impairment in nondisabled older people: the LADIS (Leukoaraiosis and Disability in the Elderly) study. J Am Geriatr Soc 2006;54:1095–101. [DOI] [PubMed] [Google Scholar]
- [10].Verdelho A, Madureira S, Ferro JM, et al. Differential impact of cerebral white matter changes, diabetes, hypertension and stroke on cognitive performance among non-disabled elderly. The LADIS study. J Neurol Neurosurg Psychiatry 2007;78:1325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ryberg C, Rostrup E, Paulson OB, et al. Corpus callosum atrophy as a predictor of age-related cognitive and motor impairment: a 3-year follow-up of the LADIS study cohort. J Neurol Sci 2011;307:100–5. [DOI] [PubMed] [Google Scholar]
- [12].Jokinen H, Ryberg C, Kalska H, et al. Corpus callosum atrophy is associated with mental slowing and executive deficits in subjects with age-related white matter hyperintensities: the LADIS study. J Neurol Neurosurg Psychiatry 2007;78:491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ryberg C, Rostrup E, Sjostrand K, et al. White matter changes contribute to corpus callosum atrophy in the elderly: the LADIS study. AJNR Am J Neuroradiol 2008;29:1498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vernooij MW, Ikram MA, Vrooman HA, et al. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry 2009;66:545–53. [DOI] [PubMed] [Google Scholar]
- [15].Alvarez-Sabin J, Roman GC. Citicoline in vascular cognitive impairment and vascular dementia after stroke. Stroke 2011;42(suppl):S40–3. [DOI] [PubMed] [Google Scholar]
- [16].Davalos A, Secades J. Citicoline preclinical and clinical update 2009–2010. Stroke 2011;42(suppl):S36–9. [DOI] [PubMed] [Google Scholar]
- [17].Fioravanti M, Buckley AE. Citicoline (Cognizin) in the treatment of cognitive impairment. Clin Interv Aging 2006;1:247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ramos-Cabrer P, Agulla J, Argibay B, et al. Serial MRI study of the enhanced therapeutic effects of liposome-encapsulated citicoline in cerebral ischemia. Int J Pharm 2011;405:228–33. [DOI] [PubMed] [Google Scholar]
- [19].Qian K, Gu Y, Zhao Y, et al. Citicoline protects brain against closed head injury in rats through suppressing oxidative stress and calpain over-activation. Neurochem Res 2014;39:1206–18. [DOI] [PubMed] [Google Scholar]
- [20].Silveri MM, Dikan J, Ross AJ, et al. Citicoline enhances frontal lobe bioenergetics as measured by phosphorus magnetic resonance spectroscopy. NMR Biomed 2008;21:1066–75. [DOI] [PubMed] [Google Scholar]


