Abstract
The instantaneous wave-free ratio (iFR) closely related to fractional flow reserve (FFR) is a adenosine-independent physiologic index of coronary stenosis severity. We sought to evaluate whether iFR derived from coronary computed tomographic angiography (iFRCT) can be used as a novel noninvasive method for diagnosis of ischemia-causing coronary stenosis.
We retrospectively enrolled 33 patients (47 lesions) with coronary artery disease (CAD) and examined with coronary computed tomographic angiography (CTA), invasive coronary angiography (ICA), and FFR. Patient-specific anatomical model of the coronary artery was built by original resting end-diastolic CTA images. Based on the model and computational fluid dynamics, individual boundary conditions were set to calculate iFRCT as the mean pressure distal to the stenosis divided by the mean aortic pressure during the diastolic wave-free period of rest state. Ischemia was assessed by an FFR of up to 0.8, while anatomically obstructive CAD was defined by a stenosis of at least 50% by ICA. The correlation between iFRCT and FFR was evaluated. The receiver operating characteristic (ROC) curve was used to select the cut-off value of iFRCT for diagnosis of ischemia-causing stenosis. The diagnostic performances of iFRCT, coronary CTA, and iFRCT plus CTA for ischemia-causing stenosis were compared with ROC curve and Delong method.
On a per-vessel basis, iFRCT and FFR had linear correlation (r = 0.75, p < 0.01). ROC analysis identified an optimal iFRCT cut-off value of 0.82 for categorization based on an FFR cut-off value 0.8, and the diagnostic accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of iFRCT were 78.72%,70.59%, 83.33%,70.59%, and 83.33%, respectively. Compared with obstructive CAD diagnosed by coronary CTA (AUC = 0.60), iFRCT yielded diagnostic improvement over stenosis assessment with AUC increasing from 0.6 by CTA to 0.87 (P < 0.01) and 0.90 (P < 0.01) when iFRCT plus CTA.
In conclusion, iFRCT is a promising index improving diagnostic performance over coronary CTA for detection of ischemia-causing coronary stenosis.
Keywords: computational fluid dynamics (CFD), computed tomography angiography (CTA), coronary stenosis, fractional flow reserve (FFR), instantaneous wave-free ratio (iFR), instantaneous wave-free ratio derived from coronary computed tomography angiography (iFRCT)
1. Introduction
In recent years, coronary computed tomography angiography (CTA) has been proved to be a favorable means of morphological examination for coronary artery stenosis.[1–4] However, simple morphological stenosis cannot reliably assess the functional significance of coronary heart disease (CHD).[5–7] Comprehensive evaluation of coronary artery stenosis should include both morphological and functional aspects. Invasively measured fractional flow reserve (FFR) is widely recognized as the gold standard in evaluating the functional changes of coronary stenosis.[8–10] Revascularization therapy based on FFR is a reliable method for improving prognosis and reducing medical costs, and it has been included in the guidelines as the gold standard for qualitative diagnosis of obstructive CHD and treatment strategy. However, in practice, the application of FFR is limited due to its invasive nature, and some patients find it hard to accept vasodilatory drugs and the high cost.[11,12]
Recently, a new derivative pressure indicator (instantaneous wave-free ratio (iFR)) in the resting state has been introduced, which can be measured without administration of vasodilatory drugs. iFR is defined as the ratio between the mean instant pressure distal to coronary stenosis and the mean pressure at the aortic root during wave-free period. The internal resistance of coronary artery is the lowest and most constant during wave-free period (defined as the diastolic window), which ranges from 25% of the way into diastole to 5 ms before its ending (close to 0). This is similar to the internal resistance of coronary artery achieved by vasodilator drugs during direct measurement of FFR.[13] The ADVISE study reported that iFR had a good correlation with FFR (r = 0.9, P < 0.001) and showed excellent diagnostic efficiency. When FFR < 0.8, the area under the receiver operating characteristic curve (AUC) of iFR amounts to 0.93.[13] The JUSTIFY-CFR study showed that iFR had a better correlation with coronary flow velocity reserve (CFVR) than FFR, which could be used in functional tests of vascular lesions independent of FFR.[14] Also, van de Hoef et al[15] investigated iFR and FFR against a combined reference of myocardial perfusion scintigraphy and hyperemic stenosis resistance (HSR), and they found that among 19% of the cases where iFR disagrees with FFR, FFR did not always produce highly accurate results, indicating that FFR is not necessarily a better discriminator of coronary ischemia than iFR. Moreover, iFR avoids the administration of vasodilatory drugs needed for FFR, and it has less variability and fewer repeated measurement differences compared with FFR (−0.0005 ± 0.002 vs 0.01 ± 0.04).[13]
So far, FFR derived from coronary CTA (FFRCT) has been used for integrated evaluation of anatomical and physiological functions of coronary stenosis, and it showed that the detection of hemodynamically significant stenosis evaluated by FFRCT correlated well with invasive FFR.[16–19] However, the application of iFR derived from coronary CTA (iFRCT) for hemodynamic assessment of coronary stenosis has not yet been studied. Therefore, the purpose of our study was to evaluate the correlation between iFRCT and FFR, explore the diagnostic efficacy of iFRCT in the diagnosis of functional ischemia, and investigate the clinical value of iFRCT combined with CTA in the evaluation of coronary stenosis.
2. Methods
2.1. Study population
Clinical data of 33 patients with suspected or known CHD from August 2011 to December 2014 in 5 Chinese hospitals (Shengjing Hospital of China Medical University, the First Affiliated Hospital of China Medical University, General Hospital of Shenyang Military Command, Dalian Central Hospital, and General Hospital of Guangdong Province) were retrospectively collected. Inclusion criteria: patients who underwent coronary CTA, invasive coronary angiography (ICA), and FFR in a nonemergent setting. Exclusion criteria: time between procedures exceeding 2 months, major interprocedural adverse cardiac events (myocardial infarction, cardiac death, or revascularization), significant decrease of left ventricular function, complicated congenital heart diseases, previous coronary artery bypass surgery or stenting, installed pacemaker, artificial heart valves, bifurcation stenosis, chronic total occlusion, nondiagnostic quality of CTA data, and body mass index (BMI)≥35. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The study was approved by the ethics committee of Shengjing Hospital of China Medical University, the ethics committee of the First Affiliated Hospital of China Medical University, the ethics committee of General Hospital of Shenyang Military Command, the ethics committee of Dalian Central Hospital, and the ethics committee of General Hospital of Guangdong Province. Informed consent from patients was not needed because of the retrospective nature of this study.
2.2. Image acquisition and analysis for CT
All the CTA scanning was carried out in accordance with the guidelines recommended by the Society of Cardiovascular Computed Tomography.[20,21] CTA was performed with ≥64-slice multidetector scanners (Brilliance iCT 256, Philips Healthcare, Surrey, UK; Somatom Definition, Siemens, Forchheim, Germany; Aquilion One, Toshiba, Otawara, Japan; Optima CT660, GE Healthcare, Milwaukee, WI). Patients with heart rate >70 bpm were treated with (β)-blockers, and no nitroglycerin was given to any patient before CT examination. During the scanning, 60–80 Ml contrast agent (Omnipaque, 350 mgI/mL, GE Healthcare; Visipaque 320 mg/dL, GE Healthcare; Iopromide, 370 mgI/mL, Bayer) was administrated through intravenous injection (4–5 mL/s), followed by rinsing with 20 to 30 mL saline. Retrospective electrocardiography (ECG)-gated spiral scanning or prospective ECG-triggering axis scanning was used. Scanning parameters included: collimator width of (2 × 64)/128/320 × 0.5/0.625 mm; 100 or 120 kV; effective tube current of 400 to 700 mA. Effective dose (ED) for CTA was calculated using the following formula: ED (mSv) = (dose length product) × 0.014. The effective radiation dose was 1.5–10.8 mSv.
The original data were reconstructed using standard convolution function, with a sharp convolution function reconstruction algorithm used for cases with severe coronary artery calcification. The best quality end-diastolic phase images were selected for further analysis.
Radiologists with 7 years of experience or more evaluated image quality and analyzed coronary stenosis. The image quality of coronary CTA was assessed using a 4-point Likert scale (1 point = poor image quality, nondiagnostic; 2 points = satisfactory, reduced image quality caused by motion artifacts, image noise or limited luminal contrast, but was good enough for luminal evaluation; 3 points = good vessel contrast without major artifacts; 4 points = excellent, no diagnostic limitations).[22] Images with quality scores of 2 to 4 points were included in the analysis. The evaluation of stenosis was carried out using blood vessels as the unit, which consisted of the left anterior descending artery (including first and second diagonal branches), left circumflex artery (including the middle branch), and right coronary artery (including the right posterior and posterior descending branches). Stenosis was classified as 4 grades through visual inspection: mild stenosis (lumen diameter reduced by <50%), moderate stenosis (lumen diameter reduced by 50%–69%), severe stenosis (lumen diameter reduced by 70%–98%), and subtotal-to-total occlusion (lumen diameter reduced by 99%–100%). Per-vessel stenosis severity was evaluated by the extent of the maximal stenosis (lesion of interest) within the vessel.
Coronary plaques were defined as visible structures >1.0 mm2 located on the vascular wall or in the surrounding lumen, which could be clearly distinguished from epicardial adipose tissues in the lumen or surrounding tissues. The CT value of calcified components was >130 HU, and that of noncalcified components was <130 HU. According to plaque composition, the coronary lesions of interest were classified as a significantly calcified plaque group (>70% of the plaque volume was occupied by calcified components), or a nonsignificantly calcified plaque group (≤70% of the plaque area was occupied by calcified components).[23]
2.3. Image acquisition and analysis for ICA
ICA was performed by an experienced interventional cardiologist. A reference guide tube was used for correction, along with an edge detection system for measuring the reference diameter, minimum luminal diameter, length of the lesion, and calculation of the percentage of stenosis. The stenosis was divided into 4 grades as follows: mild stenosis (stenosis percentage <50%), moderate stenosis (50%–69%), severe stenosis (70%–98%), and subtotal-to-total occlusion (99%–100%). Evaluation of thrombolysis in myocardial infarction flow grades during imaging was as follows: Grade 0, no blood perfusion or blood flow at the distal end of the coronary artery; Grade 1, some contrast agent reached the distal end of the coronary stenosis with incomplete filling; Grade 2: the distal end of the coronary artery stenosis could be completely filled, but the image development and elimination of contrast agent were slow; Grade 3: complete and rapid filling and elimination of contrast agent at the distal end of the coronary artery; similar to normal coronary arteries.
2.4. Invasive measurement of FFR
In case of significant clinical symptoms or ≥50% stenosis was observed on the ICA results, FFR was measured (Pressure Wire Certus, St Jude Medical Systems, Uppsala, Sweden; Combo Wire, Volcano Corporation, San Diego, CA). Prior to measurement, nitroglycerin (100–200 g) was administered into the coronary artery, and then a pressure monitoring guide wire was delivered to the distal end of the stenosis, followed by intravenous or intracoronary injection of adenosine triphosphate (ATP) (140 g/kg/min) to induce a maximum congestive state. FFR was equal to the ratio of mean distal stenosis pressure to mean aortic root pressure at the maximal coronary hyperemia status. FFR≤0.80 was specified as the diagnostic standard for functional stenosis.[10]
2.5. Calculation of iFRCT
iFRCT blood flow dynamics modeling and computational fluid dynamics numerical simulation were carried out by an independent core laboratory (Biomedical Engineering Laboratory of Beijing University of Technology, Beijing, China) blinded to FFR, coronary CTA, and ICA results. The 3 key steps were as follows: first, based on the DICOM image of conventional coronary CTA at the end-diastolic stage, Mimics 10.1 (Materialise, Leuven, Belgium), Geomagic Studio 2014 (Geomagic Inc, Cary, NC) and SolidWorks 2014 (Dassault Systèmes Inc, SOLIDWORKS Corp, Waltham, MA) were used to establish an accurate individualized anatomical model of the coronary artery. Second, based on the principle that resting coronary blood flow is proportional to myocardial oxygen demand, the individual boundary conditions at the entrance and exit of the coronary artery were calculated according to the myocardial mass and mean arterial pressure of individual patients. Third, the fluid dynamics Navier Stokes equation (ANSYS Workbench 14.5, ANSYS Inc, Canonsburg, PA) was used for iterative calculation, extraction, analysis, and visualization. The pressure difference (ΔP) between each point of the coronary artery lesions and the entrance of the coronary artery was calculated. iFRCT was defined as the mean pressure distal to the stenosis during the diastolic wave-free period of rest state (Pdwave-free period of rest state) divided by the mean aortic pressure during the same state (Pawave-free period of rest state) (Equation 1), in which Pdwave-free period of rest state was equal to Pawave-free period of rest stateminus ΔP (Equation 2), and thus the contours of iFRCT were obtained. The whole process of modeling and calculation for each case took about 4 hours.
 |
2.6. Statistical analyses
SPSS for Windows version 20.0 (SPSS, Chicago, IL) and MedCalc for Windows version 12.7.7 (MedCalc Software, Ostend, Belgium) were used for statistical analysis. All variable data were presented as mean ± standard deviation. Pearson correlation analysis was used to analyze the correlation between FFR and iFRCT, thus comparing the correlation difference of FFR and iFRCT between the significantly and nonsignificantly calcified plaque groups. FFR value was used as the gold standard for diagnosis of functional coronary stenosis. The ROC curve was used to select the most suitable cut-off value of iFRCT for the diagnosis of functional coronary artery stenosis, and to investigate the diagnostic efficiency of iFRCT for functional stenosis. The difference between the diagnostic efficiency of iFRCT in the significantly and nonsignificantly calcified plaque groups was evaluated using Fisher test. Coronary CTA luminal stenosis ≥50% was determined as the critical value for the diagnosis of functional stenosis-causing myocardial ischemia. Logistic regression was used to calculate the predictive probability of CTA combined with iFRCT, and then ROC curve analysis was used to investigate the impact of coronary CTA combined with iFRCT on diagnostic efficiency for functional stenosis. The difference of AUC in the diagnosis of functional stenosis using CTA alone and CTA combined with iFRCT was compared using Delong method. P<0.05 was considered statistically significant.
3. Results
3.1. Characteristics of study subjects
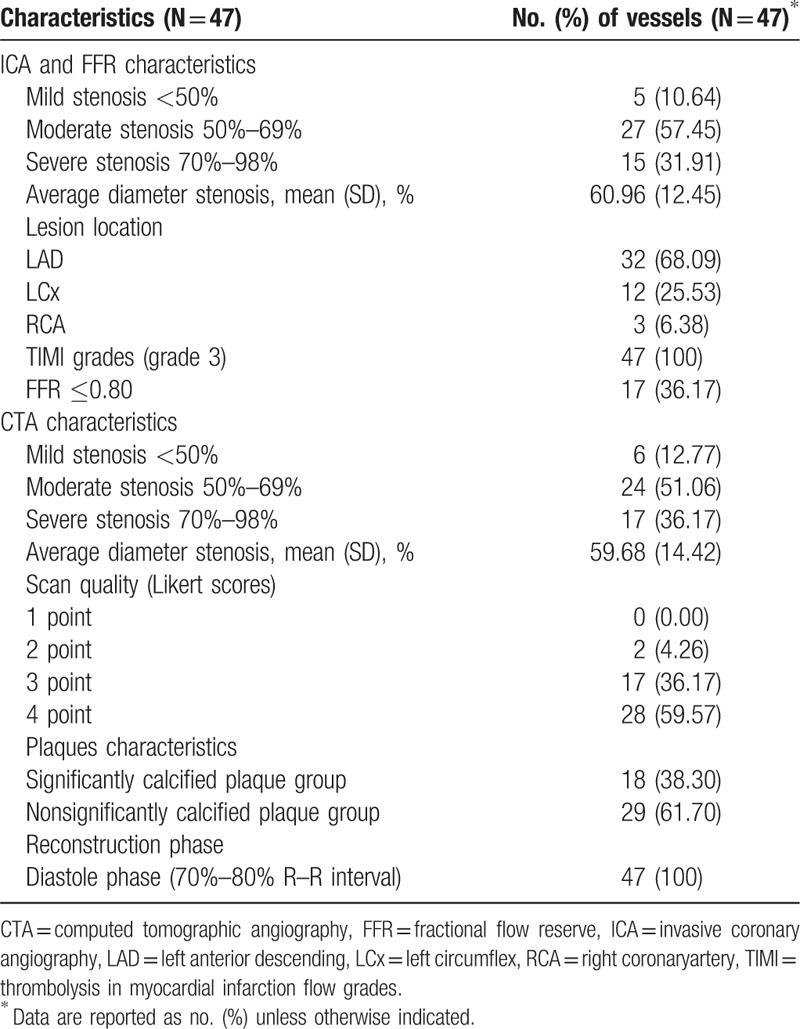
This study included 33 eligible patients with a total of 47 vascular lesions. The demographic and clinical characteristics of the study population are shown in Table 1. The analysis was based on each vessel, and the results of ICA, CTA, and FFR for all 47 lesions are listed in Table 2.
Table 1.
Baseline characteristics of the study population.
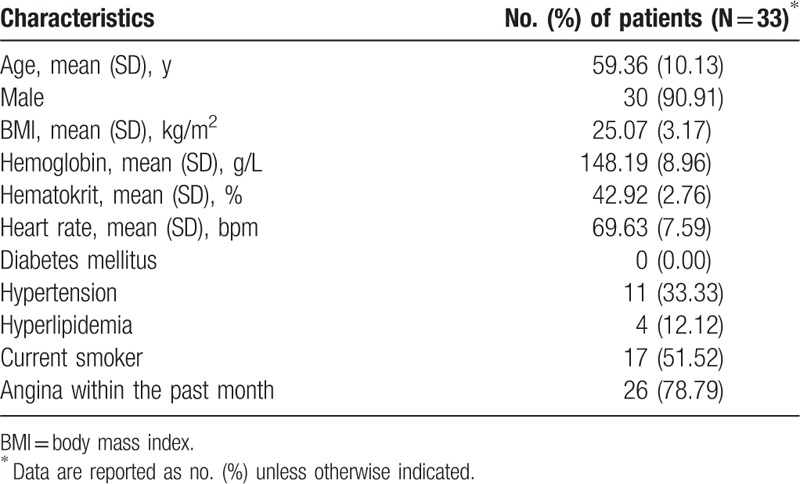
Table 2.
Vessels characteristics by ICA, FFR, and CTA.
3.2. Correlation between iFRCT and FFR
For all blood vessels, the iFRCT value was higher than the FFR value. However, the iFRCT value of the significantly calcified plaque group was lower than the FFR value (Table 3). There was a significant linear correlation between FFR and iFRCT (Fig. 1). There was no significant difference in the correlation of FFR and iFRCT between the significantly and nonsignificantly calcified plaque groups (95% confidence interval (CI) 0.72–0.95 vs 0.32–0.90, respectively) (Table 3). Representative example of anatomically obstructive stenosis without ischemia-producing stenosis is shown in Fig. 2.
Table 3.
Correlation of iFRCT and invasive FFR.
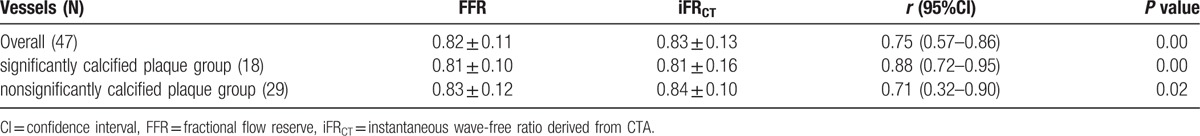
Figure 1.
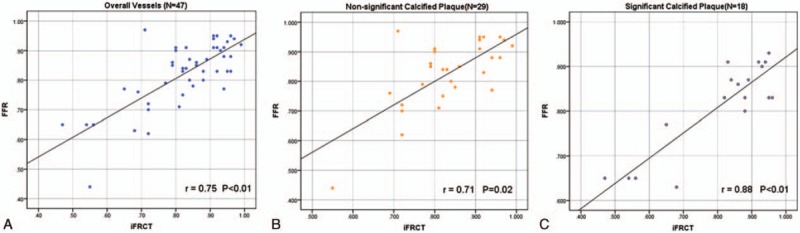
Correlation of iFRCT and invasive FFR. A strong correlation was observed between iFRCT and FFR in overall vessels (A), the group of nonsignificant calcified plaque (B), and the group of significant calcified plaque (C). FFR = fractional flow reserve, iFRCT = instantaneous wave-free ratio derived from coronary computed tomography angiography.
Figure 2.
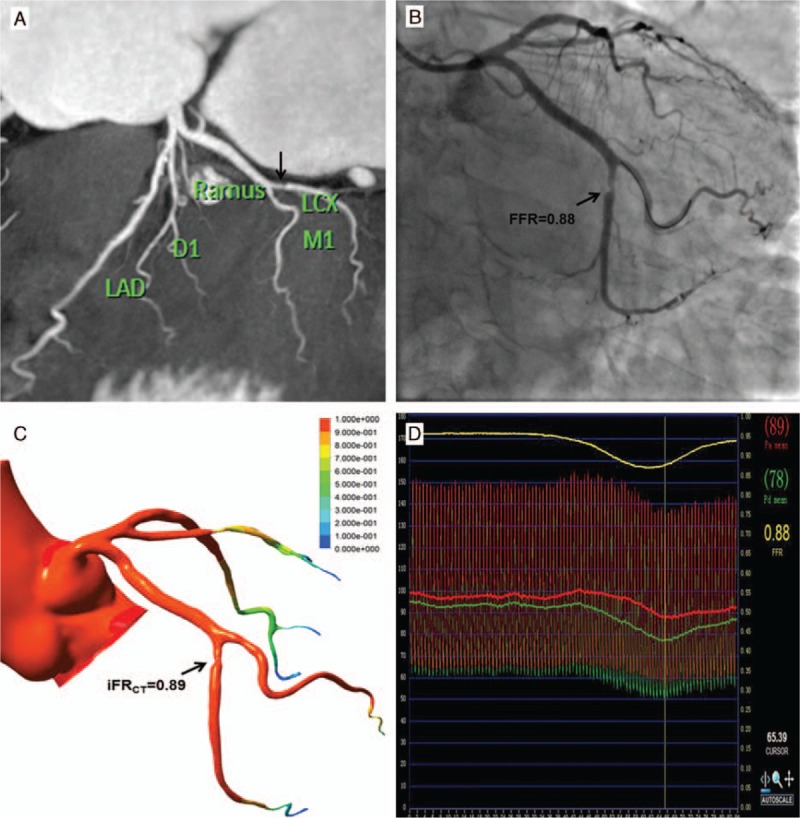
Coronary CTA, displayed as 2D with maximum intensity projection reformation of the left coronary artery, showed a moderate luminal stenosis (50–69%) in the mid portion of the LCX vessel (arrow) (A). Invasive coronary angiography confirmed the lesion (B). Catheter-based FFR of the moderate stenosis lesion of LCX was measured at 0.88, indicating lack of hemodynamic significance of this lesion (D). Noninvasive iFRCT resulted in a value of 0.89 for the lesion of LCX (arrow), in good correlation with invasive measurement (C). CTA = computed tomography angiography, FFR = fractional flow reserve, LCX = left circumflex.
3.3. The optimal cut-off value and diagnostic efficiency of iFRCT in the diagnosis of coronary stenosis
FFR≤0.80 was determined as the gold standard for the diagnosis of functional stenosis-causing myocardial ischemia. AUC for iFRCT was 0.87 (95% CI 0.75–0.98, P < 0.01), with an optimal iFRCT cut-off value of 0.82 (Fig. 3). At this point, the diagnostic accuracy of iFRCT for coronary stenosis was 78.72%, with sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 70.59%, 83.33%, 70.59%, and 83.33%, respectively.
Figure 3.
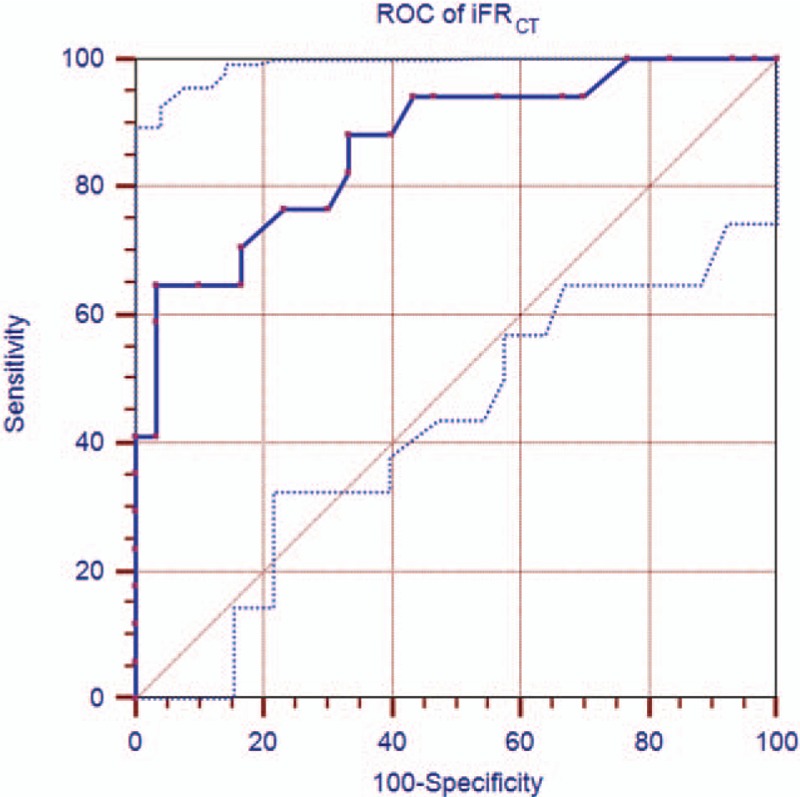
Receiver operating characteristic curve of per-vessel performance of iFRCT compared with invasive FFR≤0.80 for diagnosis of ischemia.
3.4. Comparison of iFRCT diagnostic efficiency between significantly and nonsignificantly calcified plaque groups
There were 18 vascular lesions in the significantly calcified plaque group, of which lesions with FFR ≤0.80 accounted for 33.33% (n = 6). The diagnostic accuracy, sensitivity, specificity, PPV, and NPV of iFRCT in this group were 94.44% and 83.33%, 100%, 100%, and 92.31%, respectively. There were 29 vascular lesions in the nonsignificantly calcified plaque group, of which lesions with FFR ≤0.80 accounted for 37.93% (n = 11). The diagnostic accuracy, sensitivity, specificity, PPV, and NPV of iFRCT in this group were 68.97%, 63.64%, 72.22%, 58.33%, and 76.47%, respectively. However, there was no significant difference in the diagnostic accuracy of iFRCT between these 2 groups detected by Fisher exact test (P = 0.07).
3.5. Efficacy of CTA combined with iFRCT in the diagnosis of functional coronary stenosis
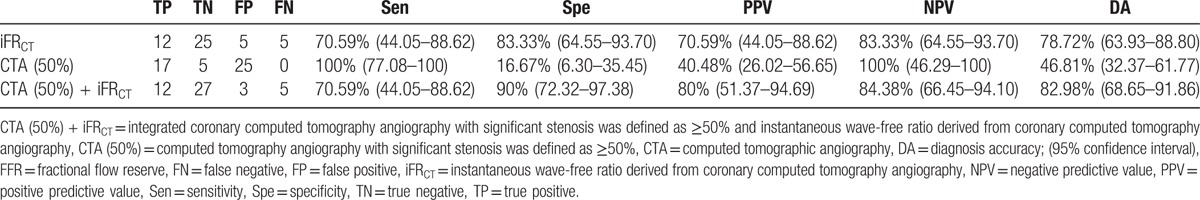
FFR≤0.80 was determined as the gold standard for the diagnosis of ischemic stenosis. The efficacy of coronary CTA and iFRCT alone, and their combination in the diagnosis of ischemic stenosis is shown in Fig. 4 and Table 4. The diagnostic accuracy of CTA (50%) was increased to 82.98% when introducing iFRCT, with its specificity increased to 90% and PPV to 80%. Compared with the AUC when using coronary CTA alone for diagnosis, the diagnostic efficacy of CTA (50%) was significantly higher after introduction of iFRCT (0.6 vs 0.9, P < 0.01), with the AUC value of the ROC curve of 0.90, demonstrating that diagnosis of coronary ischemic stenosis was significantly improved by combining CTA with iFRCT (P = 0.04).
Figure 4.
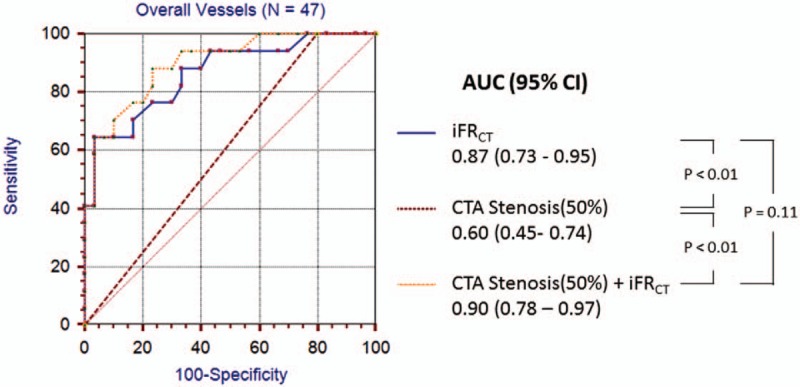
Receiver operating characteristic curve demonstrating AUC for iFRCT, CTA stenosis, and the combination of the both for discrimination of lesions that caused ischemia on a per-vessel level. The AUC for iFRCT and the combination of the both were significantly higher than CTA stenosis.
Table 4.
Diagnostic performance of iFRCT, CTA stenosis, and CTA stenosis combined with iFRCT on a per-vessel basis, using invasive FFR as the reference standardin diagnosis of functional coronary stenosis.
4. Discussion
The current study demonstrated a good correlation between iFRCT and FFR. The application of iFRCT showed high efficiency for the diagnosis of coronary stenosis, and it also had good diagnostic accuracy for functional stenosis caused by significantly calcified plaques. More importantly, using FFR≤0.80 as the gold standard for control, the diagnostic ability of iFRCT combined with coronary CTA for functional stenosis was significantly improved, with higher specificity and PPV compared with using CTA alone.
To the best of our knowledge, this study is the first blood flow dynamics simulation and calculation of resting state index iFR based on conventional coronary CTA images. Sen et al[13] found a transient wave-free interval during cardiac diastole (from 25% of the process of diastole to 5 ms before its ending), during which time the measured cardiac microcirculation resistance was naturally constant and minor. This is similar to the average microcirculation resistance of the whole cardiac cycle during maximum cardiac expansion induced by adenosine.[13] iFR is in theory similar to FFR and its result is independent of heart rate, blood pressure, and even blood pressure change caused by ectopic arrhythmia or respiration.[13] The ADVISE study showed that iFR had good reproducibility (r = 0.996, P < 0.01) and was highly correlated with FFR (r = 0.90).[13] However, the VERIFY study drew different conclusions, pointing out that iFR was influenced by vasodilators and correlated weakly with FFR.[24] In addition, the RESOLVE study and Johnson et al found hyperemic indicators provided diagnostic performance superior to that of resting iFR.[25,26] Although resistance was reduced further by administration of adenosine, the CLARIFFY study demonstrated that differences in magnitude of microvascular resistance did not influence diagnostic categorization, and iFR, iFRa (adenosine administration), and FFR had equally good diagnostic agreement with HSR.[27] Meanwhile, Petraco et al proved that hyperemic FFR flow was similar to baseline iFR flow in functionally significant lesions (FFR ≤0.75; mean FFR flow, 25.8 ± 13.7 cm/s vs mean iFR flow, 21.5 ± 11.7 cm/s; P = 0.13).[14] Furthermore, iFR showed a stronger correlation with underlying CFVR (iFR–CFVR, ρ=0.68 vs FFR–CFVR, ρ=0.50; P < 0.001) and also agreed more closely with CFVR in stenosis classification (iFR AUC = 0.82 vs FFR AUC = 0.72; P < 0.001, for a CFVR of 2).[14] Therefore, iFR can be used as an adenosine-free alternative for FFR with a good diagnostic performance. When 0.83 is used as the threshold for iFR, its diagnostic sensitivity is 91%, with specificity of 85%, PPV of 91%, and NPV of 85%.[13] The ADVISE registry study demonstrated that, based on the inherent variability of FFR, the consistency in critical lesion classification using iFR≤0.89 and FFR≤0.80 as the threshold was as high as 94%, and 81% of the critical lesions with inconsistent classification had associated FFR values located within the FFR “gray zone”(0.75–0.80).[28] Petraco et al[29] used iFR < 0.86 as the threshold (PPV 92%) for percutaneous coronary intervention (PCI), iFR>0.93 as the threshold (NPV 91%) for delay of PCI, and only performed adenosine detection of FFR in patients with iFR0.86–0.93. Such an iFR–FFR hybrid method can reduce the use of adenosine in patients by 57%, and achieve a classification consistency of 95% with FFR.[29] The results of the current study show that iFRCT is significant correlated with the invasively measured FFR, just as in previous studies,[13,24,25,27,28,30] which provides a new evidence supporting the use of iFR as an evaluation index for functional stenosis.
Our study demonstrated the high efficiency of using iFRCT for the diagnosis of coronary artery stenosis. The diagnostic accuracy, sensitivity, specificity, PPV, and NPV were 78.72%, 70.59%, 83.33%, 70.59%, and 83.33%, respectively. Such results are mostly equivalent to the diagnostic efficacy of FFRCT reported in previous multicenter studies. Previous prospective studies, DISCOVER-FLOW,[16] DeFACTO,[31] and NXT[32] have demonstrated diagnostic accuracy, sensitivity, specificity, PPV, and NPV of FFRCT of 73% to 81%, 86% to 90%, 54% to 82%, 65% to 74%, and 84% to 93%, respectively. Our study confirmed the feasibility of using a computational fluid dynamics model for noninvasive detection of the physiological and pathological changes in the coronary artery, thus providing a new noninvasive modeling index. Meanwhile, without the step of simulating maximal blood filling, our new method is closer to the true physiological state during CTA scanning.
We have demonstrated that, compared with using a stenosis diameter≥50% as the diagnostic standard for indicating obstructive coronary artery disease, iFRCT could increase diagnostic specificity fourfold, PPV by 74.38%, and accuracy by 68.17%. The significant increase in specificity will avoid unnecessary invasive examinations and reduce medical costs. Compared with the diagnostic efficacy of iFRCT alone, combining iFRCT with CTA slightly enhances the diagnostic specificity, PPV, and diagnostic accuracy. Although no significant difference was found between the diagnostic efficacy of iFRCT alone and iFRCT combined with CTA, 2 cases classified as false positive by iFRCT were reclassified as true negative using combined diagnosis. This improved the diagnostic performance of coronary CTA for functional ischemia, showing the potential of coronary CTA combined with iFRCT as the gatekeeper for ICA and revascularization therapy.
Beam hardening effects, halo artifacts (blooming), and partial volume effects could lead to overestimation by CTA of the degree of coronary stenosis caused by calcified plaques.[33,34] The current study showed good correlation between iFRCT and FFR (r = 0.88, P < 0.01) in the significantly calcified plaque group, in which iFRCT demonstrated highly accurate diagnostics and specificity of 94.44% and 100%, respectively. Although our study divided cases into different groups based on the number of calcified plaques in the lesion, the results were similar to previous studies using Agatston score. Miyoshi et al analyzed the data of the Japanese NXT study, and reported that FFRCT still had a high diagnostic efficiency (accuracy of 85% and specificity of 81%) for patients with an Agatston score of 400 to 1000.[35] Norgaard et al analyzed 333 lesions in 214 patients and showed that, compared with CTA, FFRCT had a higher diagnostic efficiency for ischemic stenosis in patients with a high Agatston score (416–3599) (AUC: 0.86 vs 0.72; P = 0.09), and in vessels with a high Agatston score (121–1703) (AUC: 0.91 vs 0.71; P = 0.004).[36] There are 2 possible reasons for this. First, the cases with severe coronary artery calcification were reconstructed using sharp convolution function reconstruction algorithm, which improved the spatial resolution and the boundary between the vessel lumen and calcified plaques, allowing easier luminal identification in modeling. Second, in addition to correlation with luminal border, hemodynamic simulation of iFRCT also involved other parameters, such as blood pressure, blood viscosity, myocardial mass, and heart rate.[22] This decreased the influence of inaccurate luminal identification on the results, which is unlike the coronary CTA that solely relies on the luminal outline. Such results suggest that iFRCT may be a good auxiliary method for CT in the evaluation of coronary artery stenosis caused by severe calcification. This avoids excluding patients with severe calcification from the indications for CTA examination, providing a new method by which coronary artery disease can be examined without invasive techniques in a high-risk population with severe calcification.
However, the current study has the following limitations: the sample size was relatively small. The proportion of patients with positive FFR was only 36.17%, indicating possible selection bias. Because of the small sample size, our study analyzed diagnosis effectiveness based only on blood vessels rather than patients. Although the iFRCT threshold value for the diagnosis of functional stenosis was provided, data from a larger sample are needed to verify the results. This study included only patients with chronic coronary artery disease without a history of coronary artery surgery and acute myocardial infarction; therefore, the applicability of iFRCT in patients with PCI, bypass surgery, and acute coronary syndrome is unknown. In this study, FFR≤0.80 was specified as the diagnostic standard for functional stenosis, as with most of the similar studies in recent years.[10,22,32,37] But FFR is not only influenced by the severity of luminal stenosis, but also by coronary artery morphology and plaque characteristics.[38–40] Therefore, the diagnosis efficiency of iFRCT for patients with different plaque characteristics and coronary artery morphology needs further validation. The modeling calculation was complicated and time-consuming. Data processing was carried out by independent laboratories, and the simulation and calculation on each patient took about 4 hours, which was affected by the performance of the computer and modeling software used. By upgrading and optimizing equipment and technology, the calculation time is expected to be significantly reduced.
5. Conclusions
iFRCT is strongly correlated with invasive FFR, and has a higher accuracy, specificity, and PPV than CTA in the diagnosis of functional coronary artery stenosis. Combining with iFRCT can improve the diagnostic accuracy of coronary CTA in the diagnosis of functional stenosis, which also has a good diagnostic ability for coronary stenosis caused by lesions with severe calcification. iFRCT is expected to become a noninvasive, adenosine-independent physiologic index for the diagnosis of ischemia-caused coronary stenosis based on resting state coronary CTA; however, additional studies are needed to determine whether iFRCT has the same diagnostic accuracy as FFRCT for the detection of ischemia-causing coronary stenosis.
Acknowledgments
The authors thank Dr Quanmin Jing (Department of Cardiology, General Hospital of Shenyang Military Command, Shenyang, China), Dr Benqiang Yang (Department of Radiology, General Hospital of Shenyang Military Command, Shenyang, China), and Dr Haishan Zhang(Department of Cardiology, the First Affiliated Hospital of China Medical University, Shenyang, China) for their help in collecting patient.
Footnotes
Abbreviations: AUC = area under the receiver operating characteristic curve, BMI = body mass index, CAD = coronary artery disease, CFD = computational fluid dynamics, CTA = computed tomography angiography, FFR = fractional flow reserve, ICA = invasive coronary angiography, iFR = instantaneous wave-free ratio, iFRCT = instantaneous wave-free ratio derived from coronary computed tomography angiography, LAD = left anterior descending, LCX = left circumflex, NPV = negative predictive value, PPV = positive predictive value, RCA = right coronary artery, ROC = receiver operating characteristic curve.
YM and HL contributed equally to this work.
The presented work was supported by Natural Science Foundation of China (81301221, China) and Program for Liaoning Innovative Research Team in University (LT2014017, Liaoning Province, China).
The authors have no conflicts of interest to disclose.
References
- [1].Yoon YE, Lim TH. Current roles and future applications of cardiac CT: risk stratification of coronary artery disease. Korean J Radiol 2014;15:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rossi A, Papadopoulou SL, Pugliese F, et al. Quantitative computed tomographic coronary angiography: does it predict functionally significant coronary stenoses? Circ Cardiovasc Imaging 2014;7:43–51. [DOI] [PubMed] [Google Scholar]
- [3].Montalescot G, Sechtem U, Achenbach S, et al. Task Force Members 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- [4].Hou Y, Ma Y, Fan W, et al. Diagnostic accuracy of low-dose 256-slice multi-detector coronary CT angiography using iterative reconstruction in patients with suspected coronary artery disease. Eur Radiol 2014;24:3–11. [DOI] [PubMed] [Google Scholar]
- [5].Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–16. [DOI] [PubMed] [Google Scholar]
- [6].Meijboom WB, Van Mieghem CA, van Pelt N, et al. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol 2008;52:636–43. [DOI] [PubMed] [Google Scholar]
- [7].Tonino PA, Fearon WF, De Bruyne B, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 2010;55:2816–21. [DOI] [PubMed] [Google Scholar]
- [8].Wijns W, Kolh P, Danchin N, et al. Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS), European Association for Percutaneous Cardiovascular Interventions (EAPCI) Guidelines on myocardial revascularization. Eur Heart J 2010;31:2501–55. [DOI] [PubMed] [Google Scholar]
- [9].Pijls NH. Fractional flow reserve to guide coronary revascularization. Circ J 2013;77:561–9. [DOI] [PubMed] [Google Scholar]
- [10].Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–24. [DOI] [PubMed] [Google Scholar]
- [11].Park SJ, Ahn JM, Park GM, et al. Trends in the outcomes of percutaneous coronary intervention with the routine incorporation of fractional flow reserve in real practice. Eur Heart J 2013;34:3353–61. [DOI] [PubMed] [Google Scholar]
- [12].Morris PD, Ryan D, Morton AC, et al. Virtual fractional flow reserve from coronary angiography: modeling the significance of coronary lesions: results from the VIRTU-1 (virtual fractional flow reserve from coronary angiography) study. JACC Cardiovasc Interv 2013;6:149–57. [DOI] [PubMed] [Google Scholar]
- [13].Sen S, Escaned J, Malik IS, et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (Adenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol 2012;59:1392–402. [DOI] [PubMed] [Google Scholar]
- [14].Petraco R, van de Hoef TP, Nijjer S, et al. Baseline instantaneous wave-free ratio as a pressure-only estimation of underlying coronary flow reserve: results of the JUSTIFY-CFR study (joined coronary pressure and flow analysis to determine diagnostic characteristics of basal and hyperemic indices of functional lesion severity-coronary flow reserve). Circ Cardiovasc Interv 2014;7:492–502. [DOI] [PubMed] [Google Scholar]
- [15].van de Hoef TP, Meuwissen M, Escaned J, et al. Head-to-head comparison of basal stenosis resistance index, instantaneous wave-free ratio, and fractional flow reserve: diagnostic accuracy for stenosis-specific myocardial ischaemia. EuroIntervention 2015;11:914–25. [DOI] [PubMed] [Google Scholar]
- [16].Koo BK, Erglis A, Doh JH, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (diagnosis of ischemia-causing stenoses obtained via noninvasive fractional flow reserve) study. J Am Coll Cardiol 2011;58:1989–97. [DOI] [PubMed] [Google Scholar]
- [17].Rajani R, Webb J, Marciniak A, et al. Comparative efficacy testing—fractional flow reserve by coronary computed tomography for the evaluation of patients with stable chest pain. Int J Cardiol 2015;183:173–7. [DOI] [PubMed] [Google Scholar]
- [18].Precious B, Blanke P, Nørgaard BL, et al. Fractional flow reserve modeled from resting coronary CT angiography: state of the science. Am J Roentgenol 2015;204:W243–248. [DOI] [PubMed] [Google Scholar]
- [19].Nørgaard BL, Jensen JM, Leipsic J. Fractional flow reserve derived from coronary CT angiography in stable coronary disease: a new standard in non-invasive testing? Eur Radiol 2015;25:2282–90. [DOI] [PubMed] [Google Scholar]
- [20].Abbara S, Arbab-Zadeh A, Callister TQ, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2009;3:190–204. [DOI] [PubMed] [Google Scholar]
- [21].Halliburton SS, Abbara S, Chen MY, et al. Society of Cardiovascular Computed Tomography. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011;5:198–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Min JK, Koo BK, Erglis A, et al. Effect of image quality on diagnostic accuracy of noninvasive fractional flow reserve: results from the prospective multicenter international DISCOVER-FLOW study. J Cardiovasc Comput Tomogr 2012;6:191–9. [DOI] [PubMed] [Google Scholar]
- [23].Yoon YE, Choi JH, Kim JH, et al. Noninvasive diagnosis of ischemia-causing coronary stenosis using CT angiography: diagnostic value of transluminal attenuation gradient and fractional flow reserve computed from coronary CT angiography compared to invasively measured fractional flow reserve. JACC Cardiovasc Imaging 2012;5:1088–96. [DOI] [PubMed] [Google Scholar]
- [24].Berry C, van ’t Veer M, Witt N, et al. VERIFY (verification of instantaneous wave-free ratio and fractional flow reserve for the assessment of coronary artery stenosis severity in everyday practice): a multicenter study in consecutive patients. J Am Coll Cardiol 2013;61:1421–7. [DOI] [PubMed] [Google Scholar]
- [25].Jeremias A, Maehara A, Généreux P, et al. Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting Pd/Pa with fractional flow reserve: the RESOLVE study. J Am Coll Cardiol 2014;63:1253–61. [DOI] [PubMed] [Google Scholar]
- [26].Johnson NP, Jeremias A, Zimmermann FM, et al. Continuum of vasodilator stress from rest to contrast medium to adenosine hyperemia for fractional flow reserve assessment. JACC Cardiovasc Interv 2016;9:757–67. [DOI] [PubMed] [Google Scholar]
- [27].Sen S, Asrress KN, Nijjer S, et al. Diagnostic classification of the instantaneous wave-free ratio is equivalent to fractional flow reserve and is not improved with adenosine administration. Results of CLARIFY (classification accuracy of pressure-only ratios against indices using flow study). J Am Coll Cardiol 2013;61:1409–20. [DOI] [PubMed] [Google Scholar]
- [28].Petraco R, Escaned J, Sen S, et al. Classification performance of instantaneous wave-free ratio (iFR) and fractional flow reserve in a clinical population of intermediate coronary stenoses: results of the ADVISE registry. EuroIntervention 2013;9:91–101. [DOI] [PubMed] [Google Scholar]
- [29].Petraco R, Park JJ, Sen S, et al. Hybrid iFR-FFR decision-making strategy: implications for enhancing universal adoption of physiology-guided coronary revascularization. EuroIntervention 2013;8:1157–65. [DOI] [PubMed] [Google Scholar]
- [30].Escaned J, Echavarría-Pinto M, Garcia-Garcia HM, et al. Prospective assessment of the diagnostic accuracy of instantaneous wave-free ratio to assess coronary stenosis relevance: results of ADVISE II International, Multicenter Study (ADenosine Vasodilator Independent Stenosis Evaluation II). JACC Cardiovasc Interv 2015;8:824–33. [DOI] [PubMed] [Google Scholar]
- [31].Min JK, Berman DS, Budoff MJ, et al. Rationale and design of the DeFACTO (determination of fractional flow reserve by anatomic computed tomographic angiography) study. J Cardiovasc Comput Tomogr 2011;5:301–9. [DOI] [PubMed] [Google Scholar]
- [32].Nørgaard BL, Leipsic J, Gaur S, et al. NXT Trial Study Group Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014;63:1145–55. [DOI] [PubMed] [Google Scholar]
- [33].Zhang S, Levin DC, Halpern EJ, et al. Accuracy of MDCT in assessing the degree of stenosis caused by calcified coronary artery plaques. Am J Roentgenol 2008;191:1676–83. [DOI] [PubMed] [Google Scholar]
- [34].Shmilovich H, Cheng VY, Dey D, et al. Optimizing image contrast display improves quantitative stenosis measurement in heavily calcified coronary arterial segments on coronary CT angiography: a proof-of-concept and comparison to quantitative invasive coronary angiography. Acad Radiol 2014;21:797–804. [DOI] [PubMed] [Google Scholar]
- [35].Miyoshi T, Osawa K, Ito H, et al. Non-invasive computed fractional flow reserve from computed tomography (CT) for diagnosing coronary artery disease—Japanese results from NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). Circ J 2015;79:406–12. [DOI] [PubMed] [Google Scholar]
- [36].Nørgaard BL, Gaur S, Leipsic J, et al. Influence of coronary calcification on the diagnostic performance of CT angiography derived FFR in coronary artery disease: a substudy of the NXT trial. JACC Cardiovasc Imaging 2015;8:1045–55. [DOI] [PubMed] [Google Scholar]
- [37].Ko BS, Wong DT, Norgaard BL, et al. Diagnostic performance of transluminal attenuation gradient and noninvasive fractional flow reserve derived from 320-detector row CT angiography to diagnose hemodynamically significant coronary stenosis: an NXT substudy. Radiology 2016;279:75–83. [DOI] [PubMed] [Google Scholar]
- [38].Park SJ, Kang SJ, Ahn JM, et al. Visual-functional mismatch between coronary angiography and fractional flow reserve. JACC Cardiovasc Interv 2012;5:1029–36. [DOI] [PubMed] [Google Scholar]
- [39].Natsumeda M, Nakazawa G, Murakami T, et al. Coronary angiographic characteristics that influence fractional flow reserve. Circ J 2015;79:802–7. [DOI] [PubMed] [Google Scholar]
- [40].Takashima H, Waseda K, Gosho M, et al. Severity of morphological lesion complexity affects fractional flow reserve in intermediate coronary stenosis. J Cardiol 2015;66:239–45. [DOI] [PubMed] [Google Scholar]


