Abstract
CYP3A4, an isoform of cytochrome P450 enzymes, is responsible for the metabolism of 45% to 60% of currently prescribed drugs. It has been shown that CYP3A4∗1G, a single nucleotide polymorphism (SNP), affects the enzymatic activity of CYP3A4. Sufentanil, a synthetic opioid commonly used for the induction and maintenance of general anesthesia, analgesia, and sedation, is mainly metabolized by CYP3A4. So far, the impact of CYP3A4∗1G on sufentanil metabolism has not been investigated. In the present study, we first determined the frequency of CYP3A4∗1G polymorphism in patients of Chinese Han nationality who underwent lung resection, and then compared the amount of sufentanil used in general anesthesia during the surgical procedure between wild type and mutant patients.
DNA sequencing was performed to genotype the CYP3A4∗1G allele in 191 patients. The sufentanil dosages consumed in general anesthesia were recorded and compared between wild-type and mutant patients.
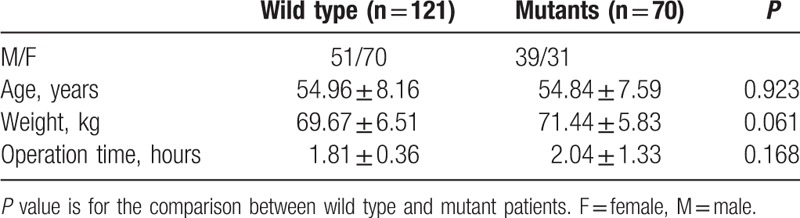
The frequency of the CYP3A4∗1G variant allele was 0.202 (77/382). No significant difference was observed in age, body weight, or operation time between wild-type and mutant patients. The amount of sufentanil consumed by patients with the point mutation was significantly lower than that in the wild type group. No significant difference in sufentanil dosages was observed between females and males within wild type or within mutant group.
High frequency of CYP3A4∗1G variants was detected in patients of Chinese Han nationality. Significantly lower amount of sufentanil was consumed in mutant patients compared with wild type subjects, likely a result of impaired CYP3A4 activity due to the point mutation. These findings suggest genotyping of CYP3A4 might be of value in providing guidance for the use of sufentanil.
Keywords: anesthesia, cytochrome P-450 CYP3A, genotyping, single nucleotide polymorphism, sufentanil
1. Introduction
Cytochrome P450 (CYP) enzymes, a superfamily of mono-oxygenases, are the major enzymes involved in drug metabolism.[1] Among all CYP isoforms, CYP3A4 is believed to be the most important one, which is responsible for the metabolism of 45% to 60% of currently prescribed drugs.[2] CYP3A4 single nucleotide polymorphisms (SNPs) affecting its enzymatic activity have been well documented.[3–5] CYP3A4∗1G, a form of SNP that is commonly detected in Asian populations, has been shown to regulate the metabolism of a variety of drugs.[6–11]
Sufentanil, a synthetic opioid, is widely used for the induction and maintenance of general anesthesia, analgesia, and sedation.[12–14] It is mainly metabolized by CYP3A4 in the liver.[15] Effects of CYP3A4∗1G on the metabolism of various drugs including analgesic compounds have been reported.[6–11] However, impact of CYP3A4∗1G on sufentanil metabolism remains elusive. In this study, we first used DNA sequencing technique to determine the frequency of CYP3A4∗1G polymorphism in patients of Chinese Han nationality who underwent lung resection, and then compared the amount of sufentanil consumed in general anesthesia between wild type and mutant patients.
2. Methods
2.1. Patients
A total of 191 patients of Chinese Han nationality, aged 29 to 65 years, undergoing lung resection in our hospital from January 2014 to December 2015 were included in this study. The study protocol, the collection, and use of clinical data were approved by the Research Ethics Committee of Weihai Municipal Hospital. Written informed consent to participating in this study was obtained from all subjects. All patients were classified as physical status I or II according to the American Society of Anesthesiologists Physical Status Classification System. None of the patients had known history of chronic pain, psychiatric diseases, diabetes mellitus, severe cardiovascular diseases, kidney, or liver diseases. No medication known to affect CYP3A4 activity was taken at least 2 week prior to the surgery.
2.2. General anesthesia
Induction of general anesthesia was accomplished by intravenous administration of sufentanil (0.3 μg/kg), midazolam (0.1 mg/kg), vecuronium bromide (0.08 mg/kg), and etomidate (0.3 mg/kg). Anesthesia was maintained with an inhalation and intravenous combined mode (inhalation: sevoflurane, 1–2 mac; and intravenous: propofol, 4 mg/kg/hour). Sufentanil was supplemented during the entire surgical procedure according to patients’ anesthetic situation. The amount of total sufentanil used for each patient and the length of operation were recorded. Patients were mechanically ventilated. Continuous ECG monitoring, end-tidal CO2 monitoring, and pulse oximetry were performed during the entire surgical procedure.
2.3. CYP3A4 genotyping
Genotyping of the CYP3A4∗1G allele was done by DNA sequencing. Briefly, genomic DNA was prepared from peripheral venous blood using a Blood DNA Extraction Kit (Tiangen Biotech Co., Ltd, Beijing, China) according to the manufacturer's instructions. A DNA fragment containing the polymorphic site was amplified by PCR using primers: 5′-GTAATAGAAAGCAGATGAACC-3′ (forward) and 5′-TCACCCTGATGTCCAGCAGAA-3′ (reverse). PCR was done in a total volume of 50 μL containing 50 ng genomic DNA, 5 μL of 10 × PCR buffer, 0.2 μM each primer, 2 μL of 5 mM dNTP, and 1 unit of TaKaRa HotStart Taq polymerase. Nuclease-free water was used to adjust the reaction volume. All PCR reagents were obtained from TaKaRa Bio Inc. (Beijing, China). PCR was run in 3 stages: stage 1, 95 °C for 10 minutes; stage 2, 30 cycles of 95 °C for 10 minutes, 60 °C for 15 seconds, and 72 °C for 30 seconds; and stage 3: 72 °C for 10 minutes. PCR product was purified using the QIAquick PCR Purification Kit (Qiagen, Shanghai, China) following the manufacturer's protocol, and the final product was then sequenced to determine the polymorphism.
2.4. Statistical analysis
All data were expressed as mean ± SD. Statistical analysis of the differences was performed by Student t test using the SPSS10.0 software (SPSS Inc., Chicago, IL). P < .05 was considered statistically significant.
3. Results
3.1. Study subjects
A total of 191 patients, 90 male and 101 female, aged from 29 to 65 years, were studied. Among these subjects, 23 had pulmonary nodules with ground-glass opacity as detected by computed tomography, 17 were diagnosed with lung cancer by preoperational biopsy, 1 had lung cyst, 1 had fungal infection, 69 were with benign nodules, and 80 had lung cancer as determined by postsurgical pathological analysis.
3.2. CYP3A4 genotyping
Of all 191 patients, 121 were of wild type homozygotes (∗1/∗1), 63 were heterozygous (∗1/∗1G), and 7 homozygous mutants (∗1G/∗1G). The frequency of the CYP3A4∗1G variant allele was 0.202 (77/382). According to their genotypes, patients were divided into wild type and mutant groups. As shown in Table 1, the age, body weight, and operation time were not significantly different between the wild type and mutant groups.
Table 1.
Genotyping of all patients.
3.3. Sufentanil consumption
The amount of sufentanil consumed during the surgical procedure for wild type and mutant subjects were 41.07 ± 5.49 and 30.43 ± 3.69 μg, respectively (Fig. 1). Statistical analysis showed that the dosage used in mutant group was significantly lower than that in wild type group (P < 0.001). No significant difference in dosages was observed between the female and the male within either wild type group or mutant group (data not shown).
Figure 1.
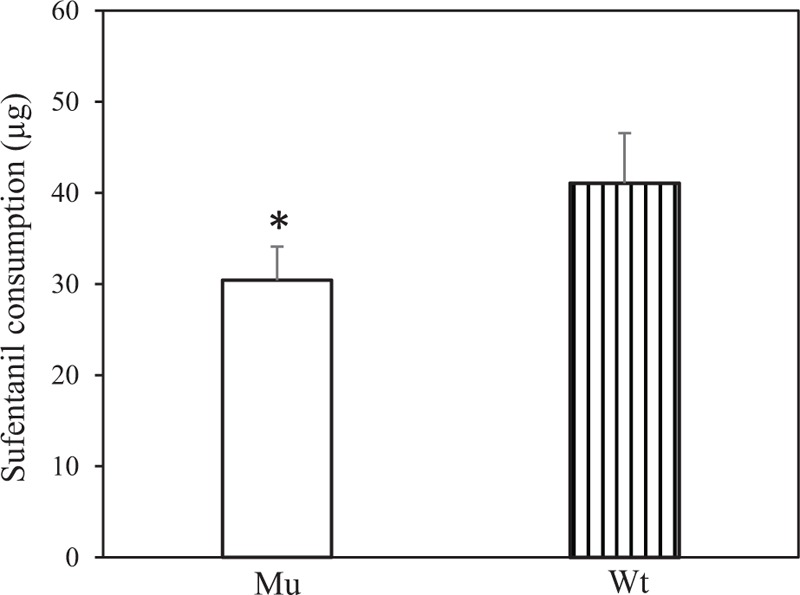
Sufentanil consumption in patients. The amount of sufentanil consumed during the surgical procedure for mutant subjects was significantly lower than that in wild type group. ∗P < 0.001 compared with wild type. Mu = mutant, Wt = wild type.
4. Discussion
The SNP in CYP3A4 has been commonly detected among different ethnic populations.[5–11,16,17] CYP3A4∗1G, in particular, is frequently found in Asian populations.[6–11,16] Miura et al[6] reported a high frequency of CYP3A4∗1G in Japanese patients with kidney disease. Gao et al[7] genotyped 217 patients with hyperlipidemia and found the frequency of CYP3A4∗1G was 0.276. Other studies have reported a frequency of CYP3A4∗1G ranging from 0.188 to 0.227 in patients of Chinese Han nationality.[8–11] In the present study, using PCR to determine the CYP3A4∗1G allele in 191 patients, we showed that the frequency of the CYP3A4∗1G variant allele was 0.202, which is in line with previously reported results, indicating a high frequency of CYP3A4∗1G polymorphism in Chinese Han population.
Several studies have shown that CYP3A4∗1G affects the metabolism of fentanyl, another synthetic opioid analgesics. Yuan et al[9] observed that 30 minutes after anesthesia induction with fentanyl, the plasma fentanyl concentration in patients with wild type CYP3A4 was significantly lower than that in individuals with the point mutation, suggesting that mutation impairs CYP3A4 activity, resulting in reduced metabolism and higher plasma concentration of fentanyl. Dong et al[10] studied the effect of fentanyl in postoperative intravenous patient-controlled analgesia in Chinese female patients after hysterectomy. They discovered that 4 hours after the initiation of patient-controlled analgesia, fentanyl consumption was significantly lower in subjects with CYP3A4∗1G than in wild-type patients. In this study, we compared the amount of sufentanil consumed between wild type and mutant patients. Although no significant difference in the age, body weight, or length of surgical procedure was observed between the 2 groups, we found significantly lower amount of sufentanil consumed by mutant patients, likely a result of impaired CYP3A4 activity due to the point mutation.
We did not observe significant difference in the sufentanil dosages used between the female and the male patients within either wild type group or mutant group (data not shown), suggesting a similar metabolic rate for sufentanil in males and females. An in vitro study using liver microsomes has demonstrated that there is no gender-related differences in the metabolic rate of fentanyl.[10] Shimada et al[2] compared the CYP3A4 activity between females and males using different substrates and found no gender-related difference in metabolizing drugs, toxic chemicals, or carcinogens. Similar findings were also reported by other groups.[18,19]
We did not have the necessary in-house equipment for the measurement of sufentanil blood concentration. Great efforts were made to explore outsources for such a service, but unfortunately it was unsuccessful. Therefore, the blood concentration of sufentanil in patients was not determined, a limit of this study. Nevertheless, we clearly showed a high frequency of CYP3A4∗1G variants in patients and significantly lower amount of sufentanil consumed in mutant patients. In view of the high frequency of CYP3A4∗1G variants in Chinese population, sufentanil is a potent drug (approximately 5–10 times more potent than fentanyl) and its accumulation may cause over-sedation, CYP3A4∗1G genotyping therefore might be of value in providing guidance for the use of sufentanil.
Footnotes
Abbreviations: CYP = cytochrome P450, SNP = single nucleotide polymorphism.
HZ and MC contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Guengerich FP. Cytochrome p450 and chemical toxicology. Chem Res Toxicol 2008;21:70–83. [DOI] [PubMed] [Google Scholar]
- [2].Shimada T, Yamazaki H, Mimura M, et al. Inter-individual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 1994;270:414–23. [PubMed] [Google Scholar]
- [3].Agrawal V, Choi JH, Giacomini KM, et al. Substrate-specific modulation of CYP3A4 activity by genetic variants of cytochrome P450 oxidoreductase. Pharmacogenet Genomics 2010;20:611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ozdemir V, Kalow W, Tang BK, et al. Evaluation of the genetic component of variability in CYP3A4 activity: a repeated drug administration method. Pharmacogenetics 2000;10:373–88. [DOI] [PubMed] [Google Scholar]
- [5].Genvigir FD, Salgado PC, Felipe CR, et al. Influence of the CYP3A4/5 genetic score and ABCB1 polymorphisms on tacrolimus exposure and renal function in Brazilian kidney transplant patients. Pharmacogenet Genomics 2016;26:462–72. [DOI] [PubMed] [Google Scholar]
- [6].Miura M, Satoh S, Kagaya H, et al. Impact of the CYP3A4∗1G polymorphism and its combination with CYP3A5 genotypes on tacrolimus pharmacokinetics in renal transplant patients. Pharmacogenomics 2011;12:977–84. [DOI] [PubMed] [Google Scholar]
- [7].Gao Y, Zhang LR, Fu Q. CYP3A4∗1G polymorphism is associated with lipid-lowering efficacy of atorvastatin but not of simvastatin. Eur J Clin Pharmacol 2008;64:877–82. [DOI] [PubMed] [Google Scholar]
- [8].Zhang W, Chang YZ, Kan QC, et al. CYP3A4∗1G genetic polymorphism influences CYP3A activity and response to fentanyl in Chinese gynecologic patients. Eur J Clin Pharmacol 2010;66:61–6. [DOI] [PubMed] [Google Scholar]
- [9].Yuan R, Zhang X, Deng Q, et al. Impact of CYP3A4∗1G polymorphism on metabolism of fentanyl in Chinese patients undergoing lower abdominal surgery. Clin Chim Acta 2011;412:755–60. [DOI] [PubMed] [Google Scholar]
- [10].Dong ZL, Li H, Chen QX, et al. Effect of CYP3A4∗1G on the fentanyl consumption for intravenous patient-controlled analgesia after total abdominal hysterectomy in Chinese Han population. J Clin Pharm Ther 2012;37:153–6. [DOI] [PubMed] [Google Scholar]
- [11].Yuan JJ, Hou JK, Zhang W, et al. CYP3A4∗1G Genetic polymorphism influences metabolism of fentanyl in human liver microsomes in Chinese patients. Pharmacology 2015;96:55–60. [DOI] [PubMed] [Google Scholar]
- [12].Monk JP, Beresford R, Ward A. Sufentanil: a review of its pharmacological properties and therapeutic use. Drugs 1988;36:286–313. [DOI] [PubMed] [Google Scholar]
- [13].Savoia G, Loreto M, Gravino E. Sufentanil: an overview of its use for acute pain management. Minerva Anestesiol 2001;67(9 Suppl 1):206–16. [PubMed] [Google Scholar]
- [14].Minkowitz HS. A review of sufentanil and the sufentanil sublingual tablet system for acute moderate to severe pain. Pain Manag 2015;5:237–50. [DOI] [PubMed] [Google Scholar]
- [15].Tateishi T, Krivoruk Y, Ueng YF, et al. Identification of human liver cytochrome P-450 3A4 as the enzyme responsible for fentanyl and sufentanil N-dealkylation. Anesth Analg 1996;82:167–72. [DOI] [PubMed] [Google Scholar]
- [16].Du J, Xing Q, Xu L, et al. Systematic screening for polymorphisms in the CYP3A4 gene in the Chinese population. Pharmacogenomics 2006;7:831–41. [DOI] [PubMed] [Google Scholar]
- [17].Lamba JK, Lin YS, Thummel K, et al. Common allelic variants of cytochrome P450 3A4 and their prevalence in different populations. Pharmacogenetics 2002;12:121–32. [DOI] [PubMed] [Google Scholar]
- [18].Sitar DS. Human drug metabolism in vivo. Pharmacol Ther 1989;43:363–75. [DOI] [PubMed] [Google Scholar]
- [19].Wrighton SA, Brian WR, Sari MA, et al. Studies on the expression and metabolic capabilities of human liver cytochrome P450IIIA5 (HLp3). Mol Pharmacol 1990;38:207–13. [PubMed] [Google Scholar]


