Supplemental Digital Content is available in the text
Keywords: breast cancer, mastectomy, negative lymph nodes, radiotherapy, SEER
Abstract
Many studies have confirmed the role of postmastectomy radiotherapy (PMRT) for breast cancer patients with at least 4 lymph nodes invasion in the postoperative therapy. Recently, the number of negative lymph nodes (NLNs) has been increasingly paid attention to and recognized as a prognostic indicator in different kinds of caners. Therefore, it is very necessary to study the association between the number of NLNs and the prognosis of PMRT in breast cancer patients. In our study, we used Surveillance, Epidemiology, and End Results (SEER) population-based data and identified 16,686 breast cancer patients to explore their correlation. The ROC curve and the log-rank χ2 test were applied to determine the appropriate cutoff point of the number of NLNs and 5 was selected as the cutoff point. Furthermore, the cutoff point 5 was validated as an independent prognostic factor affecting cancer-specific survival (CSS) and overall survival (OS) in breast cancer patients, as confirmed by both univariate and multivariate analysis (P < 0.001). In addition, subgroup analysis showed that the number of NLNs >5 can be a prognostic indicator in patients with PMRT according to different clinical variables (all, P < 0.001). Importantly, our results showed that PMRT obviously improved CSS and OS in patients regardless of the number of NLNs (P < 0.001). In conclusion, our study showed the number of NLNs is an independent prognostic factor for breast cancer patients with PMRT, and those who have higher number of NLNs have an increased CSS and OS.
1. Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among females worldwide, with an estimated 1.7 million incidence and 521,900 mortalities in 2012.[1] Breast cancer alone accounts for 25% of all cancers and 15% of all cancer-related deaths among females.[1] Lack of effective adjuvant therapies mainly account for the recurrence and metastasis of the disease, which would even result in the death of the patients. Postmastectomy radiotherapy (PMRT), a commonly used practice, can prevent locoregional recurrence and increase survival in breast cancer patients after definite surgery. PMRT has already become a standard adjuvant postoperative therapy for patients with four or more lymph nodes invasion.[2–4]
Many factors can affect the PMRT for breast cancer patients, which include lymph node involvement, large tumor size, positive or close surgical margins, et al.[5] Interestingly, the number of NLNs has recently been paid attention to and recognized as a prognostic indicator in breast cancer.[6–8] To date, studies, NLNs can be regarded as a prognostic factor for PMRT of breast cancer, are limited and controversial. Given the growing predominance of PMRT in the management of patients with breast cancer, it is vital to explore the optimal cutoff point and to further confirm the specific value of the number of NLNs in breast cancer patients with PMRT. Therefore, we assessed the effect of the number of NLNs on the prognosis of breast cancer patients with PMRT by analyzing the Surveillance, Epidemiology, and End Results (SEER)-registered database.
2. Methods
2.1. Patient selection
The SEER database was used to identify all young female patients diagnosed with breast cancer between 1990 and 2012.[9] A total of 18 population-based cancer registries in the United States were included in the current SEER database. The SEER∗Stat software (SEER∗Stat 8.2.1) was used to identify patients. The included patients should meet the following criteria: the diagnosis was confirmed microscopically, they should be female with the confirmed age, active follow-up, and only 1 primary tumor. In addition, the patients should be received modified radical mastectomy, with at least 4 positive lymph nodes removed. Patients with benign or borderline tumors, unknown age, unknown cause of death, and unknown survival months were excluded. In addition, to guarantee the accuracy, we excluded the patients diagnosed between 2009 and 2012 to have enough follow-up.
2.2. Ethical statement
This study was mainly based on the SEER database and was conducted in compliance with the Helsinki Declaration. We obtained permission to access the SEER program research data files and the reference number is 11824-Nov2014. The informed consent was not required because personal identifying information was not involved. This study was approved by the ethics committee of the Shandong Cancer Hospital affiliated to Shandong University.
2.3. Statistical analysis
For all patients, the following variables were analyzed: age, race, year of diagnosis, tumor size, grade, estrogen receptor (ER) status, progesterone receptor (PR) status, and number of NLNs. In addition, the overall survival (OS) and cancer-specific survival (CSS) were regarded as the primary endpoint of this study and extracted from the SEER database. χ2 tests were used to compare the patient baseline characteristics. The Kaplan–Meier analyses were used to generate the survival curves and the Log Rank test was applied to analyze the differences among the curves. Comparative risks of mortality were evaluated using univariate and multivariate Cox proportional hazards regression models. All statistical tests were 2-sided, and P < 0.05 was considered statistically significant. The statistical software SPSS 18.0 (SPSS Inc, Chicago, IL) was used for all data analyses.
3. Results
3.1. Patient demographics
There were 16,686 female breast cancer (FBC) patients reported in the SEER database from 1990 to 2009. The clinical characteristics and pathological features of all the patients were summarized in Table 1. Most patients were diagnosed at the age of above 40 years (89.3%). Most patients were white race (80.0%) and were diagnosed after 2000 (81.0%). A total of 44.4% of patients were in AJCC T2 stage (tumor size) and 51.0% of patients were in Grade III. In addition, 68.1% of patients were ER-positive and 56% of patients were PR-positive. The detailed statistical results were showed in Table 1.
Table 1.
Characteristics of breast cancer patients with ≥4 positive lymph nodes after PMRT from SEER Database from 1990 to 2009.

3.2. Identification of appropriate cutoff points of NLNs
The ROC curve was applied to determine the appropriate cutoff points of NLNs. The results showed that the Youden index is the largest, when the cutoff point of NLNs is 4.5 to 5.5 (Supplementary Table 1). Therefore, 5 was the appropriate cutoff point for NLNs (Area Under roc Curve = 0.628, P < 0.001) (Fig. 1). To further verify the feasibility of the 5 as appropriate cutoff point for NLNs, we treated NLNs as a continuous variable. The results showed that NLN was validated as a significant prognostic factor using univariate log-rank test (P < 0.001) (Table 2). We further analyzed individual NLNs from 5 to 16. The 5-year and 10-year CSS or OS with different numbers of NLNs were calculated, respectively. The results showed the number of NLNs from 5 to 16 has the same impact on CSS and OS (all, P < 0.001); however, the patients with ≥5 NLNs had a maximum log-rank χ2 value both in CSS (χ2 = 608.1, P < 0.001) and OS (χ2 = 636.6, P < 0.001) (Table 2). Therefore, 5 can be selected as the appropriate cutoff point for the number of NLNs for all patients. The 5-year and 10-year survival rate of the patients with the number of >5 NLNs were 81.5% and 67.7% in CSS and 77.5% and 59.1%, respectively. Therefore, the appropriate cutoff value of 5 was validated as a prognostic factor to analyze the clinical effect of the number of NLNs.
Figure 1.
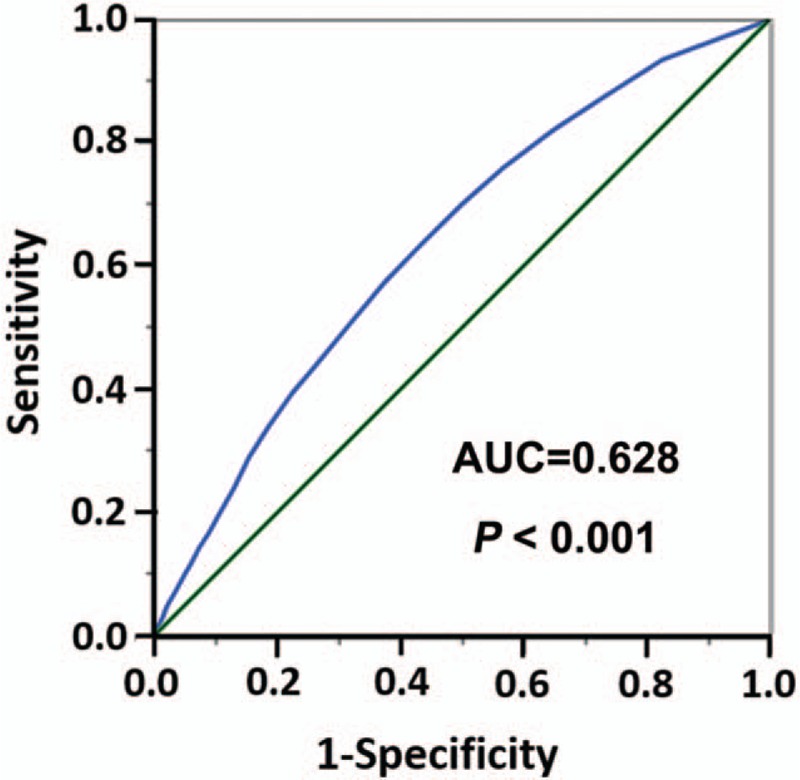
The receiver-operating characteristic curve of the appropriate cutoff point of the number of negative lymph nodes. AUC = area under the curve.
Table 2.
Univariate analysis for the influence of different cutoff points of the number of NLNs on CSS or OS in breast cancer patients with PMRT.

3.3. Prognostic value of the number of NLNs on CSS and OS
The prognoses of all the patients were further analyzed using univariate analysis. The number of NLNs and other clinicopathological factors, including race, year of diagnosis, tumor size, grade, ER, PR, were significant risk factors for CSS and OS (Table 3). The Kaplan–Meier estimates showed that the CSS and OS for patients with the number of >5 NLNs were significantly higher than the patients with the number of ≤5 NLNs (χ2 = 608.099, P < 0.001 for CSS; χ2 = 636.564, P < 0.001 for OS) (Fig. 2). Multivariate analysis with Cox regression was further performed and found that the number of NLNs (with the cutoff of 5) were independent prognostic factors for CSS and OS and the number of >5 NLNs were found to have a positive effect on CSS (hazard ratio [HR] 0.620; 95% confidence interval [CI] 0.587–0.656; P < 0.001) and OS (HR 0.649; 95% CI 0.618–0.682; P < 0.001) (Table 4).
Table 3.
Univariate survival analyses to evaluate the influence of the number of NLNs on CSS or OS in breast cancer patients with PMRT.

Figure 2.

The OS and CSS curves in breast cancer patients after PMRT with the number of NLNs >5 and ≤5 between 1990 and 2009. (A) The OS curves: the number of NLNs >5 vs. the number of NLNs ≤5 (χ2 = 636.564, P < 0.001). (B) The CSS curves: the number of NLNs >5 vs. the number of NLNs ≤5 (χ2 = 608.099, P < 0.001). CSS = cancer-specific survival, OS = overall survival, PMRT = postmastectomy radiotherapy, NLNs = negative lymph nodes.
Table 4.
Multivariate survival analyses to evaluate the influence of the number of NLNs on CSS or OS in breast cancer patients with PMRT.

3.4. Subgroup analysis to evaluate the prognostic value of the number of NLNs according to different variables
Univariate analyses have showed that race, year of diagnosis, tumor size, grade, ER status, PR status, number of NLNs were prognostic factors affecting CSS or OS (all, P < 0.001) (Table 3). Further multivariate analysis showed that the number of NLNs was an independent prognostic factor of CSS or OS. In addition, race, year of diagnosis, tumor size, grade, ER status, PR status were also independent risk factors affecting CSS or OS (all, P < 0.01) (Table 4). Then we conducted subgroup analysis of the impact of the number of NLNs on CSS or OS by different variables. As our expected, the results showed that patients with the number of more than five NLNs had better CSS in all subgroups (all, P < 0.001) (Fig. 3). Interestingly, the maximum hazard ratio (HR) could be found in Stage T4 subgroup (HR, 0.65; 95% CI, 0.58–0.73; P < 0.001), and the minimal HR could be found in Grade I subgroup (HR, 0.34; 95% CI, 0.25–0.46; P < 0.001) (Fig. 3). In addition, the results also showed that patients with the number of >5 NLNs had better OS in all subgroups (all, P < 0.001) (Fig. 4). The maximum HR could be found in Stage T4 subgroup (HR, 0.67; 95% CI, 0.61–0.74; P < 0.001), and the minimal HR could be found in Grade I subgroup (HR, 0.45; 95% CI, 0.35–0.57; P < 0.001) (Fig. 4).
Figure 3.

Hazard ratio comparing CSS between patients with >5 NLNs and patients with ≤5 NLNs according to clinical variables (all, P < 0.001). CSS = cancer-specific survival, NLNs = negative lymph nodes.
Figure 4.
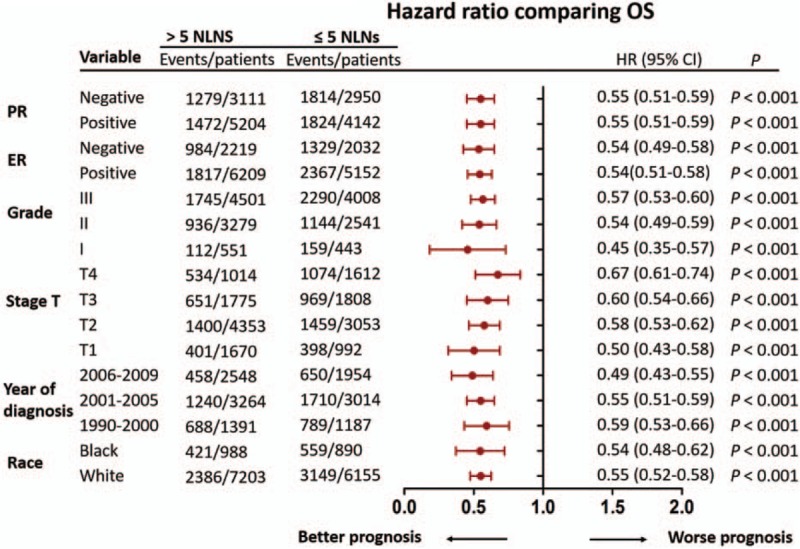
Hazard ratio comparing OS between patients with >5 NLNs and patients with ≤5 NLNs according to clinical variables (all, P < 0.001). OS = overall survival, NLNs = negative lymph nodes.
3.5. Survival rate for patients with PMRT and no-PMRT according to different cutoff point of NLNs
PMRT has been a postoperative standard therapy for patients with ≥4 lymph nodes invasion. In our study, we chose the patients with ≥4 positive lymph nodes after they underwent PMRT. The result showed that the number of NLNs were prognostic factors for CSS and OS in the part of the patients. Interestingly, we found that the patients with the number of >5 NLNs who underwent PMRT had an improved OS and CSS compared with the patients’ non-PMRT (χ2 = 255.00, P < 0.001 for OS; χ2 = 87.04, P < 0.001 for CSS) (Fig. 5A). Importantly, we then chose 10 or 16 as the cutoff point of the number of NLNs. The results also showed that the patients who underwent PMRT all had an improved OS and CSS compared with the no-PMRT patients regardless of the cutoff point of the number of NLNs (NLNs counts >10: χ2 = 106.00, P < 0.001 for OS; χ2 = 39.56, P < 0.001 for CSS; NLNs counts >16: χ2 = 38.09, P < 0.001 for OS; χ2 = 14.25, P < 0.001 for CSS) (Fig. 5 B–C). The clinical characteristics and pathological features of all the patients with non-PMRT were summarized in supply Table 2.
Figure 5.

The OS and CSS curves in breast cancer patients with PMRT and no-PMRT according to different cutoff point of NLNs. (A) The OS and CSS curves of the patients with the number of NLNs >5. Patients with PMRT vs. patients with no-PMRT (χ2 = 255.00, P < 0.001 for OS; χ2 = 87.04, P < 0.001 for CSS). (B) The OS and CSS curves of the patients with the number of NLNs >10. Patients with PMRT vs. patients with no-PMRT (χ2 = 106.00, P < 0.001 for OS; χ2 = 39.56, P < 0.001 for CSS). (C) The OS and CSS curves of the patients with the number of NLNs >16. patients with PMRT vs. patients with no-PMRT (χ2 = 38.09, P < 0.001 for OS; χ2 = 14.25, P < 0.001 for CSS). CSS = cancer-specific survival, OS = overall survival, PMRT = postmastectomy radiotherapy, NLNs = negative lymph nodes.
4. Discussion
In our study, we investigated the prognostic value of the number of NLNs in female breast cancer patients with ≥4 positive lymph nodes after PMRT, with CSS and OS as the primary study end-points. Our results showed that the number of NLNs can be used as an important prognostic factor in breast cancer patients who underwent PMRT.
The number of NLNs is defined as the number derived from the total number of removed lymph nodes by axillary dissection minus the number of positive lymph nodes. The number of NLNs has been confirmed as an independent prognosis factor in colon, esophageal, cervical, and gastric cancer.[10–13] A recent study also showed that the number of NLNs is an independent prognostic factor of disease-free survival (DFS) in breast cancer patients after mastectomy, and patients with a higher number of NLNs have a better DFS.[6–8] To analyze the correlation between NLNs and prognosis of cancer, we assumed that identification of more NLNs may reduce the overall risk of occult lesions and help determine accurate postoperative TNM stage, and then serve as an indicator of improve survival.
PMRT has become a standard adjuvant postoperative therapy for patients with positive lymph nodes.[2–4] Recent studies from China have showed that the number of NLNs can significantly predict the prognosis of patients with PMRT.[14,15] However, these studies are limited and controversial. Xin et al[16] found that less number of NLNs would benefit from PMRT in patients of ypN1 stage. Inconsistent with the study, Wu et al[7] found that patients with an elevated number of NLNs had better survival after PMRT. In our study, we showed that the number of NLNs was a prognostic indicator in patients with PMRT and an elevated number of NLNs indicated a better prognosis for patients after PMRT, using the American SEER database.
The number of NLNs has been recently paid attention to and recognized as a prognostic indicator in breast cancer patients after PMRT. However, these studies were basically from China with relatively smaller samples. In addition, some limitations can still be found from these studies.
The first limitation was the inconsistency of cutoff point and the number of NLNs. Karlsson et al[17] found that the patients with the number of NLNs <10 have an increased risk of locoregional failure and the patients with the number of NLNs ≥10 had a significantly better prognosis. In a study conducted by Wu et al, it was found that the number of NLNs was an independent prognostic factor of locoregional recurrence-free survival (LRFS), DFS, and OS, whereas it was revealed that patients with a number of NLNs >8 had better survival in patients with PMRT.[15] Another study coming from China showed that the number of NLNs was an independent prognostic factor affecting the LRFS. Patients with a number of NLNs >12 had better survival in patients with PMRT.[14] The different cutoff point of the number of NLNs may mainly be attributed to the different clinical and pathological characteristics. And the application of different methods in determining cutoff may also lead to the various cutoff values. In our study, ROC curve was applied to define specific cutoff point, using the same method as Wu et al's. The cut-off of NLN was selected as 5, as confirmed in our study, which was relatively lower as compared with the value in other studies. To further determine the feasibility of the cutoff point, we then treated the number of NLNs as a continuous variable. Univariate log-rank test was used, demonstrating NLNs as a significant prognostic factor. We further analyzed individual number of NLNs from 5 to 16 and found the patients with ≥5 NLNs had a maximum log-rank χ2 value both in CSS and OS. Although the cutoff point in our study is different from other studies, our results showed that the number of NLNs was an independent prognostic factor of CSS and OS. Patients after PMRT with higher number of NLNs had better survival, which was consistent with other studies.
The second limitation was different subgroup analysis. In our study, we found that patients with the number of >5 NLNs had better CSS and OS in all groups including different hormonal status, different AJCC T stage, different Grade stage, different year of diagnosis, and different race. Similar to our study, Wu et al[8] found that the number of NLNs had a prognostic value in patients with different pT stages and different lymph node status. However, a study from China showed the number of NLNs had no predictive value for the efficacy with PMRT in triple-negative subtypes.[15] Unfortunately, we did not analyze the Her2+ subgroup in our study. Interestingly, our results showed that the number of NLNs had the predictive value in breast cancer patients with negative ER and PR. The difference may result from the following aspects. First, different data sources: our study mainly analyzed the data from American in SEER database and had a larger sample size. However, other studies mainly analyze the data from China, which had a smaller sample size. Second, different variables were included in these studies. For example, the patients with Her2+ were not included and the patients with Grade stage were included in our study. Third, different cutoff point of NLNs numbers may be another attributing factor.
The third limitation was different conclusion between NLNs and non-PMRT. Recent studies showed that PMRT improved the LRFS, DFS, and OS in patients with ≤12 NLNs, but the survival of patients with >12 NLNs was not affected.[14] According to this study, the patients with ≥4 positive lymph nodes would not benefit from PMRT if the NLNs out numbered 12. Interestingly, PMRT has become a standard adjuvant postoperative therapy for patients with at least lymph nodes invasion. However, in our study, we found all patients with ≥4 positive lymph nodes benefited from PMRT regardless of the number of NLNs. Therefore, the number of NLNs can be used as an important prognostic indicator for patients who underwent PMRT, but not as indicators whether to carry out PMRT for breast cancer patients.
This study also has several limitations. First, some studies have showed that the systemic therapy may bring some substantial survival benefits for PMRT.[18–20] However, because of the absence of information on chemotherapy or targeted therapy included in the SEER database, its effect on survival could not be evaluated. Second, as a retrospective study, the study has its intrinsic defects. Third, the specific dose or methods of radiotherapy was not included in our study, which can affect the survival rate of breast cancer patients. Therefore, future prospective studies from different countries and regions are needed to further confirm these results.
Acknowledgments
We thank Dawei Chen (Department of Radiation Oncology, Shandong Cancer Hospital affiliated to Shandong University, Shandong Academy of Medical Sciences, jinan, China) for statistical support.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CSS = cancer-specific survival, DFS = disease-free survival, FBC = female breast cancer, HR = hazard ratio, LRFS = locoregional recurrence-free survival, NLNs = negative lymph nodes, OS = overall survival, PMRT = postmastectomy radiotherapy, SEER = Surveillance, Epidemiology, and End Results.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
This study was financially supported by the special fund for Scientific Research in Public Interest (201402011) and Natural Science Foundation of Shandong Province: (ZR2015HM040).
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].McGale P, Taylor C, Correa C, et al. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wenz F, Sperk E, Budach W, et al. DEGRO practical guidelines for radiotherapy of breast cancer IV: radiotherapy following mastectomy for invasive breast cancer. Strahlenther Onkol 2014;190:705–14. [DOI] [PubMed] [Google Scholar]
- [4].Jagsi R. Progress and controversies: radiation therapy for invasive breast cancer. CA Cancer J Clin 2014;64:135–52. [DOI] [PubMed] [Google Scholar]
- [5].Vilarino-Varela M, Chin YS, Makris A. Current indications for post mastectomy radiation. Int Semin Surg Oncol 2009;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shi Z, Peddi P, Burton G, et al. Effect of Postmastectomy Radiation on Survival of AJCC pN2/N3 Breast Cancer Patients. Anticancer Res 2016;36:261–9. [PubMed] [Google Scholar]
- [7].Wu SG, Sun JY, Zhou J, et al. Number of negative lymphnodes is associated with disease free survival in patients with breast cancer. BMC Cancer 2015;15:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wu SG, Wang Y, Zhou J, et al. Number of negative lymph nodes should be considered for incorporation into staging for breast cancer. Am J Cancer Res 2015;5:844–53. [PMC free article] [PubMed] [Google Scholar]
- [9].Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER∗Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2014 Sub (1973-2012 varying) - Linked To County Attributes - Total U.S., 1969-2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission. [Google Scholar]
- [10].Johnson PM, Porter GA, Ricciardi R, et al. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol 2006;24:3570–5. [DOI] [PubMed] [Google Scholar]
- [11].Deng J, Liang H, Wang D, et al. Enhancement the prediction of postoperative survival in gastric cancer by combining the negative lymph node count with ratio between positive and examined lymph nodes. Ann Surg Oncol 2010;17:1043–51. [DOI] [PubMed] [Google Scholar]
- [12].Zhu Z, Chen H, Yu W, et al. Number of negative lymph nodes is associated with survival in thoracic esophageal squamous cell carcinoma patients undergoing three-field lymphadenectomy. Ann Surg Oncol 2014;21:2857–63. [DOI] [PubMed] [Google Scholar]
- [13].Chen Y, Zhang L, Tian J, et al. Combining the negative lymph nodes count with the ratio of positive and removed lymph nodes can better predict the postoperative survival in cervical cancer patients. Cancer Cell Int 2013;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wu SG, Sun JY, Zhou J, et al. Number of negative lymph nodes can predict survival of breast cancer patients with four or more positive lymph nodes after postmastectomy radiotherapy. Radiat Oncol 2014;9:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu SG, Peng F, Zhou J, et al. Number of negative lymph nodes can predict survival after postmastectomy radiotherapy according to different breast cancer subtypes. J Cancer 2015;6:261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xin F, Yu Y, Yand Zj, et al. Number of negative lymph nodes as a prognostic factor for ypN0/N1 breast cancer patients undergoing neoadjuvant chemotherapy. Tumor Biol 2016;37:8445–54. [DOI] [PubMed] [Google Scholar]
- [17].Karlsson P, Cole BF, Price KN, et al. The role of the number of uninvolved lymph nodes in predicting locoregional recurrence in breast cancer. J Clin Oncol 2007;25:2019–26. [DOI] [PubMed] [Google Scholar]
- [18].Bellon JR, Harris JR. Chemotherapy and radiation therapy for breast cancer: what is the optimal sequence? J Clin Oncol 2005;23:5–7. [DOI] [PubMed] [Google Scholar]
- [19].Liu J, Mao K, Jiang S, et al. The role of postmastectomy radiotherapy in clinically node-positive, stage II-III breast cancer patients with pathological negative nodes after neoadjuvant chemotherapy: an analysis from the NCDB. Oncotarget 2016;7:24848–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Whelan TJ, Julian J, Wright J, et al. Does locoregional radiation therapy improve survival in breast cacner? A meta-analysis. J Clin Oncol 2000;18:1220–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


