Abstract
Chiari malformation type I (CM-I) is a congenital neurosurgical disease about the herniation of cerebellar tonsil through the foramen magnum. A variety of surgical techniques for CM-I have been used, and there is a controversy whether to use posterior fossa decompression with duraplasty (PFDD) or posterior fossa decompression without duraplasty (PFD) in CM-I patients. Here, we compared the clinical results and effectiveness of PFDD and PFD in adult patients with CM-I. The cases of 103 adult CM-I patients who underwent posterior fossa decompression with or without duraplasty from 2008 to 2014 were reviewed retrospectively. Patients were divided into 2 groups according to the surgical techniques: PFDD group (n = 70) and PFD group (n = 33). We compared the demographics, preoperative symptoms, radiographic characteristics, postoperative complications, and clinical outcomes between the PFD and PFDD patients. No statistically significant differences were found between the PFDD and PFD groups with regard to demographics, preoperative symptoms, radiographic characteristics, and clinical outcomes(P > 0.05); however, the postoperative complication aseptic meningitis occurred more frequently in the PFDD group than in the PFD group (P = 0.027). We also performed a literature review about the PFDD and PFD and made a summary of these preview studies. Our study suggests that both PFDD and PFD could achieve similar clinical outcomes for adult CM-I patients. The choice of surgical procedure should be based on the patient's condition. PFDD may lead to a higher complication rate and autologous grafts seemed to perform better than nonautologous grafts for duraplasty.
Keywords: Chiari malformation type I, duraplasty, posterior fossa decompression
1. Introduction
“Chiari malformation” refers to the downward displacement of the cerebellar tonsil through the foramen magnum into the upper part of the spinal canal.[1,2] No consensus exists regarding the etiology of Chiari malformations. To our knowledge, several theories have been proposed for the pathogenesis.[3] Sarnat[4] suggested that primary defects in the genetic programming of hindbrain segmentation and of growth of associated bones and cranial structures results in Chiari malformations. The hydrodynamic pulsion theory suggests that Chiari malformations are caused by early progressive fetal hydrocephalus pushing down on the brainstem and cerebellum.[5] Some scholars believe that defective closure of the neural tube during early fetal development results in the leakage of cerebrospinal fluid (CSF), which leads to insufficient distention of the embryonic ventricular system and results in a small posterior fossa and cerebral disorganization.[6] Recently, Kong et al[7] presented a new explanation called “evolutional mismatch” or “evolutional inconformity.” This theory suggests that the development of the posterior fossa volume does not match the increasing brain size, which leads to anatomic anomalies of the cerebellum, brainstem, and craniocervical junction, with downward displacement of the cerebellum, alone or together with the lower medulla into the spinal canal.
Among the subtypes of Chiari malformation, Chiari malformation type I (CM-I) is identified most commonly in adulthood and is defined by the degree cerebellar tonsillar extension below the basionopisthion line on sagittal and coronal magnetic resonance images.[1,2] However, the clinical manifestations often appear in young individuals, and the standard for diagnosing CM-I is different for people younger than 15 years of age than that for individuals older than 15 years of age.[1] In patients younger than 15 years, CM-I is defined as cerebellar tonsillar extension greater than 5 mm, whereas in patients older than 15 years, CM-I is defined as extension greater than 6 mm.[1]
Despite the fact that many surgical modalities for Chiari malformation have been used in clinical practice, controversy still exists. Two main types of surgical modalities have been advocated for the treatment of CM-I. One type is posterior fossa decompression with or without duraplasty (PFDD or PFD, respectively) and the other is reduction of the syrinx cavity using different types of shunt procedures.[8,9] Posterior fossa decompression still remains the primary surgical technique for the treatment of CM-I because the shunt technique produces a risk of iatrogenic spinal cord injury.[9–12] Extensive work has been performed concerning PFDD and PFD.[9,13–17] However, whether duraplasty is performed during posterior fossa decompression remains controversial. Most studies in this area have been conducted in pediatric patients. In order to identify the different surgical outcome between PFDD and PFD in adult patients, we retrospectively studied the clinical data of 103 CM-I patients who had undergone operations from 2008 to 2014.
2. Methods
2.1. Hospital
Peking Union Medical College Hospital (PUMCH) is a class A tertiary comprehensive hospital committed to delivering state-of-the-art clinical care, innovative scientific research, and rigorous medical education. The neurosurgery department in PUMCH has 24 faculties and 49 inpatient beds, with an average annual admission of 1400 patients.
2.2. Patients
A retrospective study of consecutive CM-I patients and their procedures from 2008 to 2014 was performed in the Neurosurgery Department of Peaking Union Medical College Hospital. All patients were older than 18 years of age. We excluded patients with other types of Chiari malformations and a history of severe diseases such as coronary artery atherosclerosis and hepatosclerosis. A retrospective study of 103 patients met the inclusion criteria and was reviewed in this study. Criteria for inclusion were age above 18 years; CM-I with or without syringomyelia in magnetic resonance imaging (MRI) examination; and absence of other craniovertebral junction malformation/tumor/infection/trauma. All of the patients underwent PFD or PFDD, and their medical records and radiographic characteristics including MRI and computed tomography were reviewed and compared.
All data were anonymously analyzed with individual patient consent in this retrospective study. This study protocol was approved by the institutional review boards at PUMCH and Peaking Union Medical College.
2.3. Demographics and preoperative symptoms
The details reviewed from medical records reviewed included sex, age, symptom duration, the total duration of the hospital stay, the duration of the postoperative hospital stay, and preoperative symptoms. The preoperative symptoms were divided into 3 main categories according to the presence or absence of symptoms and signs specific to Chiari syndrome on the Chicago Chiari Outcome Scale as follows: pain symptoms, nonpain symptoms, and functionality conditions.[18] Pain symptoms included headache, neck and back pain, and upper extremity pain. Nonpain symptoms included sensory loss, numbness and tingling, muscle weakness, extremity paresthesia, dysphagia, dizziness, ataxia, and others. Preoperative functionality conditions were classified as unable to attend, moderate impairment, mild impairment, and fully functional.
2.4. Radiographic characteristics
An initial MRI examination with contrast was performed before or on arrival at the Neurosurgery Department. CM-I was stratified into 3 subgroups according to the cerebellar tonsillar descent (CTD)[17,19] as follows: grade 1, the tonsil descended more than 5 mm below the foramen magnum but did not reach the C1 arch; grade 2, the tonsil reached the C1 arch; and grade 3, the tonsil descended over the C1 arch (Fig. 1). The location of the syrinx was classified as none, cervical, or beyond cervical.
Figure 1.
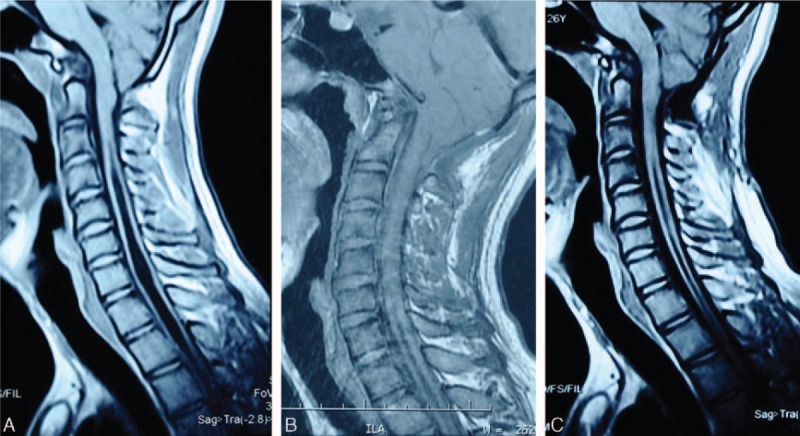
A 24-year-old CM-I male patient underwent PFDD. (A) Preoperative T1-weight image shows that CM-I with a syrinx C3 to T3. (B) One month follow-up MR examination shows slight resolution of the syrinx. (C) One year follow-up MR examination shows a wide cistern magna and slight regression of the syrinx. CM-I = Chiari malformation type I, PFDD = posterior fossa decompression with duraplasty.
2.5. Surgical procedure
The indications for surgical treatment were as follows: MRI confirmed CM-I; and patients presented with related symptoms. The specific surgical procedure (PFD or PFDD) was chosen by each surgeon on the basis of training and personal preference. No surgeon performed both procedures in this series. All patients were administered general anesthesia and placed in the prone position with slight flexion of the neck using 3-point Mayfield fixation. The skin, subcutaneous tissues, and occipital and paraspinal muscle were cut through with a midline incision extending from the occipital protuberance to the C2 spinous process. In some PFDD cases, the occipital fascia was left intact for duraplasty use. The incision exposed the edge of the occipital bone, atlantoaxial posterior arch, spinous process, and lamina. The inferior part of the occipital bone, the posterior lamina of C1, and the tip of the spinous process of C2 were removed to achieve a bony decompression (approximately 4 cm × 4 cm). After decompression, a thick fasciculation-like tissue that compressed the dura was observed. The thick fasciculation-like tissue was removed, and the dura was incised carefully through the midline under a microscope. After opening the dura, the lower pole of the cerebellar tonsil and the cervical spinal cord were exposed. In some cases, the arachnoid had scarring and adhesions and required a sharp dissection. Then, dural grafting was performed with the occipital fascia or artificial dura. Finally, the outer layers were sutured step-by-step to achieve anatomical reduction.
2.6. Follow-up and outcomes
We assessed clinical outcomes at the 1-month and 1-year follow-up visits separately. The general postoperative outcomes were evaluated based on the following criteria: excellent results, improvement of the neurological deficit; good result, cessation of progression of the neurological deficit; and poor result, further deterioration of neurological function.[20] An MRI study was performed during the follow-up consultation. The observation of the syrinx cavity solution was recorded (Fig. 1).
2.7. Statistical analysis
All statistical analyses were performed using statistical software (SPSS Version 21.0, SPSS. IBM Corp., Armonk, NY). The continuous variables were expressed as the means ± standard deviation. Comparisons between 2 groups were analyzed using the χ2 test (or Fisher exact test) for the categorical data and the t test for the continuous data. P values < 0.05 were considered statistically significant.
3. Results
3.1. Preoperative characteristics
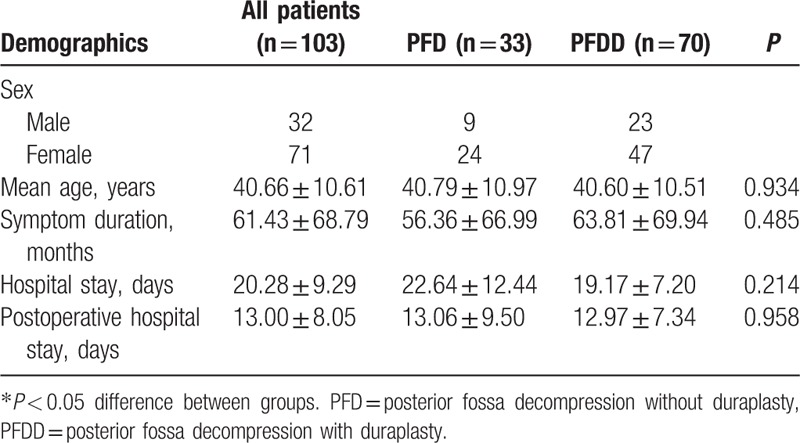
Table 1 shows the general demographic information of the patients in this study. Thirty-two males and 71 females were included in the study. All patients were more than 18 years old, and the mean age was 40.66 years (range 19–62 years). The mean symptom duration was 61.43 months. According to the medical records, the mean total hospital stay was 20.28 days and the mean postoperative hospital stay was 13.00 days. None of the above 5 characteristics were significantly different between the PFD and PFDD groups. A significant difference was found in the preoperative hospital stay (in days) between these 2 groups (P < 0.001).
Table 1.
Demographic information of PFD and PFDD.
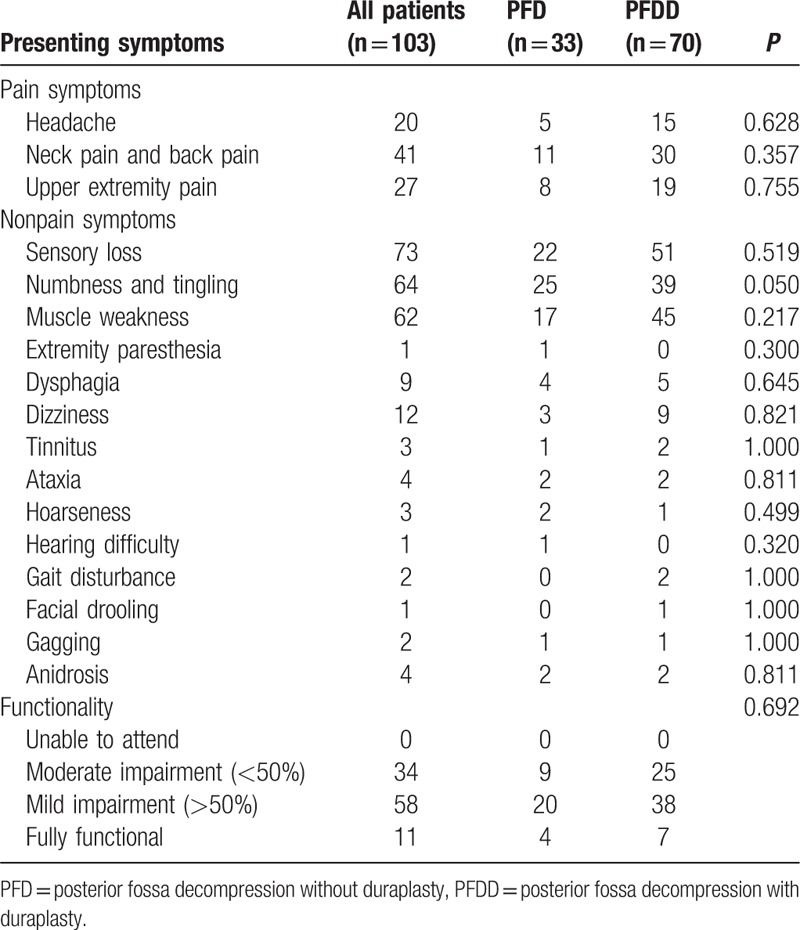
The CM-I patients presented to the Neurosurgery Department with different symptoms. The symptoms were classified into 3 main groups: pain symptoms, nonpain symptoms, and functionality. Among all the symptoms, the 3 most common symptoms included the following nonpain symptoms: sensory loss (70.9%), tingling and numbness (62.1%), and muscle weakness (60.2%). Neck pain and back pain was the most common pain symptoms (39.8%), and 56.3% of patients had a mild impairment in the functionality (Table 2). No significant difference was observed in preoperative symptoms between the PFD and PFDD groups (P > 0.05).
Table 2.
Summary of the preoperative symptoms.
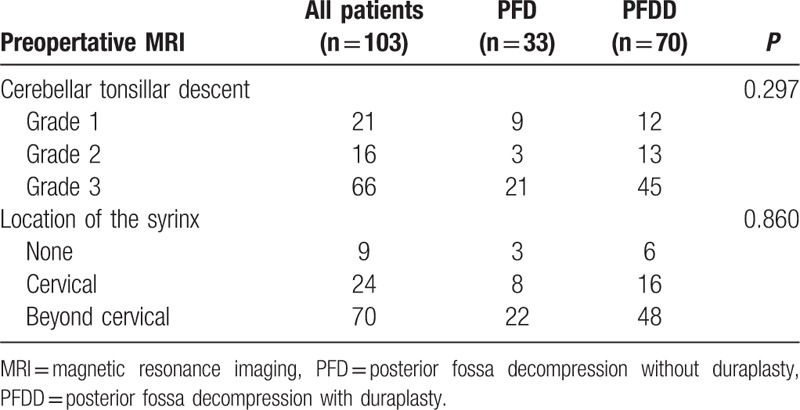
All of the patients underwent an MRI examination, and the CTD and location of the syrinx were recorded. No significant difference was found between the groups in the CTD and location of the syrinx according to the statistical analyses (P > 0.05) (Table 3).
Table 3.
The Radiographic characteristics.
3.2. Complications
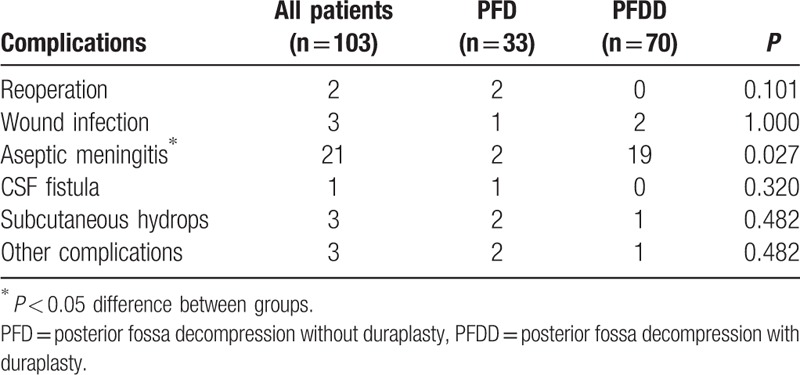
The most common complication was aseptic meningitis (21/103, 20.3%), and the occurrence rate in the PFDD group (19/70, 27.1%) was much higher than in the PFD group (2/33, 6.1%). Criteria of aseptic meningitis were positive CSF studies on lumbar puncture with negative culture. Patient with aseptic meningitis presented with fever, headache, and was response to steroids.[14] Aseptic meningitis was the only significantly different complication between the 2 groups (P = 0.027). There was no difference in other complications such as wound infections, CSF fistulas, and subcutaneous hydrops between the PFDD and PFD groups (Table 4).
Table 4.
The summary of the complications.
3.3. Outcomes
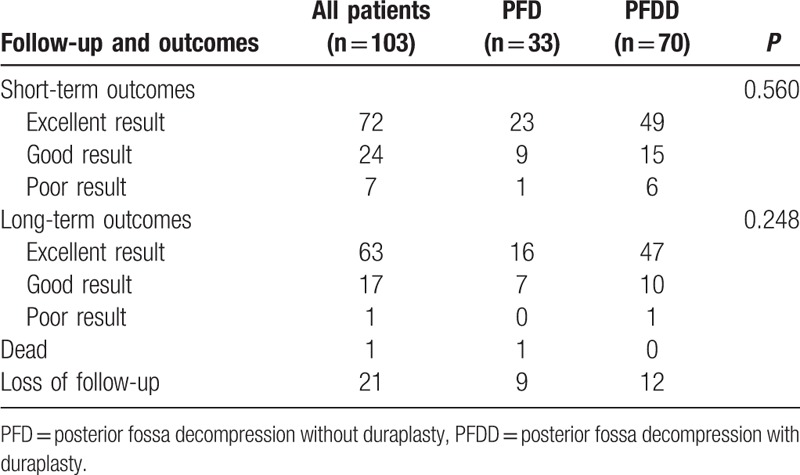
In this study, the patients’ clinical outcomes were recorded at 2 time points: short-term (after 1 month of follow-up) and long-term (after 1 year of follow-up), through clinical visits, telephone, and other means of contact. Nine patients in the PFD group and 12 patients in the PFDD groups could not be reached for long-term follow-up. One patient in the PFD group died before the 1-year follow-up. The statistical analysis showed there was no significant difference in the outcome results (excellent, good, and poor) at the short-term and long-term follow-up between the 2 groups (Table 5).
Table 5.
The follow-up and outcomes.
4. Literature review
A literature review was performed with the PubMed search engine of the National Library of Medicine of the National Institutes of Health (http://www.ncbi.nlm.nih.gov/pubmed), using the following Keywords “Chiari malformation,” “Chiari malformation type I,” “posterior fossa decompression,” “posterior fossa decompression with duraplasty,” and “posterior fossa decompression without duraplasty.” The search was restricted to English-language publications without date limitations.
5. Discussion
5.1. Preoperative symptoms and neuroimaging diagnosis
CM-I preoperative symptoms vary in different patients. According to previous studies, the most common symptom is pain, including occipital pain, neck pain, back pain, and upper limb pain.[1,2,9,16] Other clinical manifestations include sensory loss, numbness and tingling, muscle weakness, and ataxia. In this study, the preoperative symptoms were classified into pain symptoms, nonpain symptoms, and functionality based on the Chicago Chiari Outcome Scale.[18] It is noteworthy that the most frequent symptom type in our study was nonpain symptoms, which is not consistent with previous studies. A total of 58.3% of the patients had mild impairment in functionality, which may affect their daily life. Functionality should be given more attention when evaluating clinical outcomes after surgery. Klekamp[21] analyzed a series of 371 CM-I cases and concluded that children exhibited higher neurological scores than adults. This may be explained by the postnatal growth of the cerebellum.[22] The cerebellum reaches the adult volume in the 2nd year of life after starting with only 15% of its adult volume at birth.[21,22]
Although CM-I can be diagnosed through a variety of imaging modalities, MRI is considered the gold standard diagnostic tool for CM-I. MRI can be used to evaluate CSF flow, which is an important predictor of clinical outcomes.[1] Apart from the phase-contrast MRI, diffusion tensor imaging is used to evaluate the integrity of the brainstem and cerebellar white matter tracts in CM-I patients. Recently, Ucar et al[23] demonstrated a new useful sign for CM-I, namely the tonsillar blackout sign on 3-dimensional-SPACE. This sign is particularly useful for distinguishing between symptomatic and asymptomatic CM-I patients and patients who are likely to benefit from decompressive surgery.[23] Additional anomalies such as basilar invaginations and assimilations of the atlas to the occiput may also be seen in CM-I patients. Other types of imaging tools, such as computed tomography and X-ray, are more useful in identifying these bony anomalies.
Is there any relationship between the clinical manifestations and the severity of CM-I? In a study by Wu et al.[24] the severity of the clinical symptoms did not correlate with the degree of cerebellar tonsillar herniation. However, Greenberg et al[25] developed a preoperative Chiari Severity Index that integrates the clinical and neuroimaging characteristics. This is a novel tool that predicts patient-defined improvement following CM-I surgery, aids in preoperative counseling, and stratifies patients in comparative effectiveness trials.[25]
5.2. Nonduraplasty or duraplasty
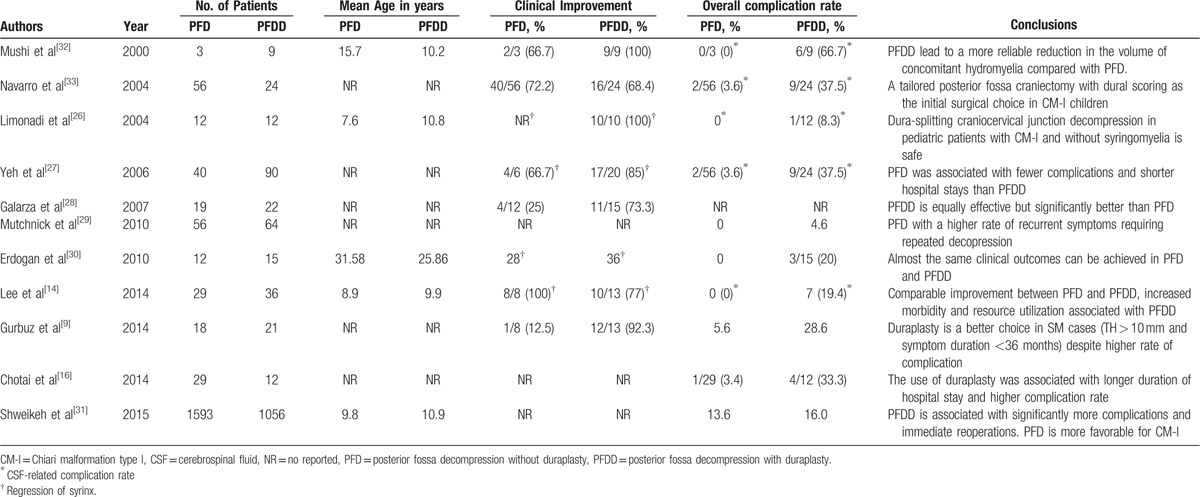
Currently, no general consensus exists for incorporating duraplasty in the surgical treatment of CM-I.[9,13–16,26–31] Some authors have advocated PFD is sufficiently effective, whereas others have suggested adding duraplasty. Table 6 shows a summary of the major studies that compare PFD and PFDD. Some authors have concluded that the surgical outcomes between PFD and PFDD are not significantly different, but the complication rate in PFDD is higher than in PFD. The results of this study are consistent with this conclusion. The short-term and long-term follow-up outcomes were similar. The only difference between the procedures was the occurrence of aseptic meningitis (6.1% in PFD vs 27.1% in PFDD). This difference may be related to the fact that PFDD has more steps than PFD, and PFDD requires opening the dura and suturing the dura with different types of materials. This destroys the integrity of the original dura and increases the risk of CSF-related complications. Patients undergoing PFD may have preoperative complaints that recur during the postoperative period and subsequently need to undergo duraplasty operations.[9]
Table 6.
Summary of the previous studies compared PFD with PFDD.
Shweikeh et al[31] evaluated 1593 patients who underwent PFD and 1056 patients who underwent PFDD and compared the complications and hospital charges in a large national study. The patients who underwent PFDD experienced more reoperations (2.1% vs 0.7%, P = 0.001), more procedure-related complications (2.3% vs 0.8%, P = 0.003), a longer length of hospital stay (4.4 vs 3.8 days, P = 0.001), and higher hospital charges (USD 35,321 vs 31,483, P = 0.01). Thus, the authors concluded that PFDD is associated with significantly more complications and immediate reoperations. PFD was shown to be more economical by requiring fewer hospital resources. Overall, PFD is more favorable for CM-I. However, according to McGirt et al, in children with displacement of the tonsils below the inferior border of the arch of the atlas, ultrasonography-indicated osseous decompression alone was associated with a 2-fold risk of symptom recurrence compared to decompression with duraplasty.[34] Duraplasty may be warranted in cases of tonsillar herniation that extends below the C-1 lamina regardless of the intraoperative ultrasonography findings.
5.3. The choice of the duraplasty materials
In PFDD, the choice of the materials for duraplasty remains controversial. Autologous pericranium, fascia lata (autograft or allograft), bovine pericardium, fetal bovine tissue, processed collagen matrix, and synthetic fabrics have all been used for duraplasty in CM-I surgery.[35–37] In our study, autograft fascia lata was used in 9 patients, and no complications were observed, which is consistent with the results of previous studies. Nonautologous dural grafts have been associated with numerous complications including hemorrhage, bacteria and virus transmission, fatal Creutzfeldt–Jakob disease transmission, foreign body reactions, systemic immune responses, excessive scarring, slower healing, premature graft dissolution, and wound dehiscence. Autogenous tissues have the advantage of being nonimmunogenic, nontoxic, readily available, and inexpensive.[37] Abuzayed et al[36] used an on-site muscle flap with a pedicle to supply and vascularize the autologous fascia lata in 6 patients with postoperative CSF leaks. In 5 of those patients, the CSF leaks were successfully controlled without recurrence. Compared to synthetic materials, autologous fascia lata are more viable and produce less tissue response, which results in healthy healing of the graft and/or flap, consequent closure of the defect, and prevention of CSF leakage.[36] Autologous grafts were more often preferred by neurosurgeons.
6. Limitations
The present study has all of the limitations of any retrospective study design. The results of this study should be interpreted with caution. A prospective multicenter study with a large and equal number of patients in the PFD and PFDD groups might provide sufficient data for an adequate comparison of these 2 techniques to better define the indications and benefits. Also the follow-up evaluation is needed to be replaced by a more objective method in the further study.
7. Conclusion
To our knowledge, the clinical outcome of PFD is nearly identical to that of PFDD in adult patients; however, the complication rate is higher for PFDD, particularly for aseptic meningitis. Compared with other synthetic materials, autologous fascia lata is more reliable for PFDD because it produces fewer complications.
Acknowledgments
The authors thank all the participants of this study and all the staff who participated in data collection, data analysis, and interpretation.
Footnotes
Abbreviations: CM-I = Chiari malformation type I, CTD = cerebellar tonsillar descent, MRI = magnetic resonance imaging, PFD = posterior fossa decompression without duraplasty, PFDD = posterior fossa decompression with duraplasty, PUMCH = Peking Union Medical College Hospital, TBS = tonsillar blackout sign.
JC and YL are first co-author.
Authorship: JC and YL have contributed equally to the study. JG and JX are corresponding authors.
Ethical and conflict statement: All data were anonymously analyzed with individual patient consent in this retrospective study. This study protocol was approved by the institutional review boards at Peking Union Medical College Hospital and Peking Union Medical College.
The authors have no conflicts of interest to disclose.
References
- [1].McVige JW, Leonardo J. Neuroimaging and the clinical manifestations of Chiari malformation type I (CMI). Curr Pain Headache Rep 2015;19:18. [DOI] [PubMed] [Google Scholar]
- [2].Fischbein R, Saling JR, Marty P, et al. Patient-reported Chiari malformation type I symptoms and diagnostic experiences: a report from the national Conquer Chiari Patient Registry database. Neurol Sci 2015;36:1617–24. [DOI] [PubMed] [Google Scholar]
- [3].Cesmebasi A, Loukas M, Hogan E, et al. The Chiari malformations: a review with emphasis on anatomical traits. Clin Anat 2015;28:184–94. [DOI] [PubMed] [Google Scholar]
- [4].Sarnat HB. Regional ependymal upregulation of vimentin in Chiari II malformation, aqueductal stenosis, and hydromyelia. Pediatr Dev Pathol 2004;7:48–60. [DOI] [PubMed] [Google Scholar]
- [5].Royo-Salvador MB, Solé-Llenas J, Doménech JM, et al. Results of the section of the filum terminale in 20 patients with syringomyelia, scoliosis and Chiari malformation. Acta Neurochir (Wien) 2005;147:515–23. discussion 523. [DOI] [PubMed] [Google Scholar]
- [6].McLone DG, Knepper PA. The cause of Chiari II malformation: a unified theory. Pediatr Neurosci 1989;15:1–2. [DOI] [PubMed] [Google Scholar]
- [7].Kong X, Yang Y, Gao J, et al. A new pathogenetic explanation of human chiari malformations. J Pak Med Assoc 2015;65:804–5. [PubMed] [Google Scholar]
- [8].Ma J, You C, Chen H, et al. Cerebellar tonsillectomy with suboccipital decompression and duraplasty by small incision for Chiari I malformation (with syringomyelia): long term follow-up of 76 surgically treated cases. Turk Neurosurg 2012;22:274–9. [DOI] [PubMed] [Google Scholar]
- [9].Gurbuz MS, Karaaslan N, Caliskan T, et al. Comparison of the surgical results for foramen magnum decompression with and without duraplasty in Chiari malformation type 1. Turk Neurosurg 2015;25:419–24. [DOI] [PubMed] [Google Scholar]
- [10].Hida K, Iwasaki Y, Koyanagi I, et al. Surgical indication and results of foramen magnum decompression versus syringosubarachnoid shunting for syringomyelia associated with Chiari I malformation. Neurosurgery 1995;37:673–8. discussion 678–9. [DOI] [PubMed] [Google Scholar]
- [11].da SJA, dos SAA, Melo LR, et al. Posterior fossa decompression with tonsillectomy in 104 cases of basilar impression, Chiari malformation and/or syringomyelia. Arq Neuropsiquiatr 2011;69:817–23. [DOI] [PubMed] [Google Scholar]
- [12].Di LN, Palma L, Palatinsky E, et al. “Conservative” cranio-cervical decompression in the treatment of syringomyelia-Chiari I complex. A prospective study of 20 adult cases. Spine (Phila Pa 1976) 1995;20:2479–83. [DOI] [PubMed] [Google Scholar]
- [13].Durham SR, Fjeld-Olenec K. Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation Type I in pediatric patients: a meta-analysis. J Neurosurg Pediatr 2008;2:42–9. [DOI] [PubMed] [Google Scholar]
- [14].Lee A, Yarbrough CK, Greenberg JK, et al. Comparison of posterior fossa decompression with or without duraplasty in children with Type I Chiari malformation. Childs Nerv Syst 2014;30:1419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hankinson T, Tubbs RS, Wellons JC. Duraplasty or not? An evidence-based review of the pediatric Chiari I malformation. Childs Nerv Syst 2011;27:35–40. [DOI] [PubMed] [Google Scholar]
- [16].Chotai S, Medhkour A. Surgical outcomes after posterior fossa decompression with and without duraplasty in Chiari malformation-I. Clin Neurol Neurosurg 2014;125:182–8. [DOI] [PubMed] [Google Scholar]
- [17].Yilmaz A, Kanat A, Musluman AM, et al. When is duraplasty required in the surgical treatment of Chiari malformation type I based on tonsillar descending grading scale. World Neurosurg 2011;75:307–13. [DOI] [PubMed] [Google Scholar]
- [18].Aliaga L, Hekman KE, Yassari R, et al. A novel scoring system for assessing Chiari malformation type I treatment outcomes. Neurosurgery 2012;70:656–64. discussion 664–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Deng X, Yang C, Gan J, et al. Long-term outcomes after small-bone-window posterior fossa decompression and duraplasty in adults with Chiari malformation type I. World Neurosurg 2015;84:998–1004. [DOI] [PubMed] [Google Scholar]
- [20].Tator CH, Meguro K, Rowed DW. Favorable results with syringosubarachnoid shunts for treatment of syringomyelia. J Neurosurg 1982;56:517–23. [DOI] [PubMed] [Google Scholar]
- [21].Klekamp J. Surgical treatment of Chiari I malformation–analysis of intraoperative findings, complications, and outcome for 371 foramen magnum decompressions. Neurosurgery 2012;71:365–80. discussion 380. [DOI] [PubMed] [Google Scholar]
- [22].Klekamp J, Riedel A, Harper C, et al. Morphometric study on the postnatal growth of non-cortical brain regions in Australian aborigines and Caucasians. Brain Res 1989;485:79–88. [DOI] [PubMed] [Google Scholar]
- [23].Ucar M, Tokgoz N, Koc AM, et al. Assessment of 3D T2-weighted high-sampling-efficiency technique (SPACE) for detection of cerebellar tonsillar motion: new useful sign for Chiari I malformation. Clin Imaging 2015;39:42–50. [DOI] [PubMed] [Google Scholar]
- [24].Wu YW, Chin CT, Chan KM, et al. Pediatric Chiari I malformations: do clinical and radiologic features correlate. Neurology 1999;53:1271–6. [DOI] [PubMed] [Google Scholar]
- [25].Greenberg JK, Yarbrough CK, Radmanesh A, et al. The Chiari Severity Index: a preoperative grading system for Chiari malformation type 1. Neurosurgery 2015;76:279–85. discussion 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Limonadi FM, Selden NR. Dura-splitting decompression of the craniocervical junction: reduced operative time, hospital stay, and cost with equivalent early outcome. J Neurosurg 2004;101(2 Suppl):184–8. [DOI] [PubMed] [Google Scholar]
- [27].Yeh DD, Koch B, Crone KR. Intraoperative ultrasonography used to determine the extent of surgery necessary during posterior fossa decompression in children with Chiari malformation type I. J Neurosurg 2006;105(1 Suppl):26–32. [DOI] [PubMed] [Google Scholar]
- [28].Galarza M, Sood S, Ham S. Relevance of surgical strategies for the management of pediatric Chiari type I malformation. Childs Nerv Syst 2007;23:691–6. [DOI] [PubMed] [Google Scholar]
- [29].Mutchnick IS, Janjua RM, Moeller K, et al. Decompression of Chiari malformation with and without duraplasty: morbidity versus recurrence. J Neurosurg Pediatr 2010;5:474–8. [DOI] [PubMed] [Google Scholar]
- [30].Erdogan E, Cansever T, Secer HI, et al. The evaluation of surgical treatment options in the Chiari Malformation Type I. Turk Neurosurg 2010;20:303–13. [DOI] [PubMed] [Google Scholar]
- [31].Shweikeh F, Sunjaya D, Nuno M, et al. National trends, complications, and hospital charges in pediatric patients with Chiari malformation type I treated with posterior fossa decompression with and without duraplasty. Pediatr Neurosurg 2015;50:31–7. [DOI] [PubMed] [Google Scholar]
- [32].Munshi I, Frim D, Stine-Reyes R, et al. Effects of posterior fossa decompression with and without duraplasty on Chiari malformation-associated hydromyelia. Neurosurgery 2000;46:1384–9. discussion 1389-90. [DOI] [PubMed] [Google Scholar]
- [33].Navarro R, Olavarria G, Seshadri R, et al. Surgical results of posterior fossa decompression for patients with Chiari I malformation. Childs Nerv Syst 2004;20:349–56. [DOI] [PubMed] [Google Scholar]
- [34].McGirt MJ, Attenello FJ, Datoo G, et al. Intraoperative ultrasonography as a guide to patient selection for duraplasty after suboccipital decompression in children with Chiari malformation Type I. J Neurosurg Pediatr 2008;2:52–7. [DOI] [PubMed] [Google Scholar]
- [35].Bowers CA, Brimley C, Cole C, et al. AlloDerm for duraplasty in Chiari malformation: superior outcomes. Acta Neurochir (Wien) 2015;157:507–11. [DOI] [PubMed] [Google Scholar]
- [36].Abuzayed B, Kafadar AM, Oğuzoğlu, et al. Duraplasty using autologous fascia lata reenforced by on-site pedicled muscle flap: technical note. J Craniofac Surg 2009;20:435–8. [DOI] [PubMed] [Google Scholar]
- [37].Stevens EA, Powers AK, Sweasey TA, et al. Simplified harvest of autologous pericranium for duraplasty in Chiari malformation Type I. Technical note. J Neurosurg Spine 2009;11:80–3. [DOI] [PubMed] [Google Scholar]


